Organogels for Low-Polar Organic Solvents: Potential Applications on Cultural Heritage Materials
Abstract
:1. Introduction
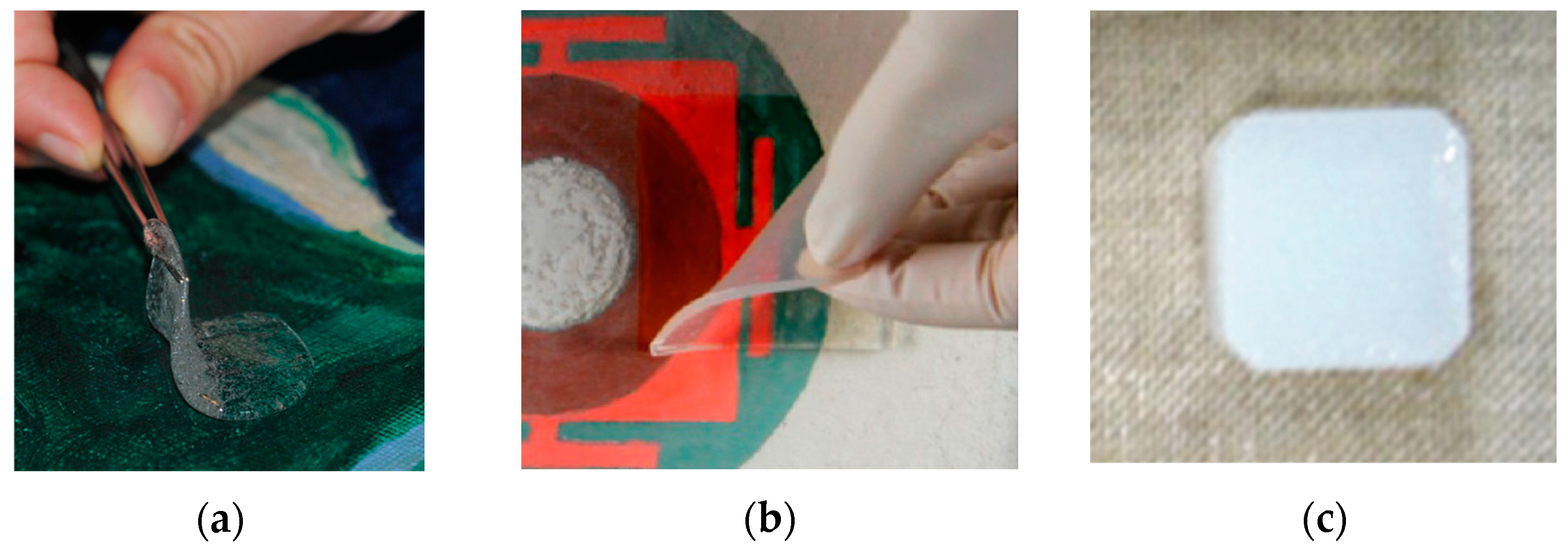
2. Gels for Low-Polar Systems in Cultural Heritage Conservation
2.1. Chemical Solvent–Surfactant Gels
2.2. Silicone-Based Thickeners
2.3. Emulsions and Microemulsions
2.3.1. O/W Emulsions
2.3.2. Inorganic Emulsions
3. Organogels Structure
4. Low-Molecular-Weight Organogelators (LMWGs) for Non-Polar and Low-Polar Solvents
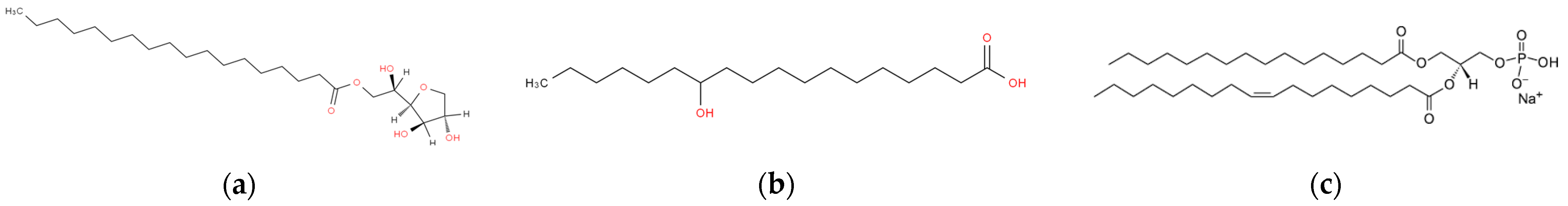
4.1. Cholesterol Derivatives
4.2. Fatty Acid-Derived Organogels
4.3. Anthryl- and Anthraquinone-Derived Organogelators
4.4. Amino Acid-Type Organogelators
4.5. Saccharide-Based Organogelators
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phenix, A.; Townsend, J.A.; Townsend, J. A Brief Survey of Historical Varnishes. In Conservation of Easel Paintings; Routledge: Abingdon, UK, 2012; pp. 252–263. [Google Scholar]
- Arslanoglu, J.; Learner, T. The Evaluation of Laropal A81: Paraloid B-72 Polymer Blend Varnishes for Painted and Decorative Surfaces—Appearance and Practical Considerations. Conservator 2001, 25, 62–72. [Google Scholar] [CrossRef]
- Rene De La Rie, E.; Mcglinchey, C.W. New Synthetic Resins for Picture Varnishes. Stud. Conserv. 1990, 35, 168–173. [Google Scholar] [CrossRef]
- Bartoletti, A.; Barker, R.; Chelazzi, D.; Bonelli, N.; Baglioni, P.; Lee, J.; Angelova, L.V.; Ormsby, B. Reviving WHAAM! A Comparative Evaluation of Cleaning Systems for the Conservation Treatment of Roy Lichtenstein’s Iconic Painting. Herit. Sci. 2020, 8, 9. [Google Scholar] [CrossRef]
- Swartz, N.; Clare, T.L. On the Protective Nature of Wax Coatings for Culturally Significant Outdoor Metalworks: Microstructural Flaws, Oxidative Changes, and Barrier Properties. J. Am. Inst. Conserv. 2015, 54, 181–201. [Google Scholar] [CrossRef]
- Seung-Jun, O.; Koang-Chul, W. Convergence Study on the Development and Material Property of Wax for Surface Conservation of Iron Alloy Outdoor Sculpture. J. Korea Converg. Soc. 2018, 9, 151–160. [Google Scholar] [CrossRef]
- Barreca, S.; Bruno, M.; Oddo, L.; Orecchio, S. Preliminary Study on Analysis and Removal of Wax from a Carrara Marble Statue. Nat. Prod. Res. 2019, 33, 947–955. [Google Scholar] [CrossRef]
- Shedlosky, T.J.; Stanek, K.M.; Bierwagen, G. On-Line Survey Results of Techniques Used for Outdoor Bronze Conservation. In AIC Objects Speciality Group Postprints; AIC: Washington, DC, USA, 2000; Volume 320, pp. 3–13. [Google Scholar]
- De La Rie, E.R. Photochemical and Thermal Degradation of Films of Dammar Resin. Stud. Conserv. 1988, 33, 53–70. [Google Scholar] [CrossRef]
- Dei, L. Conservation Treatments: Cleaning, Consolidation and Protection. In Nanoscience for the Conservation of Works of Art; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Phenix, A.; Wolbers, R. Removal of Varnish: Organic Solvents as Cleaning Agents. In Conservation of Easel Paintings; Routledge: London, UK, 2012; pp. 524–554. [Google Scholar]
- Ricci, C.; Gambino, F.; Nervo, M.; Piccirillo, A.; Scarcella, A.; Zenucchini, F.; Pozo-Antonio, J.S. Developing New Cleaning Strategies of Cultural Heritage Stones: Are Synergistic Combinations of a Low-Toxic Solvent Ternary Mixtures Followed by Laser the Solution? Coatings 2020, 10, 466. [Google Scholar] [CrossRef]
- Macchia, A.; Zaratti, C.; Biribicchi, C.; Colasanti, I.A.; Barbaccia, F.I.; Favero, G. Evaluation of Green Solvents’ Applicability for Chromatic Reintegration of Polychrome Artworks. Heritage 2023, 6, 3353–3364. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Haghbakhsh, R.; Marques, R.; Paiva, A.; Carlyle, L.; Duarte, A.R.C. Evaluation of Deep Eutectic Systems as an Alternative to Solvents in Painting Conservation. ACS Sustain. Chem. Eng. 2021, 9, 15451–15460. [Google Scholar] [CrossRef]
- Biribicchi, C.; Macchia, A.; Favero, G.; Strangis, R.; Gabriele, B.; Mancuso, R.; La Russa, M.F. Sustainable Solutions for Removing Aged Wax-Based Coatings from Cultural Heritage: Exploiting Hydrophobic Deep Eutectic Solvents (DESs). New J. Chem. 2023, 47, 5991–6000. [Google Scholar] [CrossRef]
- Macchia, A.; Biribicchi, C.; Carnazza, P.; Montorsi, S.; Sangiorgi, N.; Demasi, G.; Prestileo, F.; Cerafogli, E.; Colasanti, I.A.; Aureli, H.; et al. Multi-Analytical Investigation of the Oil Painting “Il Venditore Di Cerini” by Antonio Mancini and Definition of the Best Green Cleaning Treatment. Sustainability 2022, 14, 3972. [Google Scholar] [CrossRef]
- Jia, Y.; Sciutto, G.; Botteon, A.; Conti, C.; Focarete, M.L.; Gualandi, C.; Samorì, C.; Prati, S.; Mazzeo, R. Deep Eutectic Solvent and Agar: A New Green Gel to Remove Proteinaceous-Based Varnishes from Paintings. J. Cult. Herit. 2021, 51, 138–144. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Volpi, F.; Rehorn, C.; Vurro, R.; Blümich, B.; Mazzocchetti, L.; Giorgini, L.; Samorì, C.; Galletti, P.; et al. Cleaning Oil Paintings: NMR Relaxometry and SPME to Evaluate the Effects of Green Solvents and Innovative Green Gels. New J. Chem. 2019, 43, 8229–8238. [Google Scholar] [CrossRef]
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. Assessing the Value of Green Conservation for Cultural Heritage: Positive and Critical Aspects of Already Available Methodologies. Int. J. Conserv. Sci. 2016, 7, 185–202. [Google Scholar]
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Microemulsions, Micelles, and Functional Gels: How Colloids and Soft Matter Preserve Works of Art. Angew. Chem. Int. Ed. 2018, 57, 7296–7303. [Google Scholar] [CrossRef]
- Gels. Available online: https://www.conservation-wiki.com/wiki/Gels (accessed on 25 September 2023).
- Feller, R.L.; Wilt, M. Evaluation of Cellulose Ethers for Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 1990; Volume III. [Google Scholar]
- Angelova, L.; Ormsby, B.; Townsend, J.H.; Wolbers, R. Gels in the Conservation of Art; Archetype Publications: London, UK, 2017. [Google Scholar]
- Experimental Study of Complex Fluids. Available online: https://andrewsun.net/en/research/gels-and-gelation-of-clay-particles/ (accessed on 29 October 2023).
- Maheux, A.F. Cross-Disciplinary Uses for Gellan Gum in Conservation. Book Pap. Group Annu. 2015, 34, 69–79. [Google Scholar]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, B.; Keefe, M.; Phenix, A.; von Aderkas, E.; Learner, T.; Tucker, C.; Kozak, C. Mineral Spirits-Based Microemulsions: A Novel Cleaning System for Painted Surfaces. J. Am. Inst. Conserv. 2016, 55, 12–31. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage; Springer: Dutch, The Netherlands, 2015. [Google Scholar]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- Carretti, E.; Matarrese, C.; Fratini, E.; Baglioni, P.; Dei, L. Physicochemical Characterization of Partially Hydrolyzed Poly(Vinyl Acetate)-Borate Aqueous Dispersions. Soft Matter 2014, 10, 4443–4450. [Google Scholar] [CrossRef]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, Microemulsions, and Gels for the Conservation of Cultural Heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef]
- Barclay, B.; Hett, C. The Cleaning, Polishing, and Protective Waxing of Brass and Copper. CGI Notes 2007, 9, 1–4. [Google Scholar]
- Knutinen, U.; Norman, A. Wax Analysis in Conservation Objects by Solubility Studies, FTIR and DSC. In Proceedings of the 15th World Conference on Nondestructive Testing, Rome, Italy, 15–21 October 2000. [Google Scholar]
- Seung-Jun, O.; Koang-Chul, W. A Study on the Development and Application of Perilla Oil Based Compound Wax Agent for Preserving Outdoor Metal Sculpture: A Case Study on Iron Sculptures. J. Conserv. Sci. 2017, 33, 121–130. [Google Scholar]
- Cremonesi, P.; Signorini, E. L’uso Dei Solventi Organici Neutri Nella Pulitura Dei Dipinti: Un Nuovo Test Di Solubilità. Progett. Restauro 2004, 31, 2–15. [Google Scholar]
- Byrne, A. Wolbers Cleaning Methods: Introduction. AICCM Bull. 1991, 17, 3–11. [Google Scholar] [CrossRef]
- Jia, Y.; Sciutto, G.; Mazzeo, R.; Samorì, C.; Focarete, M.L.; Prati, S.; Gualandi, C. Organogel Coupled with Microstructured Electrospun Polymeric Nonwovens for the Effective Cleaning of Sensitive Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 39620–39629. [Google Scholar] [CrossRef] [PubMed]
- Stulik, D.; Miller, D.; Khanjian, H.; Khandekar, N.; Wolbers, R.; Carlson, J.; Petersen, W.C. Solvent Gels for the Cleaning of Works of Art: The Residue Question, 2004th ed.; Stulik, D., Ed.; Getty Publications: Los Angeles, CA, USA, 2004. [Google Scholar]
- Poggi, G.; Santan, H.D.; Smets, J.; Chelazzi, D.; Noferini, D.; Petruzzellis, M.L.; Pensabene Buemi, L.; Fratini, E.; Baglioni, P. Nanostructured Bio-Based Castor Oil Organogels for the Cleaning of Artworks. J. Colloid Interface Sci. 2023, 638, 363–374. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organogels as Matrices for Controlled Drug Delivery: A Review on the Current State. Soft Mater. 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into Low Molecular Mass Organic Gelators: A Focus on Drug Delivery and Tissue Engineering Applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and Their Use in Drug Delivery—A Review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hanabusa, K. L-Lysine-Based Low-Molecular-Weight Gelators. Chem. Soc. Rev. 2009, 38, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Jenifer, R.V.; Akshay, M.S.; Dhivahar, J.; Mohan Das, T. Emerging Perspectives of Sugar-Based Gelators of Diverse Applications. Trends Carbohydr. Res. 2021, 13, 15–34. [Google Scholar]
- McKibbin, A.; McKibbin, C.; Allington-Jones, L.; Verveniotou, E. Giant Sequoia: An Extraordinary Case Study Involving Carbopol® Gel. In Solvent Gels in the Conservation of Art; Archtype Publishing: London, UK, 2018. [Google Scholar]
- De Gennes, P.G.; Taupln, C. Microemulsions and the Flexibility of Oil/Water Interfaces. J. Phys. Chem. 1982, 86, 2294–2304. [Google Scholar] [CrossRef]
- Moretti, P.; Rosi, F.; Miliani, C.; Daugherty, M.; van den Berg, K.J.; Cartechini, L. Non-Invasive Reflection FT-IR Spectroscopy for on-Site Detection of Cleaning System Residues on Polychrome Surfaces. Microchem. J. 2020, 157, 105033. [Google Scholar] [CrossRef]
- Casoli, A.; Di Diego, Z.; Isca, C. Cleaning Painted Surfaces: Evaluation of Leaching Phenomenon Induced by Solvents Applied for the Removal of Gel Residues. Environ. Sci. Pollut. Res. 2014, 21, 13252–13263. [Google Scholar] [CrossRef]
- Hansen, A.; Russo, A.; Di Pasqua, P.; Villafranca Soissons, I.; Campanella, L. Experimental Methodologies in the Conservation of Design Objects. Case Studies from the RECOPLART Project. Archeomatica 2018, 9, 24–29. [Google Scholar]
- Stavroudis, C. Modular Cleaning Program. Available online: https://cool.culturalheritage.org/byauth/stavroudis/mcp/ (accessed on 3 November 2023).
- Albano, M.; Grassi, S.; Fiocco, G.; Invernizzi, C.; Rovetta, T.; Licchelli, M.; Marotti, R.; Merlo, C.; Comelli, D.; Malagodi, M. A Preliminary Spectroscopic Approach to Evaluate the Effectiveness of Water-and Silicone-Based Cleaning Methods on Historical Varnished Brass. Appl. Sci. 2020, 10, 3982. [Google Scholar] [CrossRef]
- Briffa, C. Investigating the Removal of Permanent Marker Ink from Historical Parchment, Using Acetone and Benzyl Alcohol with Velvesil Plus Gel. Available online: https://www.westdean.ac.uk/blog/investigating-the-removal-of-permanent-marker-ink-from-historical-parchment-using-acetone-and-benzyl-alcohol-with-velvesil-plus-gel (accessed on 19 July 2023).
- Dekant, W.; Klaunig, J.E. Toxicology of Decamethylcyclopentasiloxane (D5). Regul. Toxicol. Pharmacol. 2016, 74, S67–S76. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Colloid and Surface Chemistry. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–400. [Google Scholar]
- Wolbers, R.; Stavroudis, C. Aqueous Methods for the Cleaning of Paintings. In Conservation of Easel Paintings; Routledge: Oxford, UK, 2012; pp. 500–523. [Google Scholar]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials Fromworks of Art: SAXS Characterization and Application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef] [PubMed]
- lo Dico, G.; Semilia, F.; Milioto, S.; Parisi, F.; Cavallaro, G.; Inguì, G.; Makaremi, M.; Pasbakhsh, P.; Lazzara, G. Microemulsion Encapsulated into Halloysite Nanotubes and Their Applications for Cleaning of a Marble Surface. Appl. Sci. 2018, 8, 1455. [Google Scholar] [CrossRef]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Release of Surfactant Cargo from Interfacially-Active Halloysite Clay Nanotubes for Oil Spill Remediation. Langmuir 2014, 30, 13533–13541. [Google Scholar] [CrossRef]
- Prathapet, A.; Sureshan, K.M. Sugar-Based Organogelators for Various Applications. Langmuir 2019, 35, 6005–6014. [Google Scholar] [CrossRef]
- Hassan, P.A.; Verma, G.; Ganguly, R. Polymer Gels. In Soft Materials—Properties and Applications; Elsevier: London, UK, 2012; pp. 1–59. [Google Scholar]
- Araceli, M.-I.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as Drug Delivery Systems: From Their Components to Their Applications. Drug Discov. Today 2022, 27, 1008–1026. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Li, S.; Brehm, M.; Bartschat, A.; Theato, P.; Levkin, P.A. Inherently UV Photodegradable Poly(Methacrylate) Gels. Adv. Funct. Mater. 2021, 31, 2105681. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.Q.; Guo, H.; Li, W.H. Recent Advances of Organogels: From Fabrications and Functions to Applications. Prog. Org. Coat. 2021, 159, 106417. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Ren, X.; Xie, Z.; Wu, H.; Shi, W.; Zhang, Y. Multiple-Responsive Organogels with Self-Colorimetric Chemo Sensing Responsiveness towards Hg2+ Ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 124003. [Google Scholar] [CrossRef]
- Basak, S.; Bhattacharya, S.; Datta, A.; Banerjee, A. Charge-Transfer Complex Formation in Gelation: The Role of Solvent Molecules with Different Electron-Donating Capacities. Chem. A Eur. J. 2014, 20, 5721–5726. [Google Scholar] [CrossRef]
- Yang, Y.S.; Liang, C.; Yang, C.; Zhang, Y.P.; Wang, B.X.; Liu, J. A Novel Coumarin-Derived Acylhydrazone Schiff Base Gelator for Synthesis of Organogels and Identification of Fe3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118391. [Google Scholar] [CrossRef]
- Lescanne, M.; Colin, A.; Mondain-Monval, O.; Fages, F.; Pozzo, J.L. Structural Aspects of the Gelation Process Observed with Low Molecular Mass Organogelators. Langmuir 2003, 19, 2013–2020. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef]
- Liu, X.Y. Gelation with Small Molecules: From Formation Mechanism to Nanostructure Architecture. Top. Curr. Chem. 2005, 256, 1–37. [Google Scholar] [CrossRef]
- Gronwald, O.; Shinkai, S. Sugar-Integrated Gelators of Organic Solvents. Chem. Eur. J. 2001, 7, 4328–4334. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of Low-Molecular-Weight Gelators and Polymer-Based Gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- George, M.; Tan, G.; John, V.T.; Weiss, R.G. Urea and Thiourea Derivatives as Low Molecular-Mass Organogelators. Chem. A Eur. J. 2005, 11, 3243–3254. [Google Scholar] [CrossRef]
- Huang, Y.D.; Dong, X.L.; Zhang, L.L.; Chai, W.; Chang, J.Y. Structure-Property Correlation of Benzoyl Thiourea Derivatives as Organogelators. J. Mol. Struct. 2013, 1031, 43–48. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Santos, C.M.M.; Silva, A.M.S. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2019, 24, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Briand, V.A.; Sharma, N.; Ahn, S.; Kasi, R.M. Polymers Comprising Cholesterol: Synthesis, Self-Assembly, and Applications. Materials 2009, 2, 636–660. [Google Scholar] [CrossRef]
- Bot, A.; Agterof, W.G.M. Structuring of Edible Oils by Mixtures of γ-Oryzanol with β-Sitosterol or Related Phytosterols. J. Am. Oil Chem. Soc. 2006, 83, 513–521. [Google Scholar] [CrossRef]
- Peng, J.; Xia, H.; Liu, K.; Gao, D.; Yang, M.; Yan, N.; Fang, Y. Water-in-Oil Gel Emulsions from a Cholesterol Derivative: Structure and Unusual Properties. J. Colloid Interface Sci. 2009, 336, 780–785. [Google Scholar] [CrossRef]
- Malik, S.; Kawano, S.; Fujita, N.; Shinkai, S. Pyridine-Containing Versatile Gelators for Post-Modification of Gel Tissues toward Construction of Novel Porphyrin Nanotubes. Tetrahedron 2007, 63, 7326–7333. [Google Scholar] [CrossRef]
- Babu, P.; Sangeetha, N.M.; Maitra, U. Supramolecular Chemistry of Bile Acid Derivatives: Formation of Gels. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2006; Volume 241, pp. 60–67. [Google Scholar]
- He, P.; Liu, J.; Liu, K.; Ding, L.; Yan, J.; Gao, D.; Fang, Y. Preparation of Novel Organometallic Derivatives of Cholesterol and Their Gel-Formation Properties. Colloids Surf. A Physicochem. Eng. Asp. 2010, 362, 127–134. [Google Scholar] [CrossRef]
- Sahoo, P.; Puranik, V.G.; Patra, A.K.; Sastry, P.U.; Dastidar, P. Ferrocene Based Organometallic Gelators: A Supramolecular Synthon Approach. Soft Matter 2011, 7, 3634–3641. [Google Scholar] [CrossRef]
- Klawonn, T.; Gansäuer, A.; Winkler, I.; Lauterbach, T.; Franke, D.; Nolte, R.J.M.; Feiters, M.C.; Börner, H.; Hentschel, J.; Dötz, K.H. A Tailored Organometallic Gelator with Enhanced Amphiphilic Character and Structural Diversity of Gelation. Chem. Commun. 2007, 19, 1894–1895. [Google Scholar] [CrossRef]
- Gansäuer, A.; Winkler, I.; Klawonn, T.; Nolte, R.J.M.; Feiters, M.C.; Börner, H.G.; Hentschel, J.; Dötz, K.H. Novel Organometallic Gelators with Enhanced Amphiphilic Character: Structure—Property Correlations, Principles for Design, and Diversity of Gelation. Organometallics 2009, 28, 1377–1382. [Google Scholar] [CrossRef]
- Mufioz, S.; Gokel, G.W. Organometallic Amphiphiles: Novel Mechanisms for Redox Chemical Control of Aggregation Behavior. Lnorganica Chim. Acta 1996, 250, 59–67. [Google Scholar] [CrossRef]
- Weng, W.; Benjamin Beck, J.; Jamieson, A.M.; Rowan, S.J. Understanding the Mechanism of Gelation and Stimuli-Responsive Nature of a Class of Metallo-Supramolecular Gels. J. Am. Chem. Soc. 2006, 128, 11663–11672. [Google Scholar] [CrossRef]
- Paulusse, J.M.J.; Van Beek, D.J.M.; Sijbesma, P.P. Reversible Switching of the Sol-Gel Transition with Ultrasound in Rhodium(I) and Iridium(I) Coordination Networks. J. Am. Chem. Soc. 2007, 129, 2392–2397. [Google Scholar] [CrossRef]
- Tu, T.; Assenmacher, W.; Peterlik, H.; Weisbarth, R.; Nieger, M.; Dötz, K.H. An Air-Stable Organometallic Low-Molecular-Mass Gelator: Synthesis, Aggregation, and Catalytic Application of a Palladium Pincer Complex. Angew. Chem. Int. Ed. 2007, 46, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yan, N.; Peng, J.; Liu, J.; Zhang, Q.; Fang, Y. Supramolecular Gels Based on Organic Diacid Monoamides of Cholesteryl Glycinate. J. Colloid Interface Sci. 2008, 327, 233–242. [Google Scholar] [CrossRef]
- Willemen, H.M.; Vermonden, T.; Marcelis, A.T.M.; Sudhölter, E.J.R. N-Cholyl Amino Acid Alkyl Esters A Novel Class of Organogelators. Eur. J. Org. Chem. 2001, 2001, 2329–2335. [Google Scholar] [CrossRef]
- Noponen, V.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E.; Sievänen, E. Bile Acid-Amino Acid Ester Conjugates: Gelation, Structural Properties, and Thermoreversible Solid to Solid Phase Transition. Soft Matter 2010, 6, 3789–3796. [Google Scholar] [CrossRef]
- Daniel, J.; Rajasekharan, R. Organogelation of Plant Oils and Hydrocarbons by Long-Chain Saturated FA, Fatty Alcohols, Wax Esters, and Dicarboxylic Acids. J. Am. Oil. Chem. Soc. 2003, 80, 417–421. [Google Scholar] [CrossRef]
- Murdan, S.; Gregoriadis, G.; Florence, A.T. Non-Ionic Surfactant Based Organogels Incorporating Niosomes. STP Pharma Sci. 1996, 6, 44–48. [Google Scholar]
- Murdan, S.; Van Den Bergh, B.; Gregoriadis, G.; Florence, A.T. Water-in-Sorbitan Monostearate Organogels (Water-in-Oil Gels). J. Pharm. Sci. 1999, 88, 615–619. [Google Scholar] [CrossRef]
- Murdan, S.; Gregoriadis, G.; Florence, A.T. Interaction of a Nonionic Surfactant-Based Organogel with Aqueous Media. Int. J. Pharm. 1999, 180, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S.; Gregoriadis, G.; Florence, A.T. Novel Sorbitan Monostearate Organogels. J. Pharm. Sci. 1999, 88, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Wright, A.J.; Marangoni, A.G. Engineering the Oil Binding Capacity and Crystallinity of Self-Assembled Fibrillar Networks of 12-Hydroxystearic Acid in Edible Oils. Soft Matter 2008, 4, 1483–1490. [Google Scholar] [CrossRef]
- Mallia, V.A.; George, M.; Blair, D.L.; Weiss, R.G. Robust Organogels from Nitrogen-Containing Derivatives of (R)-12-Hydroxystearic Acid as Gelators: Comparisons with Gels from Stearic Acid Derivatives. Langmuir 2009, 25, 8615–8625. [Google Scholar] [CrossRef] [PubMed]
- Brizard, A.; Oda, R.; Huc, I. Chirality Effects in Self-Assembled Fibrillar Networks. Top. Curr. Chem. 2005, 256, 167–218. [Google Scholar] [CrossRef] [PubMed]
- Grahame, D.A.S.; Olauson, C.; Lam, R.S.H.; Pedersen, T.; Borondics, F.; Abraham, S.; Weiss, R.G.; Rogers, M.A. Influence of Chirality on the Modes of Self-Assembly of 12-Hydroxystearic Acid in Molecular Gels of Mineral Oil. Soft Matter 2011, 7, 7359–7365. [Google Scholar] [CrossRef]
- Schurtenberger’, P.; Cavaco, C. Polymer-like Lecithin Reverse Micelles. 1. A Light Scattering Study. Langmuir 1994, 10, 100–108. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, O.P. Lecithin Organogels as a Potential Phospholipid-Structured System for Topical Drug Delivery: A Review. Aaps Pharmscitech 2005, 6, E298–E310. [Google Scholar] [CrossRef]
- Shumilina, E.V.; Khromova, Y.L.; Shchipunov, Y.A. Lecithin Organogels: The Effect of Phosphatidylethanolamine Additives. Colloid J. Russ. Acad. Sci. 1997, 59, 514–518. [Google Scholar] [CrossRef]
- Shchipunov, Y.A. Lecithin Organogel: A Micellar System with Unique Properties. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 541–554. [Google Scholar] [CrossRef]
- Shchipunov, Y.; Shumilina, E. Lecithin Organogels: Role of Polar Solvent and Nature of Intermolecular Interactions. Colloid J. Russ. Acad. Sci. 1996, 58, 117–125. [Google Scholar]
- Clavier, G.M.; Pozzo, J.L.; Bouas-Laurent, H.; Liere, C.; Roux, C.; Sanchez, C. Organogelators for Malting Porous Sol-Gel Derived Silica at Two Different Length Scales. J. Mater. Chem. 2000, 10, 1725–1730. [Google Scholar] [CrossRef]
- Cabrera, S.; Rojas, J.; Moreno, A. Oleogels, Contribution in the Production of Healthier Food Products: The Fats of the Future. J. Food Nutr. Res. 2020, 8, 172–182. [Google Scholar]
- Brosse, N.; Barth, D.; Jamart-Grégoire, B. A Family of Strong Low-Molecular-Weight Organogelators Based on Aminoacid Derivatives. Tetrahedron Lett. 2004, 45, 9521–9524. [Google Scholar] [CrossRef]
- Pinazo, A.; Pons, R.; Pérez, L.; Infante, M.R. Amino Acids as Raw Material for Biocompatible Surfactants. Ind. Eng. Chem. Res. 2011, 50, 4805–4817. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, H.; Hanabusa, K. Two-Component Organogelators Based on Two L-Ainino Acids: Effect of Combination of L-Lysine with Various L-Amino Acids on Organogelation Behavior. Langmuir 2009, 25, 8579–8585. [Google Scholar] [CrossRef]
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable Surfactants for Biochemical Applications and Nanotechnology. J. Surfactants Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef]
- Murdan, S. Organogels in Drug Delivery. Expert Opin Drug Deliv 2005, 2, 489–505. [Google Scholar] [CrossRef]
- Plourde, F.; Motulsky, A.; Couffin-Hoarau, A.C.; Hoarau, D.; Ong, H.; Leroux, J.C. First Report on the Efficacy of L-Alanine-Based in Situ-Forming Implants for the Long-Term Parenteral Delivery of Drugs. J. Control. Release 2005, 108, 433–441. [Google Scholar] [CrossRef]
- Motulsky, A.; Lafleur, M.; Couffin-Hoarau, A.C.; Hoarau, D.; Boury, F.; Benoit, J.P.; Leroux, J.C. Characterization and Biocompatibility of Organogels Based on L-Alanine for Parenteral Drug Delivery Implants. Biomaterials 2005, 26, 6242–6253. [Google Scholar] [CrossRef]
- Suzuki, M.; Sato, T.; Kurose, A.; Shirai, H.; Hanabusa, K. New Low-Molecular Weight Gelators Based on L-Valine and L-Isoleucine with Various Terminal Groups. Tetrahedron Lett. 2005, 46, 2741–2745. [Google Scholar] [CrossRef]
- Patra, T.; Pal, A.; Dey, J. Birefringent Physical Gels of N-(4-n-Alkyloxybenzoyl)-l-Alanine Amphiphiles in Organic Solvents: The Role of Hydrogen-Bonding. J. Colloid Interface Sci. 2010, 344, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Czaun, M.; Takafuji, M.; Ihara, H. Synthesis, Self-Assembling Properties, and Atom Transfer Radical Polymerization of an Alkylated L-Phenylalanine-Derived Monomeric Organogel from Silica: A New Approach to Prepare Packing Materials for High-Performance Liquid Chromatography. Chem. A Eur. J. 2008, 14, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ghosh, Y.K.; Bhattacharya, S. Molecular Mechanism of Physical Gelation of Hydrocarbons by Fatty Acid Amides of Natural Amino Acids. Tetrahedron 2007, 63, 7334–7348. [Google Scholar] [CrossRef]
- Cui, J.; Liu, A.; Guan, Y.; Zheng, J.; Shen, Z.; Wan, X. Tuning the Helicity of Self-Assembled Structure of a Sugar-Based Organogelator by the Proper Choice of Cooling Rate. Langmuir 2010, 26, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chakraborty, A.; Maiti, D.K.; Puranik, V.G. Crystal or Low Molecular Mass Organogel Based on Sugar-Derived Chiral Pyrano[2,3-b]Naphtho[1,2-e]Pyrans. Org. Lett. 2006, 8, 1061–1064. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Handore, K.; Sureshan, K.M. Soft Optical Devices from Self-Healing Gels Formed by Oil and Sugar-Based Organogelators. Angew. Chem. Int. Ed. 2011, 50, 8021–8024. [Google Scholar] [CrossRef]
- Soundarajan, K.; Das, T.M. Design, Synthesis, Characterization and Gelation Studies of Sugar-Oxadiazole Based N-Glycosylamines. Trends Carbohydr. Res. 2019, 11, 14–21. [Google Scholar]
- Pal, K.B.; Mukhopadhyay, B. Carbohydrate-BasedSafe Fuel Gel with Significant Self–Healing Property. ChemistrySelect 2017, 2, 967–974. [Google Scholar] [CrossRef]
- Moore, J.W.; Shorb, J.; Prat-Resina, X.; Wendorff, T.; Hahn, A. Solubility and Molecular Structure. In ChemPRIME; University of Wisconsin: Madison, WI, USA, 2021. [Google Scholar]
- Soundarajan, K.; Mohan Das, T. Sugar-Benzohydrazide Based Phase Selective Gelators for Marine Oil Spill Recovery and Removal of Dye from Polluted Water. Carbohydr. Res. 2019, 481, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biribicchi, C.; Giuliani, L.; Macchia, A.; Favero, G. Organogels for Low-Polar Organic Solvents: Potential Applications on Cultural Heritage Materials. Sustainability 2023, 15, 16305. https://doi.org/10.3390/su152316305
Biribicchi C, Giuliani L, Macchia A, Favero G. Organogels for Low-Polar Organic Solvents: Potential Applications on Cultural Heritage Materials. Sustainability. 2023; 15(23):16305. https://doi.org/10.3390/su152316305
Chicago/Turabian StyleBiribicchi, Chiara, Laura Giuliani, Andrea Macchia, and Gabriele Favero. 2023. "Organogels for Low-Polar Organic Solvents: Potential Applications on Cultural Heritage Materials" Sustainability 15, no. 23: 16305. https://doi.org/10.3390/su152316305





