Recent Advances in Capacitive Deionization: Research Progress and Application Prospects
Abstract
:1. Introduction
2. Reaction Mechanism of CDI

3. Optimization Strategies of CDI
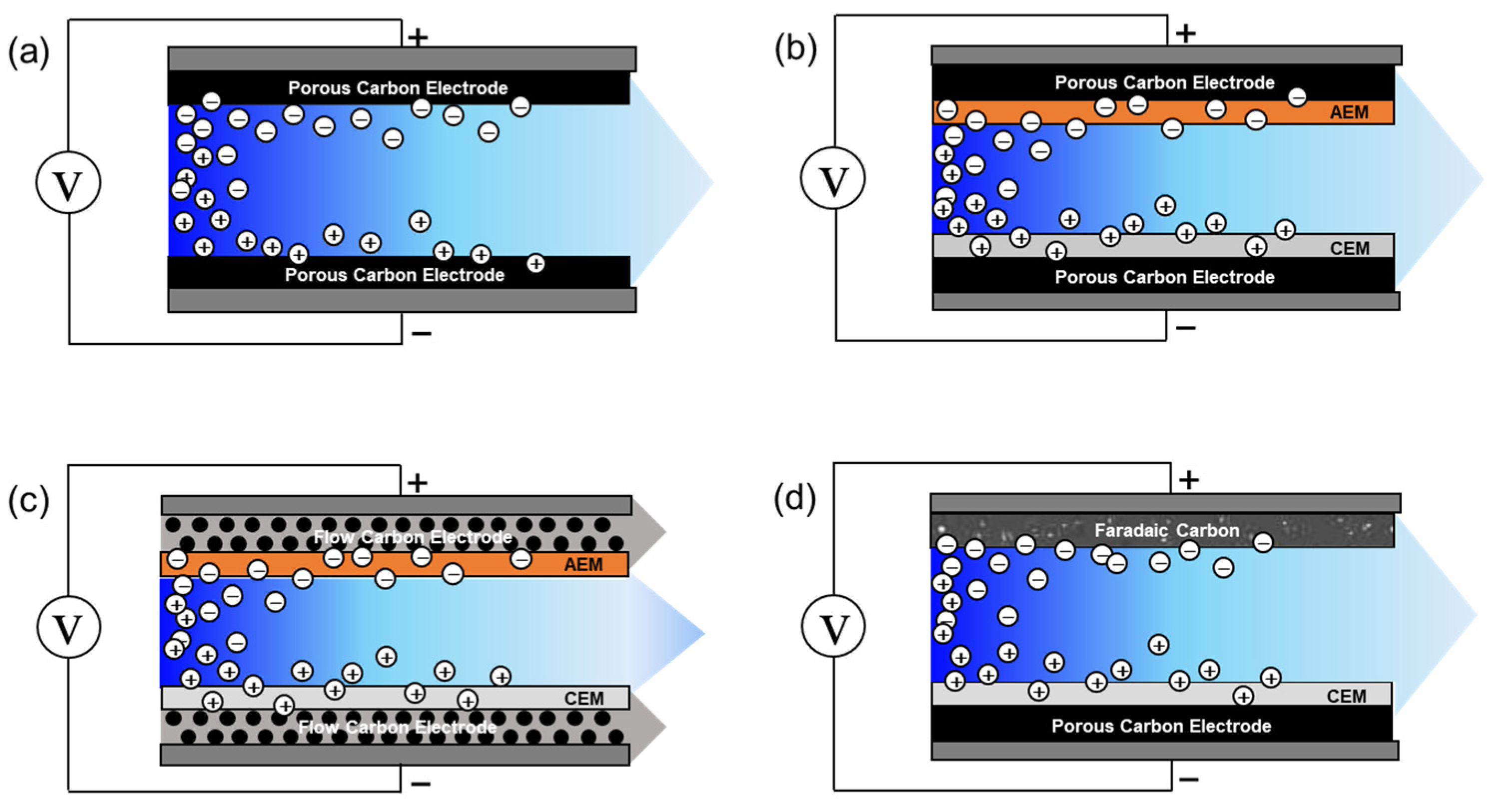
3.1. Cell Architecture
3.1.1. Classic CDI
3.1.2. Membrane Capacitive Deionization (MCDI)
3.1.3. Flow Electrode Capacitive Deionization (FCDI)
3.1.4. Hybrid Capacitive Deionization (HCDI)
3.2. Electrode Material Design
3.2.1. Carbon Material

3.2.2. Insertion Electrode Materials
3.2.3. Polymer Electrode Materials
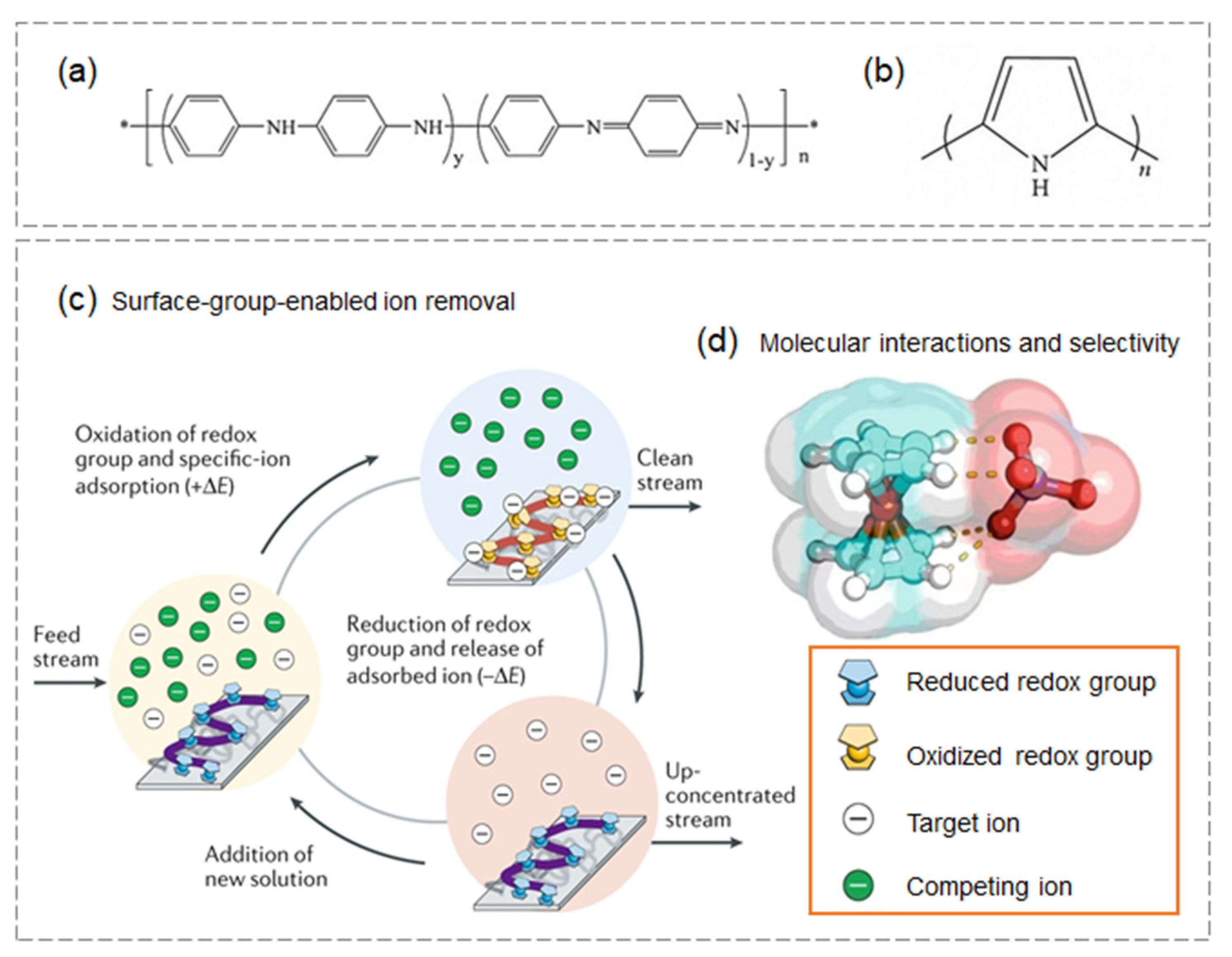
3.2.4. Fibrous Membranes Materials
3.3. Operation Mode
3.4. Technoeconomic Evaluation
4. Emerging Application Fields
4.1. Contaminant Removal
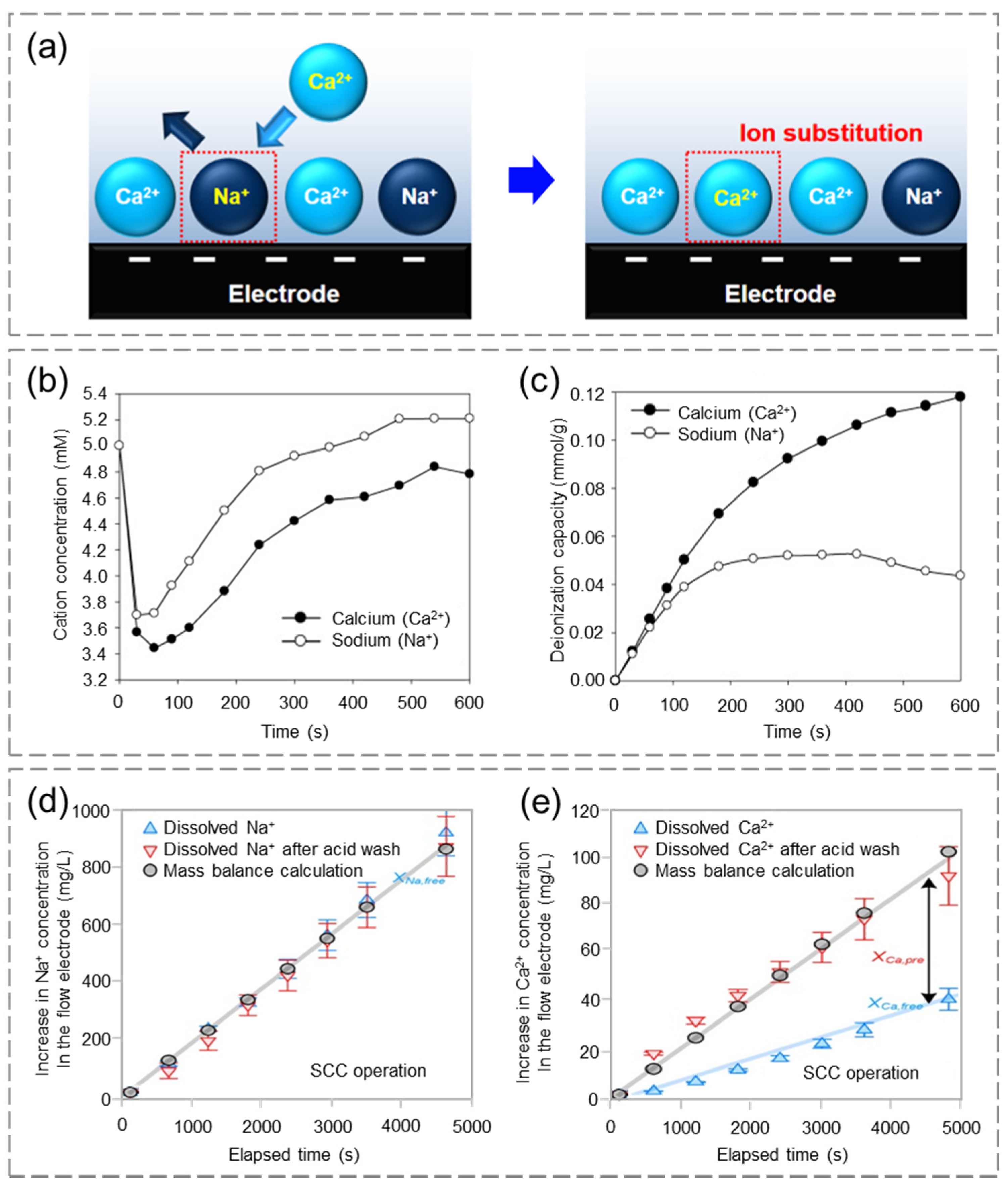
4.1.1. Water Softening
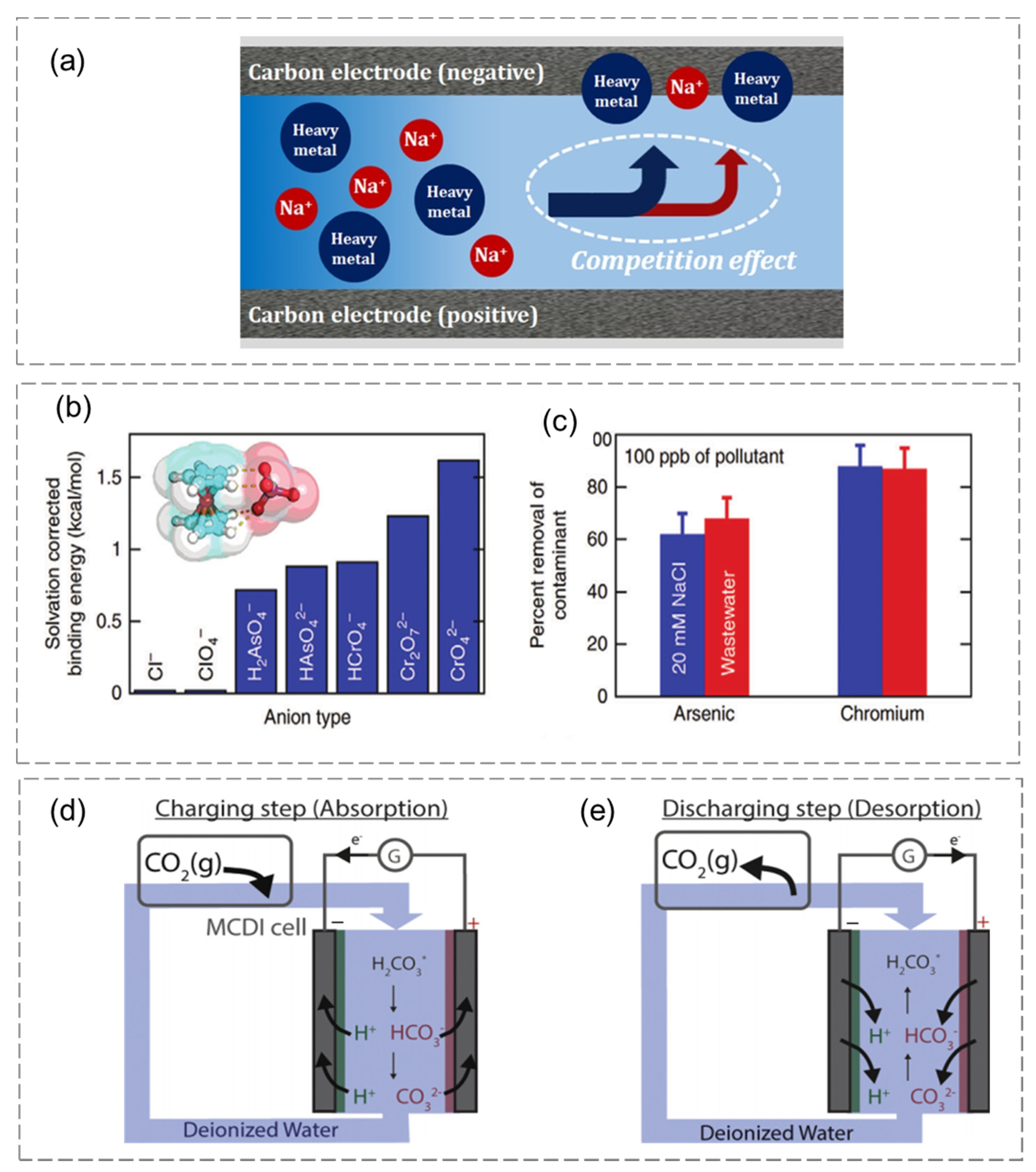
4.1.2. Heavy-Metal Removal
4.1.3. CO2 Capture
4.2. Resource Recovery
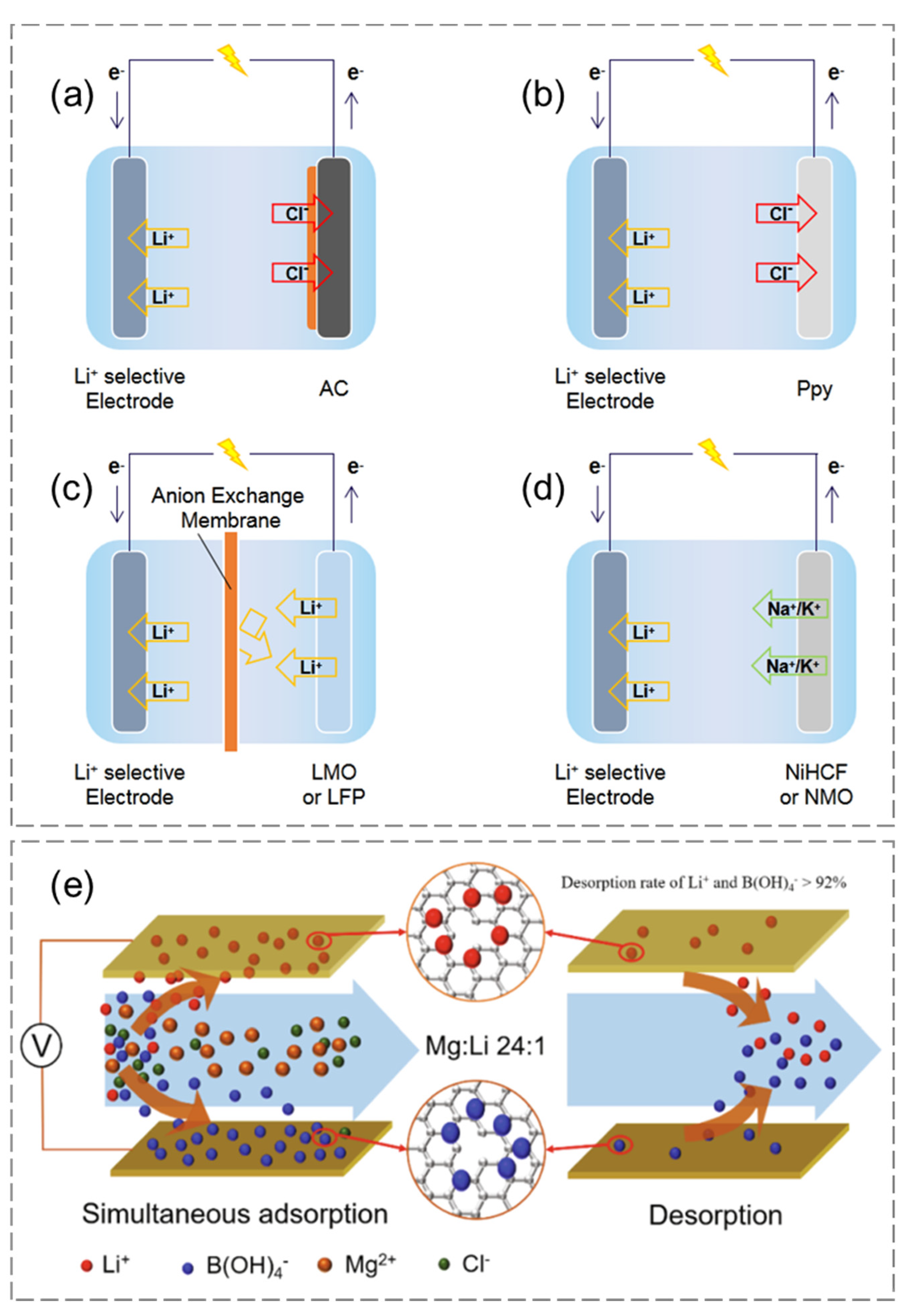
4.2.1. Lithium Extraction
4.2.2. Biological Nutrient Removal
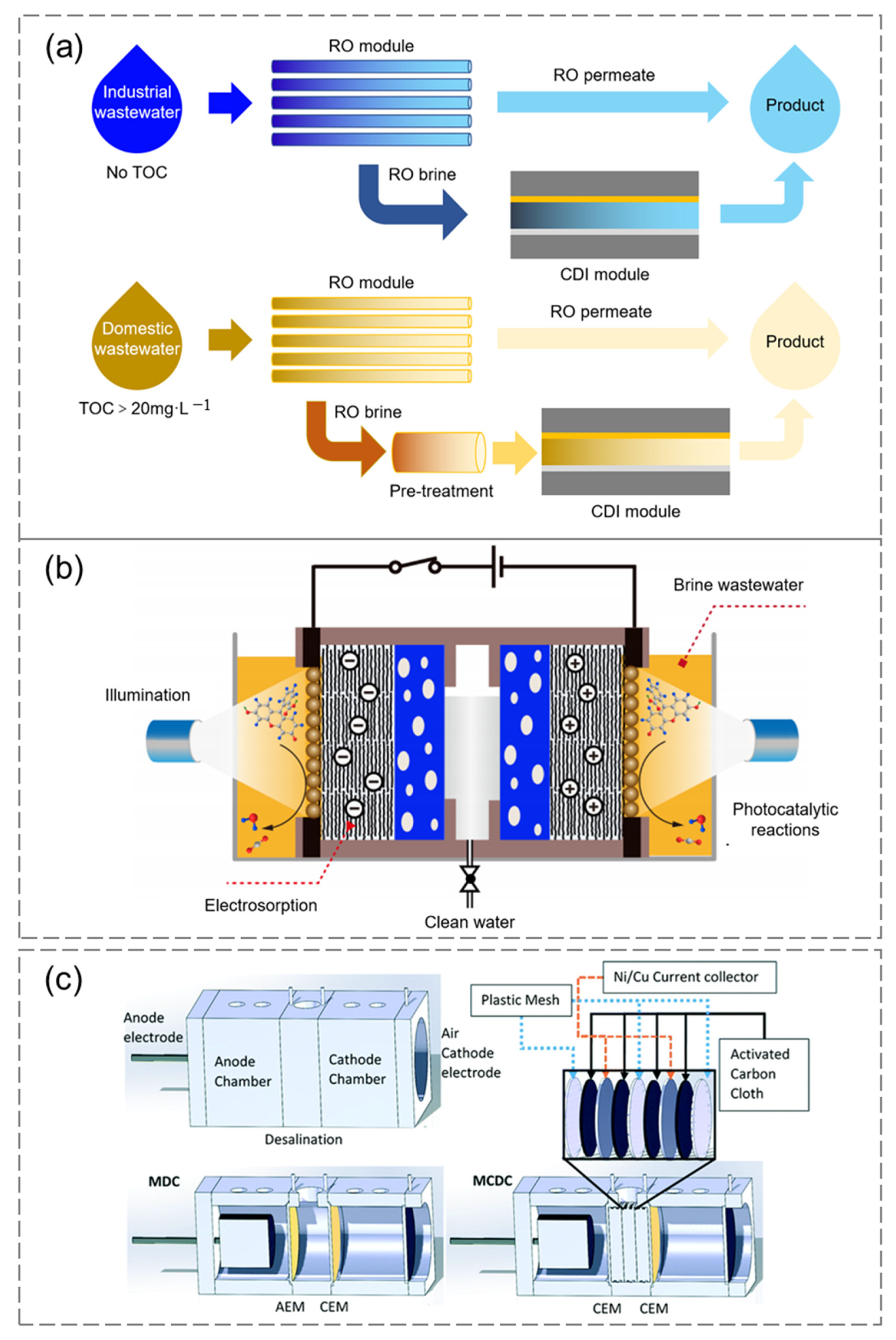
4.3. Coupling System and Application
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.W.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268. [Google Scholar] [CrossRef]
- Murphy, G.W.; Caudle, D.D. Mathematical theory of electrochemical demineralization in flowing systems. Electrochim. Acta 1967, 12, 1655–1664. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; van Limpt, B.; Van der Wal, A. Dynamic adsorption/desorption process model for capacitive deionization. J. Phys. Chem. C 2009, 113, 5636. [Google Scholar] [CrossRef]
- Bockris, J.O.M. The structure of water in the double layer. Inorg. Chim. Acta 1980, 40, X14. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Miedema, H.; Bruning, H.; van der Wal, A. Charge efficiency: A functional tool to probe the double-layer structure inside of porous electrodes and application in the modeling of capacitive deionization. J. Phys. Chem. Lett. 2010, 1, 205–210. [Google Scholar] [CrossRef]
- Evans, S.; Hamilton, W.S. The mechanism of demineralization at carbon electrodes. J. Electrochem. Soc. 1966, 113, 1314. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Fu, Y.; Bazant, M.Z. Diffuse charge and Faradaic reactions in porous electrodes. Phys. Rev. E 2011, 83, 061507. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Dykstra, J.E.; Porada, S.; van der Wal, A.; Biesheuvel, P.M. Energy consumption in capacitive deionization-constant current versus constant voltage operation. Water Res. 2018, 143, 367–375. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Eum, H.M.; Lee, C.W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Jeon, S.-I.; Park, H.-R.; Yeo, J.-G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, Z.-H.; Cui, T.; Gui, X.; Kang, F.; Wang, K.; Wu, D. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J. Mater. Chem. 2011, 21, 18295–18299. [Google Scholar] [CrossRef]
- Li, Z.; Song, B.; Wu, Z.; Lin, Z.; Yao, Y.; Moon, K.-S.; Wong, C.P. 3D porous graphene with ultrahigh surface area for microscale capacitive deionization. Nano Energy 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Xu, X.T.; Allah, A.E.; Wang, C.; Tan, H.B.; Farghali, A.A.; Khedr, M.H.; Malgras, V.; Yang, T.; Yamauchi, Y. Capacitive deionization using nitrogen-doped mesostructured carbons for highly efficient brackish water desalination. Chem. Eng. J. 2019, 362, 887–896. [Google Scholar] [CrossRef]
- Kumar, R.; Sen Gupta, S.; Katiyar, S.; Raman, V.K.; Varigala, S.K.; Pradeep, T.; Sharma, A. Carbon aerogels through organo-inorganic co-assembly and their application in water desalination by capacitive deionization. Carbon 2016, 99, 375–383. [Google Scholar] [CrossRef]
- Beguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Suss, M.E.; Presser, V. Water desalination with energy storage electrode materials. Joule 2018, 2, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Chang, B.; Kim, S.; Lee, J.; Yoon, J.; Choi, J.W. Battery electrode materials with omnivalent cation storage for fast and charge-efficient ion removal of asymmetric capacitive deionization. Adv. Funct. Mater. 2018, 28, 1802665. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.-R.; Kang, J.; Kim, S.; Kim, C.; Yoon, J. Capacitive deionization with Ca-alginate coated-carbon electrode for hardness control. Desalination 2016, 392, 46–53. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Lee, J.; Yu, S.-H.; Kim, C.; Sung, Y.-E.; Yoon, J. Highly selective lithium recovery from brine using a lambda-MnO2-Ag battery. Phys. Chem. Chem. Phys. 2013, 15, 7690–7695. [Google Scholar] [CrossRef] [PubMed]
- Siekierka, A.; Tomaszewska, B.; Bryjak, M. Lithium capturing from geothermal water by hybrid capacitive deionization. Desalination 2018, 436, 8–14. [Google Scholar] [CrossRef]
- Pasta, M.; Battistel, A.; La Mantia, F. Batteries for lithium recovery from brines. Energy Environ. Sci. 2012, 5, 9487–9491. [Google Scholar] [CrossRef]
- Trocoli, R.; Battistel, A.; La Mantia, F. Nickel hexacyanoferrate as suitable alternative to Ag for electrochemical lithium recovery. ChemSusChem 2015, 8, 2514–2519. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Kim, S.; Lee, J.; Yoon, J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci. Water Res. Technol. 2018, 4, 175–182. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.; Shin, D.; Lee, J.; Yoon, J. Electrochemical selective ion separation in capacitive deionization with sodium manganese oxide. J. Colloid Interface Sci. 2017, 506, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Electrochemical sodium ion impurity removal system for producing high purity KCl. Hydrometallurgy 2018, 175, 354–358. [Google Scholar] [CrossRef]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization of NH4ClO4 solutions with carbon aerogel electrodes. J. Appl. Electrochem. 1996, 26, 1007–1018. [Google Scholar] [CrossRef]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes. J. Electrochem. Soc. 1996, 143, 159–169. [Google Scholar] [CrossRef]
- Legrand, L.; Shu, Q.; Tedesco, M.; Dykstra, J.E.; Hamelers, H.V.M. Role of ion exchange membranes and capacitive electrodes in membrane capacitive deionization (MCDI) for CO2 capture. J. Colloid Interface Sci. 2020, 564, 478–490. [Google Scholar] [CrossRef]
- Legrand, L.; Schaetzle, O.; de Kler, R.C.F.; Hamelers, H.V.M. Solvent-free CO2 capture using membrane capacitive deionization. Environ. Sci. Technol. 2018, 52, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Singh, A.; Mishra, B.K. Capacitive deionized hybrid systems for wastewater treatment and desalination: A review on synergistic effects, mechanisms and challenges. Chem. Eng. J. 2021, 417, 128129. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of capacitive deionization in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Wang, Y.; Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 2019, 850, 113389. [Google Scholar] [CrossRef]
- Długołęcki, P.; van der Wal, A. Energy recovery in membrane capacitive deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, T.; Shin, H.; Lee, J.; Ha, J.-I.; Yoon, J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; van der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Bryjak, M.; van der Wal, A.; Biesheuvel, P.M. Effect of electrode thickness variation on operation of capacitive deionization. Electrochim. Acta 2012, 75, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; van Soestbergen, M.; Rijnaarts, H.H.M.; van der Wal, A.; Bazant, M.Z.; Biesheuvel, P.M. Time-dependent ion selectivity in capacitive charging of porous electrodes. J. Colloid Interface Sci. 2012, 384, 38–44. [Google Scholar] [CrossRef]
- Kamran, K.; van Soestbergen, M.; Pel, L. Electrokinetic salt removal from porous building materials using ion exchange membranes. Transp. Porous Med. 2013, 96, 221–235. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Fu, Y.; Bazant, M.Z. Electrochemistry and capacitive charging of porous electrodes in asymmetric multicomponent electrolytes. Russ. J. Electrochem. 2012, 48, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Kastening, B.; Heins, M. Properties of electrolytes in the micropores of activated carbon. Electrochim. Acta 2005, 50, 2487–2498. [Google Scholar] [CrossRef]
- Arafat, H.A.; Franz, M.; Pinto, N.G. Effect of salt on the mechanism of adsorption of aromatics on activated carbon. Langmuir 1999, 15, 5997–6003. [Google Scholar] [CrossRef]
- Liu, E.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Treatment of industrial brine using capacitive deionization (CDI) towards zero liquid discharge-challenges and optimization. Water Res. 2020, 183, 116059. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Liu, Y.; Lu, T.; Pan, L. Ultrahigh desalinization performance of asymmetric flow-electrode capacitive deionization device with an improved operation voltage of 1.8 V. ACS Sustain. Chem. Eng. 2017, 5, 189–195. [Google Scholar] [CrossRef]
- Andres, G.L.; Yoshihara, Y. A capacitive deionization system with high energy recovery and effective re-use. Energy 2016, 103, 605–617. [Google Scholar] [CrossRef]
- Hou, C.H.; Liu, N.L.; Hsu, H.L.; Den, W. Development of multi-walled carbon nanotube/poly(vinyl alcohol) composite as electrode for capacitive deionization. Sep. Purif. Technol. 2014, 130, 7–14. [Google Scholar] [CrossRef]
- Srimuk, P.; Kaasik, F.; Kruener, B.; Tolosa, A.; Fleischmann, S.; Jaeckel, N.; Tekeli, M.C.; Aslan, M.; Suss, M.E.; Presser, V. MXene as a novel intercalation-type pseudocapacitive cathode and anode for capacitive deionization. J. Mater. Chem. A 2016, 4, 18265–18271. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Srimuk, P.; Zwingelstein, R.; Zornitta, R.L.; Choi, J.; Kim, C.; Presser, V. Sodium ion removal by hydrated vanadyl phosphate for electrochemical water desalination supplementary information (ESI) available. J. Mater. Chem. A 2019, 7, 4175–4184. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Fletcher, J.; Waite, T.D. Energy recovery in pilot scale membrane CDI treatment of brackish waters. Water Res. 2020, 168, 115146. [Google Scholar] [CrossRef]
- Zhan, F.; Wang, Z.; Wu, T.; Dong, Q.; Zhao, C.; Wang, G.; Qiu, J. High performance concentration capacitors with graphene hydrogel electrodes for harvesting salinity gradient energy. J. Mater. Chem. A 2018, 6, 4981–4987. [Google Scholar] [CrossRef]
- Tang, W.; He, D.; Zhang, C.; Kovalsky, P.; Waite, T.D. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes. Water Res. 2017, 120, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Bae, W.-S.; Choi, J.-H. Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process. Desalination 2010, 258, 159–163. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Levi, M.; Bazant, M.Z. Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 2014, 18, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.M.; Song, H.O.; Zhang, Q.X.; Wang, B.J.; Li, A.M. Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration. Desalination 2015, 365, 407–415. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. Study of fouling and scaling in capacitive deionization by using dissolved organic and inorganic salts. J. Hazard. Mater. 2013, 244, 387–393. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wang, Y.; Ma, D.Y.; Xu, S.C.; Wang, J.X. Investigations on the fouling characteristics of ion-doped polypyrrole/carbon nanotube composite electrodes in capacitive deionization by using half cycle running mode. Sep. Purif. Technol. 2018, 192, 15–20. [Google Scholar] [CrossRef]
- Luo, J.; Tian, D.; Ding, Z.; Lu, T.; Xu, X.; Pan, L. Enhanced cycling stability of capacitive deionization via effectively inhibiting H2O2 formation: The role of nitrogen dopants. J. Electroanal. Chem. 2019, 855, 113488. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zou, L. Ion-exchange membrane capacitive deionization: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- Laxman, K.; Myint, M.; Abri, M.A.; Sathe, P.; Dobretsov, S.; Dutta, J. Desalination and disinfection of inland brackish ground water in a capacitive deionization cell using nanoporous activated carbon cloth electrodes. Desalination 2015, 362, 126–132. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, D.; Ma, J.X.; Tang, W.W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gui, Y.; Blackwood, D.J. Development of a nanostructured alpha-MnO2/carbon paper composite for removal of Ni2+/Mn2+ ions by electrosorption. ACS Appl. Mater. Interfaces 2018, 10, 19615–19625. [Google Scholar] [CrossRef] [PubMed]
- Alaei Shahmirzadi, M.A.; Hosseini, S.S.; Luo, J.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef]
- Yang, S.; Choi, J.; Yeo, J.-G.; Jeon, S.-I.; Park, H.-R.; Kim, D.K. Flow-electrode capacitive deionization using an aqueous electrolyte with a high salt concentration. Environ. Sci. Technol. 2016, 50, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Biesheuvel, P.M.; van der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Park, H.R.; Choi, J.; Yang, S.; Kwak, S.J.; Jeon, S.I.; Han, M.H.; Kim, D.K. Surface-modified spherical activated carbon for high carbon loading and its desalting performance in flow-electrode capacitive deionization. RSC Adv. 2016, 6, 69720–69727. [Google Scholar] [CrossRef]
- Liang, P.; Sun, X.; Bian, Y.; Zhang, H.; Yang, X.; Jiang, Y.; Liu, P.; Huang, X. Optimized desalination performance of high voltage flow-electrode capacitive deionization by adding carbon black in flow-electrode. Desalination 2017, 420, 63–69. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Gendel, Y.; Wessling, M. Single module flow-electrode capacitive deionization for continuous water desalination. Electrochem. Commun. 2015, 60, 34–37. [Google Scholar] [CrossRef]
- Gendel, Y.; Rommerskirchen, A.K.E.; David, O.; Wessling, M. Batch mode and continuous desalination of water using flowing carbon deionization (FCDI) technology. Electrochem. Commun. 2014, 46, 152–156. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Linnartz, C.J.; Muller, D.; Willenberg, L.K.; Wessling, M. Energy recovery and process design in continuous flow electrode capacitive deionization processes. ACS Sustain. Chem. Eng. 2018, 6, 13007–13015. [Google Scholar] [CrossRef]
- Fang, K.; Gong, H.; He, W.; Peng, F.; He, C.; Wang, K. Recovering ammonia from municipal wastewater by flow-electrode capacitive deionization. Chem. Eng. J. 2018, 348, 301–309. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Zhang, C.; Song, J.; Waite, T.D. Short-circuited closed-cycle operation of flow-electrode CDI for brackish water softening. Environ. Sci. Technol. 2018, 52, 9350–9360. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; He, C.; He, D.; Zhang, C.Y.; Waite, T.D. Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water Res. 2018, 144, 296–303. [Google Scholar] [CrossRef]
- Yang, S.C.; Kim, H.; Jeon, S.I.; Choi, J.; Yeo, J.G.; Park, H.R.; Jin, J.; Kim, D.K. Analysis of the desalting performance of flow-electrode capacitive deionization under short-circuited closed cycle operation. Desalination 2017, 424, 110–121. [Google Scholar] [CrossRef]
- Yang, S.C.; Jeon, S.-i.; Kim, H.; Choi, J.; Yeo, J.-g. Stack design and operation for scaling up the capacity of flow-electrode capacitive deionization technology. ACS Sustain. Chem. Eng. 2016, 4, 4174–4180. [Google Scholar] [CrossRef]
- Lee, K.S.; Cho, Y.; Choo, K.Y.; Yang, S.; Han, M.H.; Kim, D.K. Membrane-spacer assembly for flow-electrode capacitive deionization. Appl. Surf. Sci. 2018, 433, 437–442. [Google Scholar] [CrossRef]
- Yang, S.C.; Park, H.R.; Yoo, J.; Kim, H.; Choi, J.; Han, M.H.; Kim, D.K. Plate-shaped graphite for improved performance of flow-electrode capacitive deionization. J. Electrochem. Soc. 2017, 164, E480–E488. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A desalination battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.; Guo, L.; Sun, L.; Wang, Y.; Yang, H.Y. Dual-ions electrochemical deionization: A desalination generator. Energy Environ. Sci. 2017, 10, 2081–2089. [Google Scholar] [CrossRef]
- Nam, D.H.; Choi, K.S. Bismuth as a New Chloride-storage electrode enabling the construction of a practical high capacity desalination battery. J. Am. Chem. Soc. 2017, 139, 11055–11063. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.; Cusick, R.D. Characterizing the impacts of deposition techniques on the performance of MnO2 cathodes for sodium electrosorption in hybrid capacitive deionization. Environ. Sci. Technol. 2017, 51, 12027–12034. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, G.; Wang, S.Y.; Zhan, F.; Fu, Y.; Qiao, H.Y.; Qiu, J.S. Highly stable hybrid capacitive deionization with a MnO2 anode and a positively charged cathode. Environ. Sci. Technol. Lett. 2018, 5, 98–102. [Google Scholar] [CrossRef]
- Yang, S.; Luo, M. In-situ embedding ZrO2 nanoparticles in hierarchically porous carbon matrix as electrode materials for high desalination capacity of hybrid capacitive deionization. Mater. Lett. 2019, 248, 197–200. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Zhang, S.; Sun, N.; Zhou, H.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Porous carbon nanosheets functionalized with Fe3O4 nanoparticles for capacitive removal of heavy metal ions from water. Environ. Sci. Water Res. Technol. 2020, 6, 331–340. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.A.; Zhou, K.F.; Wu, P.C.; Hou, C.H. Enhanced desalination performance via mixed capacitive-Faradaic ion storage using RuO2-activated carbon composite electrodes. Electrochim. Acta 2019, 295, 769–777. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Li, Y.; Lu, T.; Yao, Y.; Pan, L. Improved sodium-ion storage performance of Ti3C2Tx MXenes by sulfur doping. J. Mater. Chem. A 2018, 6, 1234–1243. [Google Scholar] [CrossRef]
- Srimuk, P.; Halim, J.; Lee, J.; Tao, Q.Z.; Rosen, J.; Presser, V. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation. ACS Sustain. Chem. Eng. 2018, 6, 3739–3747. [Google Scholar] [CrossRef] [Green Version]
- Torkamanzadeh, M.; Wang, L.; Zhang, Y.; Budak, Ö.; Srimuk, P.; Presser, V. MXene/Activated-carbon hybrid capacitive deionization for permselective ion removal at low and high salinity. ACS Appl. Mater. Interfaces 2020, 12, 26013–26025. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile synthesis of crumpled nitrogen-doped MXene nanosheets as a new sulfur host for lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef] [Green Version]
- Srimuk, P.; Lee, J.; Fleischmann, S.; Choudhury, S.; Jackel, N.; Zeiger, M.; Kim, C.; Aslan, M.; Presser, V. Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few-layered molybdenum disulfide. J. Mater. Chem. A 2017, 5, 15640–15649. [Google Scholar] [CrossRef]
- Bao, W.Z.; Tang, X.; Guo, X.; Choi, S.; Wang, C.Y.; Gogotsi, Y.; Wang, G.X. Porous cryo-dried MXene for efficient capacitive deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-G.; Li, W.; Liu, D.-P.; Feng, X.-L.; Wang, J.; Yang, X.-Y.; Zhang, X.-B.; Zhu, Y.; Zhang, Y. Flexible electrodes for sodium-ion batteries: Recent progress and perspectives. Adv. Mater. 2017, 29, 1703012. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Srimuk, P.; Lee, J.; Fleischmann, S.; Aslan, M.; Presser, V. Influence of pore structure and cell voltage of activated carbon cloth as a versatile electrode material for capacitive deionization. Carbon 2017, 122, 329–335. [Google Scholar] [CrossRef]
- Bouhadana, Y.; Avraham, E.; Noked, M.; Ben-Tzion, M.; Soffer, A.; Aurbach, D. Capacitive deionization of NaCl solutions at non-steady-state conditions: Inversion functionality of the carbon electrodes. J. Phys. Chem. C 2011, 115, 16567–16573. [Google Scholar] [CrossRef]
- Avraham, E.; Noked, M.; Soffer, A.; Aurbach, D. The feasibility of boron removal from water by capacitive deionization. Electrochim. Acta 2011, 56, 6312–6317. [Google Scholar] [CrossRef]
- Guo, L.; Kong, D.; Pam, M.E.; Huang, S.; Ding, M.; Shang, Y.; Gu, C.; Huang, Y.; Yang, H.Y. The efficient Faradaic Li4Ti5O12@C electrode exceeds the membrane capacitive desalination performance. J. Mater. Chem. A 2019, 7, 8912–8921. [Google Scholar] [CrossRef]
- Liu, P.; Yan, T.; Shi, L.; Park, H.S.; Chen, X.; Zhao, Z.; Zhang, D. Graphene-based materials for capacitive deionization. J. Mater. Chem. A 2017, 5, 13907–13943. [Google Scholar] [CrossRef]
- Khan, Z.U.; Yan, T.; Han, J.; Shi, L.; Zhang, D. Capacitive deionization of saline water using graphene nanosphere decorated N-doped layered mesoporous carbon frameworks. Environ. Sci. Nano 2019, 6, 3442–3453. [Google Scholar] [CrossRef]
- Duan, H.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. A facile strategy for the fast construction of porous graphene frameworks and their enhanced electrosorption performance. Chem. Commun. 2017, 53, 7465–7468. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J. Mater. Chem. A 2017, 5, 14748–14757. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Liu, G.; Li, J.; Tian, H.; Li, J.; Ma, Y. Scalable self-propagating high-temperature synthesis of graphene for supercapacitors with superior power density and cyclic stability. Adv. Mater. 2017, 29, 1604690. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, J.; Lei, H.; Shi, L.; An, Z.; Park, H.S.; Zhang, D. Capacitive deionization of saline water using sandwich-like nitrogen-doped graphene composites via a self-assembling strategy. Environ. Sci. Nano 2018, 5, 2722–2730. [Google Scholar] [CrossRef]
- Khan, Z.U.; Yan, T.; Shi, L.; Zhang, D. Improved capacitive deionization by using 3D intercalated graphene sheet–sphere nanocomposite architectures. Environ. Sci. Nano 2018, 5, 980–991. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, F.M.; Guo, L.; Yang, H.Y. Ultrahigh performance of a novel electrochemical deionization system based on a NaTi2(PO4)3/rGO nanocomposite. J. Mater. Chem. A 2017, 5, 18157–18165. [Google Scholar] [CrossRef]
- Lee, B.; Park, N.; Kang, K.S.; Ryu, H.J.; Hong, S.H. Enhanced Capacitive deionization by dispersion of CNTs in activated carbon dlectrode. ACS Sustain. Chem. Eng. 2018, 6, 1572–1579. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Lu, T.; Sun, Z.; Chua, D.H.C.; Pan, L. Rational design and fabrication of graphene/carbon nanotubes hybrid sponge for high-performance capacitive deionization. J. Mater. Chem. A 2015, 3, 13418–13425. [Google Scholar] [CrossRef]
- Moronshing, M.; Subramaniam, C. Scalable approach to highly efficient and rapid capacitive deionization with CNT-thread as electrodes. ACS Appl. Mater. Interfaces 2017, 9, 39907–39915. [Google Scholar] [CrossRef]
- Vinod, S.; Tiwary, C.S.; Machado, L.D.; Ozden, S.; Vajtai, R.; Galvao, D.S.; Ajayan, P.M. Synthesis of ultralow density 3D graphene-CNT foams using a two-step method. Nanoscale 2016, 8, 15857–15863. [Google Scholar] [CrossRef]
- Sriramulu, D.; Yang, H.Y. Free-standing flexible film as a binder-free electrode for an efficient hybrid deionization system. Nanoscale 2019, 11, 5896–5908. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Pan, L.; Zhang, Y.; Chen, Y.; Sun, Z. Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res. 2008, 42, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.T.; Wang, M.; Liu, Y.; Lu, T.; Pan, L.K. Metal-organic framework-engaged formation of a hierarchical hybrid with carbon nanotube inserted porous carbon polyhedra for highly efficient capacitive deionization. J. Mater. Chem. A 2016, 4, 5467–5473. [Google Scholar] [CrossRef]
- Nie, C.; Pan, L.; Liu, Y.; Li, H.; Chen, T.; Lu, T.; Sun, Z. Electrophoretic deposition of carbon nanotubes–polyacrylic acid composite film electrode for capacitive deionization. Electrochim. Acta 2012, 66, 106–109. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, F.; Ma, W.; Li, H. Metal-organic-framework derived carbon polyhedron and carbon nanotube hybrids as electrode for electrochemical supercapacitor and capacitive deionization. Electrochim. Acta 2018, 263, 85–93. [Google Scholar] [CrossRef]
- Srimuk, P.; Lee, J.; Fleischmann, S.; Aslan, M.; Kim, C.; Presser, V. Potential-dependent, switchable ion selectivity in aqueous media using titanium disulfide. Chemsuschem 2018, 11, 2091–2100. [Google Scholar] [CrossRef]
- Agartan, L.; Hantanasirisakul, K.; Buczek, S.; Akuzum, B.; Mahmoud, K.A.; Anasori, B.; Gogotsi, Y.; Kumbur, E.C. Influence of operating conditions on the desalination performance of a symmetric pre-conditioned Ti3C2Tx-MXene membrane capacitive deionization system. Desalination 2020, 477, 114267. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Yoon, J. Rocking chair desalination battery based on prussian blue electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef]
- Porada, S.; Shrivastava, A.; Bukowska, P.; Biesheuvel, P.M.; Smith, K.C. Nickel hexacyanoferrate electrodes for continuous cation intercalation desalination of brackish water. Electrochim. Acta 2017, 255, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Gorski, C.A.; Logan, B.E. Low energy desalination using battery electrode deionization. Environ. Sci. Technol. Lett. 2017, 4, 444–449. [Google Scholar] [CrossRef]
- Vafakhah, S.; Beiramzadeh, Z.; Saeedikhani, M.; Yang, H.Y. A review on free-standing electrodes for energy-effective desalination: Recent advances and perspectives in capacitive deionization. Desalination 2020, 493, 114662. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, C.; Yoon, J. Na2FeP2O7 as a novel material for hybrid capacitive deionization. Electrochim. Acta 2016, 203, 265–271. [Google Scholar]
- Wang, K.; Liu, Y.; Ding, Z.B.; Li, Y.Q.; Lu, T.; Pan, L.K. Metal-organic-frameworks-derived NaTi2(PO4)3/carbon composites for efficient hybrid capacitive deionization. J. Mater. Chem. A 2019, 7, 12126–12133. [Google Scholar] [CrossRef]
- Srimuk, P.; Lee, J.; Tolosa, A.; Kim, C.; Aslan, M.; Presser, V. Titanium disulfide: A promising low-dimensional electrode material for sodium ion intercalation for seawater desalination. Chem. Mater. 2017, 29, 9964–9973. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar]
- Guo, L.; Wang, X.; Leong, Z.Y.; Mo, R.; Sun, L.; Yang, H.Y. Ar plasma modification of 2D MXene Ti3C2Tx nanosheets for efficient capacitive desalination. FlatChem 2018, 8, 17–24. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, X.; Li, J.; Li, Y.; Wang, K.; Lu, T.; Hossain, M.S.A.; Amin, M.A.; Zhang, S.; Pan, L. Nanoarchitectonics from 2D to 3D: MXenes-derived nitrogen-doped 3D nanofibrous architecture for extraordinarily-fast capacitive deionization. Chem. Eng. J. 2022, 430, 133161. [Google Scholar] [CrossRef]
- Giorgetti, M.; Scavetta, E.; Berrettoni, M.; Tonelli, D. Nickel hexacyanoferrate membrane as a coated wire cation-selective electrode. Analyst 2001, 126, 2168–2171. [Google Scholar]
- Gao, X.; Omosebi, A.; Landon, J.; Liu, K.L. Surface charge enhanced carbon electrodes for stable and efficient capacitive deionization using inverted adsorption-desorption behavior. Energy Environ. Sci. 2015, 8, 897–909. [Google Scholar]
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Shi, W.; Liu, X.; Deng, T.; Huang, S.; Ding, M.; Miao, X.; Zhu, C.; Zhu, Y.; Liu, W.; Wu, F.; et al. Enabling superior sodium capture for efficient water desalination by a tubular polyaniline decorated with prussian blue nanocrystals. Adv. Mater. 2020, 32, 1907404. [Google Scholar] [CrossRef] [PubMed]
- Neff, V.D. Electrochemical oxidation and reduction of thin films of prussian blue. J. Electrochem. Soc. 1978, 125, 886–887. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kang, J.S.; Jo, K.; Kim, S.; Sung, Y.-E.; Yoon, J. Lithium recovery from brine using a λ-MnO2/activated carbon hybrid supercapacitor system. Chemosphere 2015, 125, 50–56. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Zhou, S.; Liu, N.; Wang, X.; Li, J.; Wang, F. Aqueous dispersed conducting polyaniline nanofibers: Promising high specific capacity electrode materials for supercapacitor. J. Power Sources 2011, 196, 10484–10489. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Li, S.; Wang, J.; Wang, C.; Wallace, G.G.; Liu, H.-K. Electrodeposited polypyrrole (PPy)/para (toluene sulfonic acid) (pTS) free-standing film for lithium secondary battery application. Electrochim. Acta 2012, 60, 201–205. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, D.; Mu, S. A rechargeable Zn- poly(aniline-co-m-aminophenol) battery. J. Power Sources 2006, 161, 685–691. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wang, C.-H.; Nataraj, S.K.; Huang, H.-C.; Du, H.-Y.; Chang, S.-T.; Chen, L.-C.; Chen, K.-H. Stand-up structure of graphene-like carbon nanowalls on CNT directly grown on polyacrylonitrile-based carbon fiber paper as supercapacitor. Diam. Relat. Mater. 2012, 25, 176–179. [Google Scholar] [CrossRef]
- Shown, I.; Ganguly, A.; Chen, L.-C.; Chen, K.-H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Bandaru, P.R.; Yamada, H.; Narayanan, R.; Hoefer, M. Charge transfer and storage in nanostructures. Mater. Sci. Eng. R Rep. 2015, 96, 1–69. [Google Scholar] [CrossRef] [Green Version]
- Amarnath, C.A.; Chang, J.H.; Kim, D.; Mane, R.S.; Han, S.-H.; Sohn, D. Electrochemical supercapacitor application of electroless surface polymerization of polyaniline nanostructures. Mater. Chem. Phys. 2009, 113, 14–17. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Graphene oxide doped polyaniline for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161. [Google Scholar] [CrossRef]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “nanofiber seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Xu, X.; Wang, Q. A polyoxometalate-based binder-free capacitive deionization electrode for highly efficient sea water desalination. Chem. Eur. J. 2020, 26, 4403–4409. [Google Scholar] [CrossRef]
- Lai, L.; Yang, H.; Wang, L.; Teh, B.K.; Zhong, J.; Chou, H.; Chen, L.; Chen, W.; Shen, Z.; Ruoff, R.S.; et al. Preparation of supercapacitor electrodes through selection of graphene surface functionalities. ACS Nano 2012, 6, 5941–5951. [Google Scholar] [CrossRef]
- Yan, C.J.; Zou, L.; Short, R. Single-walled carbon nanotubes and polyaniline composites for capacitive deionization. Desalination 2012, 290, 125–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Z.-Y.; Li, L.; Zhang, Y.; Wei, Y.-L.; Wang, L.-F.; Zhu, M.-F. Polyaniline/multi-walled carbon nanotube composites with core–shell structures as supercapacitor electrode materials. Electrochim. Acta 2010, 55, 3904–3908. [Google Scholar] [CrossRef]
- Fonner, J.M.; Schmidt, C.E.; Ren, P. A combined molecular dynamics and experimental study of doped polypyrrole. Polymer 2010, 51, 4985–4993. [Google Scholar] [CrossRef] [Green Version]
- Killian, J.G.; Coffey, B.M.; Gao, F.; Poehler, T.O.; Searson, P.C. Polypyrrole composite electrodes in an all-polymer battery system. J. Electrochem. Soc. 1996, 143, 936–942. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Wang, C.Y.; Zhou, D.; Too, C.O.; Wallace, G.G. Electrochemical synthesis of polypyrrole films using stainless steel mesh as substrate for battery application. Synth. Met. 2005, 153, 117–120. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Mao, X.W.; Hatton, T.A. An Asymmetric Electrochemical system with complementary tunability in hydrophobicity for selective separations of organics. ACS Cent. Sci. 2019, 5, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, F.; Wang, L.; Yang, J.; Wu, X.; Li, M.; Jiang, S.; Lin, S.; Chen, Z. Highly compact, free-standing porous electrodes from polymer-derived nanoporous carbons for efficient electrochemical capacitive deionization. J. Mater. Chem. A 2019, 7, 1768–1778. [Google Scholar]
- Ezika, A.C.; Sadiku, E.R.; Ray, S.S.; Hamam, Y.; Folorunso, O.; Adekoya, G.J. Emerging advancements in polypyrrole MXene hybrid nanoarchitectonics for capacitive energy storage applications. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1521–1540. [Google Scholar] [CrossRef]
- Su, X.; Kulik, H.J.; Jamison, T.F.; Hatton, T.A. Anion-selective redox electrodes: Electrochemically mediated separation with heterogeneous organometallic interfaces. Adv. Funct. Mater. 2016, 26, 3394–3404. [Google Scholar] [CrossRef]
- Su, X.; Tan, K.J.; Elbert, J.; Ruttiger, C.; Gallei, M.; Jamison, T.F.; Hatton, T.A. Asymmetric Faradaic systems for selective electrochemical separations. Energy Environ. Sci. 2017, 10, 1272–1283. [Google Scholar] [CrossRef]
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T.A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 4701. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Bannuru, K.K.R.; Wang, Y.; Guo, L.; Baji, A.; Yang, H.Y. Free-standing electrodes derived from metal–organic frameworks/nanofibers hybrids for membrane capacitive deionization. Adv. Mater. Technol. 2018, 3, 1800135. [Google Scholar]
- Liu, Y.; Ma, J.Q.; Lu, T.; Pan, L.K. Electrospun carbon nanofibers reinforced 3D porous carbon polyhedra network derived from metal-organic frameworks for capacitive deionization. Sci. Rep. 2016, 6, 32784. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Wang, Y.; Xiong, Z.; Yang, J.; Xie, Z.; Liu, J. Fabrication of electrospun trace NiO-doped hierarchical porous carbon nanofiber electrode for capacitive deionization. J. Colloid Interface Sci. 2018, 532, 343–351. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Q.; Wu, T.; Zhan, F.; Zhou, M.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon fiber/graphene electrodes for capacitive deionization: Importance and unique role of electrical conductivity. Carbon 2016, 103, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Dykstra, J.E.; Porada, S.; van der Wal, A.; Yoon, J.; Biesheuvel, P.M. Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage. J. Colloid Interface Sci. 2015, 446, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ko, C.J.; Leffell, D.J. Cutaneous squamous cell carcinomas of the lower extremity: A distinct subset of squamous cell carcinomas. J. Am. Acad. Dermatol. 2014, 70, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Porada, S.; Biesheuvel, P.M.; Van der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J. CDI ragone plot as a functional tool to evaluate desalination performance in capacitive deionization. RSC Adv. 2015, 5, 1456–1461. [Google Scholar] [CrossRef]
- Jande, Y.A.C.; Kim, W.S. Desalination using capacitive deionization at constant current. Desalination 2013, 329, 29–34. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of perchlorate in drinking water: A critical review. Sep. Purif. Technol. 2009, 69, 7–21. [Google Scholar] [CrossRef]
- Qu, Y.T.; Campbell, P.G.; Gu, L.; Knipe, J.M.; Dzenitis, E.; Santiago, J.G.; Stadermann, M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionization. Desalination 2016, 400, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yin, X.; Zhu, L.; Qiu, Y. Energy recovery and electrode regeneration under different charge/discharge conditions in membrane capacitive deionization. Desalination 2018, 439, 93–101. [Google Scholar] [CrossRef]
- Guo, H.; You, F.; Yu, S.; Li, L.; Zhao, D. Mechanisms of chemical cleaning of ion exchange membranes: A case study of plant-scale electrodialysis for oily wastewater treatment. J. Membr. Sci. 2015, 496, 310–317. [Google Scholar] [CrossRef]
- Saleem, M.W.; Jande, Y.A.C.; Asif, M.; Kim, W.-S. Hybrid CV-CC operation of capacitive deionization in comparison with constant current and constant voltage. Sep. Sci. Technol. 2016, 51, 1063–1069. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Han, X.; Zhang, L.; Xu, S.; Wang, J. Optimization on electrode assemblies based on ion-doped polypyrrole/carbon nanotube composite in capacitive deionization process. J. Electroanal. Chem. 2016, 768, 72–80. [Google Scholar] [CrossRef]
- Steven, H.; Jeremy, G.; Roland, D. Technoeconomic analysis of brackish water capacitive deionization: Navigating tradeoffs between performance, lifetime, and material costs. Environ. Sci. Technol. 2019, 53, 13353–13363. [Google Scholar]
- Lin, S. Energy Efficiency of desalination: Fundamental insights from intuitive interpretation. Environ. Sci. Technol. 2020, 54, 76–84. [Google Scholar] [CrossRef]
- Park, J.S.; Song, J.H.; Yeon, K.H.; Moon, S.H. Removal of hardness ions from tap water using electromembrane processes. Desalination 2007, 202, 1–8. [Google Scholar] [CrossRef]
- Gabrielli, C.; Maurin, G.; Francy-Chausson, H.; Thery, P.; Tran, T.T.M.; Tlili, M. Electrochemical water softening: Principle and application. Desalination 2006, 201, 150–163. [Google Scholar] [CrossRef]
- Dean, J.G.; Bosqui, F.L.; Lanouette, K.H. Removing heavy metals from waste water. Environ. Sci. Technol. 1972, 6, 518–522. [Google Scholar] [CrossRef]
- Nagarajan, M.K.; Paine, H.L. Water hardness control by detergent builders. J. Am. Soc. Brew. Chem. 1984, 61, 1475–1478. [Google Scholar]
- Wiers, B.H.; Grosse, R.J.; Cilley, W.A. Divalent and trivalent ion exchange with zeolite A. Environ. Sci. Technol. 1982, 16, 617–624. [Google Scholar]
- Ghizellaoui, S.; Chibani, A.; Ghizellaoui, S. Use of nanofiltration for partial softening of very hard water. Desalination 2005, 179, 315–322. [Google Scholar] [CrossRef]
- Hauck, A.R.; Sourirajan, S. Performance of porous cellulose acetate membranes for the reverse osmosis treatment of hard and waste waters. Environ. Sci. Technol. 1969, 3, 1269–1275. [Google Scholar] [CrossRef]
- Seo, S.-J.; Jeon, H.; Lee, J.K.; Kim, G.-Y.; Park, D.; Nojima, H.; Lee, J.; Moon, S.-H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-H.; Huang, C.-Y. A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 2013, 314, 124–129. [Google Scholar] [CrossRef]
- Aslani, P.; Kennedy, R.A. Studies on diffusion in alginate gels. I. Effect of cross-linking with calcium or zinc ions on diffusion of acetaminophen. J. Control. Release 1996, 42, 75–82. [Google Scholar] [CrossRef]
- Chen, J.P.; Wang, L. Characterization of a Ca-alginate based ion-exchange resin and its applications in lead, copper, and zinc removal. Sep. Sci. Technol. 2001, 36, 3617–3637. [Google Scholar] [CrossRef]
- Doornbusch, G.J.; Dykstra, J.E.; Biesheuvel, P.M.; Suss, M.E. Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization. J. Mater. Chem. A 2016, 4, 3642–3647. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Zhang, W.; Mossad, M.; Yazdi, J.S.; Zou, L. A statistical experimental investigation on arsenic removal using capacitive deionization. Desalin. Water Treat. 2016, 57, 3254–3260. [Google Scholar] [CrossRef]
- Fan, C.-S.; Tseng, S.-C.; Li, K.-C.; Hou, C.-H. Electro-removal of arsenic (III) and arsenic (V) from aqueous solutions by capacitive deionization. J. Hazard. Mater. 2016, 312, 208–215. [Google Scholar] [CrossRef]
- Lado, J.J.; Pérez-Roa, R.E.; Wouters, J.J.; Tejedor-Tejedor, M.I.; Anderson, M.A. Evaluation of operational parameters for a capacitive deionization reactor employing asymmetric electrodes. Sep. Purif. Technol. 2014, 133, 236–245. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Ma, J.; Liang, P. Effective removal and selective capture of copper from salty solution in flow electrode capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 341–350. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Collins, R.N.; Tsarev, S.; Aoyagi, N.; Kinsela, A.S.; Jones, A.M.; Waite, T.D. Flow-electrode CDI removes the uncharged Ca–UO2–CO3 ternary complex from brackish potable groundwater: Complex dissociation, transport, and sorption. Environ. Sci. Technol. 2019, 53, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, M.; Shen, P.; Uytterhoeven, C.; Mamrol, N.; Shen, J.; Gao, C.; Van der Bruggen, B. Composite anti-scaling membrane made of interpenetrating networks of nanofibers for selective separation of lithium. J. Membr. Sci. 2021, 618, 118668. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, G.; Suib, S.L.; Liu, F.; Zheng, L.; Tan, W.; Qin, L. Enhancement of Zn2+ and Ni2+ removal performance using a deionization pseudocapacitor with nanostructured birnessite and its carbon nanotube composite electrodes. Chem. Eng. J. 2017, 328, 464–473. [Google Scholar] [CrossRef]
- Liu, L.H.; Peng, Q.C.; Qiu, G.H.; Zhu, J.; Tan, W.F.; Liu, C.S.; Zheng, L.R.; Dang, Z. Cd2+ adsorption performance of tunnel-structured manganese oxides driven by electrochemically controlled redox. Environ. Pollut. 2019, 244, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Shyam, B.; Stone, K.H.; Weker, J.N.; Pasta, M.; Lee, H.-W.; Toney, M.F.; Cui, Y. Reversible multivalent (monovalent, divalent, trivalent) ion insertion in open framework materials. Adv. Energy Mater. 2015, 5, 1401869. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Q.; Li, F.; Dai, J. Removal of heavy metal ions from wastewater by capacitive deionization using polypyrrole/chitosan composite electrode. Adsorpt. Sci. Technol. 2019, 37, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Xue, J.Q.; Li, F.; Dai, J.Z.; Zhang, X.Z.Y. Preparation of polypyrrole/chitosan/carbon nanotube composite nano-electrode and application to capacitive deionization process for removing Cu2+. Chem. Eng. Process. 2019, 139, 121–129. [Google Scholar] [CrossRef]
- Dugas, R.; Rochelle, G. Absorption and desorption rates of carbon dioxide with monoethanolamine and piperazine. Energy Procedia 2009, 1, 1163–1169. [Google Scholar] [CrossRef]
- Conway, W.; Wang, X.; Fernandes, D.; Burns, R.; Lawrance, G.; Puxty, G.; Maeder, M. Comprehensive Kinetic and Thermodynamic Study of the Reactions of CO2(aq) and HCO3− with Monoethanolamine (MEA) in Aqueous Solution. J. Phys. Chem. A 2011, 115, 14340–14349. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, K.; Verweij, H.; Winston Ho, W.S. Membrane processes for carbon capture from coal-fired power plant flue gas: A modeling and cost study. J. Membr. Sci. 2012, 421–422, 299–310. [Google Scholar] [CrossRef]
- Datta, S.; Henry, M.P.; Lin, Y.J.; Fracaro, A.T.; Millard, C.S.; Snyder, S.W.; Stiles, R.L.; Shah, J.; Yuan, J.; Wesoloski, L.; et al. Electrochemical CO2 capture using resin-wafer electrodeionization. Ind. Eng. Chem. Res. 2013, 52, 15177–15186. [Google Scholar] [CrossRef]
- Eisaman, M.D.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011, 4, 1319–1328. [Google Scholar] [CrossRef]
- Kokoszka, B.; Jarrah, N.K.; Liu, C.; Moore, D.T.; Landskron, K. Supercapacitive swing adsorption of carbon dioxide. Angew. Chem. Int. Ed. 2014, 53, 3698–3701. [Google Scholar] [CrossRef]
- Ozdemir, E. Biomimetic CO2 Sequestration: 1. Immobilization of carbonic anhydrase within polyurethane foam. Energy Fuels 2009, 23, 5725–5730. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Wanjari, S.; Prabhu, C.; Kumar, V.; Labhsetwar, N.; Satyanarayanan, T.; Kotwal, S.; Rayalu, S. Immobilized carbonic anhydrase for the biomimetic carbonation reaction. Energy Fuels 2010, 24, 6198–6207. [Google Scholar] [CrossRef]
- Dai, N.; Shah, A.D.; Hu, L.; Plewa, M.J.; McKague, B.; Mitch, W.A. Measurement of nitrosamine and nitramine formation from NOx reactions with amines during amine-based carbon dioxide capture for postcombustion carbon sequestration. Environ. Sci. Technol. 2012, 46, 9793–9801. [Google Scholar] [CrossRef]
- Lepaumier, H.; da Silva, E.F.; Einbu, A.; Grimstvedt, A.; Knudsen, J.N.; Zahlsen, K.; Svendsen, H.F. Comparison of MEA degradation in pilot-scale with lab-scale experiments. Energy Procedia 2011, 4, 1652–1659. [Google Scholar] [CrossRef]
- da Silva, E.F.; Lepaumier, H.; Grimstvedt, A.; Vevelstad, S.J.; Einbu, A.; Vernstad, K.; Svendsen, H.F.; Zahlsen, K. Understanding 2-ethanolamine degradation in postcombustion CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 13329–13338. [Google Scholar] [CrossRef]
- Wagner, E.D.; Hsu, K.-M.; Lagunas, A.; Mitch, W.A.; Plewa, M.J. Comparative genotoxicity of nitrosamine drinking water disinfection byproducts in Salmonella and mammalian cells. Mutat. Res. 2012, 741, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Lepaumier, H.; Picq, D.; Kittel, J.; de Bruin, T.; Faraj, A.; Carrette, P.-L. New amines for CO2 capture. IV. degradation, corrosion, and quantitative structure property relationship model. Ind. Eng. Chem. Res. 2012, 51, 6283–6289. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Xu, W.; He, L.; Zhao, Z. Lithium extraction from high Mg/Li brine via electrochemical intercalation/de-intercalation system using LiMn2O4 materials. Desalination 2021, 503, 114935. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of lithium production and recovery from minerals, brines, and lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Hoshino, T. Development of technology for recovering lithium from seawater by electrodialysis using ionic liquid membrane. Fusion Eng. Des. 2013, 88, 2956–2959. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium resources and production: Critical assessment and global projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Flexer, V.; Fernando Baspineiro, C.; Ines Galli, C. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; La Mantia, F.; Trocoli, R. Electrochemical methods for lithium recovery: A comprehensive and critical review. Adv. Mater. 2020, 32, 1905440. [Google Scholar] [CrossRef]
- Swain, B.J.S. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Hatton, T.A.; Lienhard, J.H.V. Lithium recovery from oil and gas produced water: A need for a growing energy industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef] [Green Version]
- Bajestani, M.B.; Moheb, A.; Dinari, M. Preparation of lithium ion-selective cation exchange membrane for lithium recovery from sodium contaminated lithium bromide solution by electrodialysis process. Desalination 2020, 486, 114476. [Google Scholar] [CrossRef]
- Gamaethiralalage, J.G.; Singh, K.; Sahin, S.; Yoon, J.; Elimelech, M.; Suss, M.E.; Liang, P.; Biesheuvel, P.M.; Zornitta, R.L.; de Smet, L.C.P.M. Recent advances in ion selectivity with capacitive deionization. Energy Environ. Sci. 2021, 14, 1095–1120. [Google Scholar] [CrossRef]
- Siekierka, A.; Kujawa, J.; Kujawski, W.; Bryjak, M. Lithium dedicated adsorbent for the preparation of electrodes useful in the ion pumping method. Sep. Purif. Technol. 2018, 194, 231–238. [Google Scholar] [CrossRef]
- Missoni, L.L.; Marchini, F.; del Pozo, M.; Calvo, E.J. A LiMn2O4-polypyrrole system for the extraction of LiCl from natural brine. J. Electrochem. Soc. 2016, 163, A1898–A1902. [Google Scholar] [CrossRef]
- Marchini, F.; Rubi, D.; del Pozo, M.; Williams, F.J.; Calvo, E.J. Surface chemistry and lithium-ion exchange in LiMn2O4 for the electrochemical selective extraction of LiCl from natural salt lake brines. J. Phys. Chem. C 2016, 120, 15875–15883. [Google Scholar] [CrossRef]
- Zhao, Z.; Si, X.; Liu, X.; He, L.; Liang, X. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials. Hydrometallurgy 2013, 133, 75–83. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zhao, Z.; Liang, X. Effect of Na+ on Li extraction from brine using LiFePO4/FePO4 electrodes. Hydrometallurgy 2014, 146, 24–28. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Moazeni, M.; Hajipour, H.; Askari, M.; Nusheh, M. Hydrothermal synthesis and characterization of titanium dioxide nanotubes as novel lithium adsorbents. Mater. Res. Bull. 2015, 61, 70–75. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, D.; He, G.; Yao, Q.; Wang, F.; Zhou, J. Synthesis of H2TiO3− lithium adsorbent loaded on ceramic foams. Mater. Lett. 2015, 145, 351–354. [Google Scholar] [CrossRef]
- Siekierka, A. Lithium and magnesium separation from brines by hybrid capacitive deionization. Desalination 2022, 527, 115569. [Google Scholar] [CrossRef]
- Siekierka, A. Lithium iron manganese oxide as an adsorbent for capturing lithium ions in hybrid capacitive deionization with different electrical modes. Sep. Purif. Technol. 2020, 236, 116234. [Google Scholar] [CrossRef]
- Jin, W.; Hu, M.; Sun, Z.; Huang, C.-H.; Zhao, H. Simultaneous and precise recovery of lithium and boron from salt lake brine by capacitive deionization with oxygen vacancy-rich CoP/Co3O4− graphene aerogel. Chem. Eng. J. 2021, 420, 127661. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Q.; Zhou, X.; Lee, S.; Xia, Y.; Liu, Z. New-concept batteries based on aqueous Li+/Na+ mixed-ion electrolytes. Sci. Rep. 2013, 3, 1946. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.-Y.; Ji, Z.-Y.; Zhang, Y.-G.; Guo, Z.-Y.; Zhao, Y.-Y.; Liu, J.; Yuan, J.-S. Study on lithium extraction from brines based on LiMn2O4/Li1-xMn2O4 by electrochemical method. Electrochim. Acta 2017, 252, 350–361. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, Y.-H.; Choi, S.; Shin, J.; Dinh, H.-C.; Choi, J.W. An electrochemical cell for selective lithium capture from seawater. Environ. Sci. Technol. 2015, 49, 9415–9422. [Google Scholar] [CrossRef]
- Neufeld, R.D.; Thodos, G. Removal of orthophosphates from aqueous solutions with activated alumina. Environ. Sci. Technol. 1969, 3, 661–667. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Zuthi, M.F.R.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I. Enhanced biological phosphorus removal and its modeling for the activated sludge and membrane bioreactor processes. Bioresour. Technol. 2013, 139, 363–374. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, X.; Lu, L.; Liang, P.; Ren, Z.J. Concurrent nitrogen and phosphorus recovery using flow-electrode capacitive deionization. ACS Sustain. Chem. Eng. 2019, 7, 7844–7850. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Waite, T.D. Ammonia-rich solution production from wastewaters using chemical-free flow-electrode capacitive deionization. ACS Sustain. Chem. Eng. 2019, 7, 6480–6485. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Song, J.; He, C.; Waite, T.D. Continuous ammonia recovery from wastewaters using an integrated capacitive flow electrode membrane stripping system. Environ. Sci. Technol. 2018, 52, 14275–14285. [Google Scholar] [CrossRef] [PubMed]
- Kf, A.; Whb, A.; Fei, P.; Kw, A. Ammonia recovery from concentrated solution by designing novel stacked FCDI cell. Sep. Purif. Technol. 2020, 250, 117066. [Google Scholar]
- Kim, T.; Gorski, C.A.; Logan, B.E. Ammonium removal from domestic wastewater using selective battery electrodes. Environ. Sci. Technol. Lett. 2018, 5, 578. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2019, 215, 190–207. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Choi, J.-H. Enhancement of nitrate removal from a solution of mixed nitrate, chloride and sulfate ions using a nitrate-selective carbon electrode. Desalination 2013, 320, 10–16. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; He, D.; Waite, T.D. Capacitive membrane stripping for ammonia recovery (CapAmm) from dilute wastewaters. Environ. Sci. Technol. Lett. 2018, 5, 43–49. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Jiang, Y.; Wang, T.-J.; Wang, H. Effects of the hydration ratio on the electrosorption selectivity of ions during capacitive deionization. Desalination 2016, 399, 171–177. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Wu, C.; Wang, Y.; Li, W. A study of electrosorption selectivity of anions by activated carbon electrodes in capacitive deionization. Desalination 2015, 369, 46–50. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tang, L.; Tang, W.; Zhong, Y.; Luo, K.; Duan, M.; Xing, W.; Liang, J. Removal and recovery of phosphorus from low-strength wastewaters by flow-electrode capacitive deionization. Sep. Purif. Technol. 2020, 237, 116322. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Xiao, W.; Ma, J.; Sun, J.; Mo, H.; Waite, T.D. Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI). Water Res. 2021, 189, 116653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, X.; Wang, M.; Ma, J.; Collins, R.; Kinsela, A.; Zhang, Y.; Waite, T.D. Phosphate recovery as vivianite using a flow-electrode capacitive desalination (FCDI) and fluidized bed crystallization (FBC) coupled system. Water Res. 2021, 194, 116939. [Google Scholar] [CrossRef]
- Xu, L.; Ding, R.; Mao, Y.; Peng, S.; Li, Z.; Zong, Y.; Wu, D. Selective recovery of phosphorus and urea from fresh human urine using a liquid membrane chamber integrated flow-electrode electrochemical system. Water Res. 2021, 202, 117423. [Google Scholar] [CrossRef]
- Tao, G.; Viswanath, B.; Kekre, K.; Lee, L.Y.; Ng, H.Y.; Ong, S.L.; Seah, H. RO brine treatment and recovery by biological activated carbon and capacitive deionization process. Water Sci. Technol. 2011, 64, 77–82. [Google Scholar] [CrossRef]
- Ye, G.; Yu, Z.; Li, Y.; Li, L.; Song, L.; Gu, L.; Cao, X. Efficient treatment of brine wastewater through a flow-through technology integrating desalination and photocatalysis. Water Res. 2019, 157, 134–144. [Google Scholar] [CrossRef]
- Feng, C.; Hou, C.-H.; Chen, S.; Yu, C.-P. A microbial fuel cell driven capacitive deionization technology for removal of low level dissolved ions. Chemosphere 2013, 91, 623–628. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine management in desalination industry: From waste to resources generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Yu, J.; Qin, J.; Kekre, K.A.; Viswanath, B.; Tao, G.; Seah, H. Impact of operating conditions on performance of capacitive deionisation for reverse osmosis brine recovery. J. Water Reuse Desalin. 2013, 4, 59–64. [Google Scholar] [CrossRef]
- Minhas, M.B.; Jande, Y.A.C.; Kim, W.S. Combined reverse osmosis and constant—current operated capacitive deionization system for seawater desalination. Desalination 2014, 344, 299–305. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, J.; Kim, D.I.; Gwak, G.; Hong, S. Feasibility study of reverse osmosis-flow capacitive deionization (RO-FCDI) for energy-efficient desalination using seawater as the flow-electrode aqueous electrolyte. Desalination 2020, 479, 114326. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of persistent organic pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: Synergistic effects, mechanisms and challenges. Renew. Sust. Energ. Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayashi, T.; Iwasaki, H.; Awatsu, M.; Yokoyama, H. Ultra-low-power energy harvester for microbial fuel cells and its application to environmental sensing and long-range wireless data transmission. J. Power Sources 2019, 430, 1–11. [Google Scholar] [CrossRef]
- Forrestal, C.; Xu, P.; Ren, Z. Sustainable desalination using a microbial capacitive desalination cell. Energy Environ. Sci. 2012, 5, 7161–7167. [Google Scholar] [CrossRef]
- Forrestal, C.; Stoll, Z.; Xu, P.; Ren, Z.J. Microbial capacitive desalination for integrated organic matter and salt removal and energy production from unconventional natural gas produced water. Environ. Sci. Water Res. Technol. 2015, 1, 47–55. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; He, M.; Han, J.; Sun, Y.; Jiang, H.; Li, Z.; Li, Y.; Zhang, H. Recent Advances in Capacitive Deionization: Research Progress and Application Prospects. Sustainability 2022, 14, 14429. https://doi.org/10.3390/su142114429
Liu M, He M, Han J, Sun Y, Jiang H, Li Z, Li Y, Zhang H. Recent Advances in Capacitive Deionization: Research Progress and Application Prospects. Sustainability. 2022; 14(21):14429. https://doi.org/10.3390/su142114429
Chicago/Turabian StyleLiu, Meijun, Mengyao He, Jinglong Han, Yueyang Sun, Hong Jiang, Zheng Li, Yuna Li, and Haifeng Zhang. 2022. "Recent Advances in Capacitive Deionization: Research Progress and Application Prospects" Sustainability 14, no. 21: 14429. https://doi.org/10.3390/su142114429





