Proof-of-Concept Study on the Feasibility of Supercritical Carbon Dioxide-Assisted Consolidation Treatment for a Pair of Goalkeeper Gloves on Synthetic Latex-Based Foam Mock-Ups
Abstract
:1. Introduction
Aim and Viability of the Research
2. Materials and Methods
2.1. Mock-Up Sample Preparation
2.2. Materials
2.3. Carbon Dioxide (CO2) Apparatus, Experimental Conditions and Procedure
2.4. Sample Characterisation
2.4.1. Empirical Observations
2.4.2. Colour Measurements
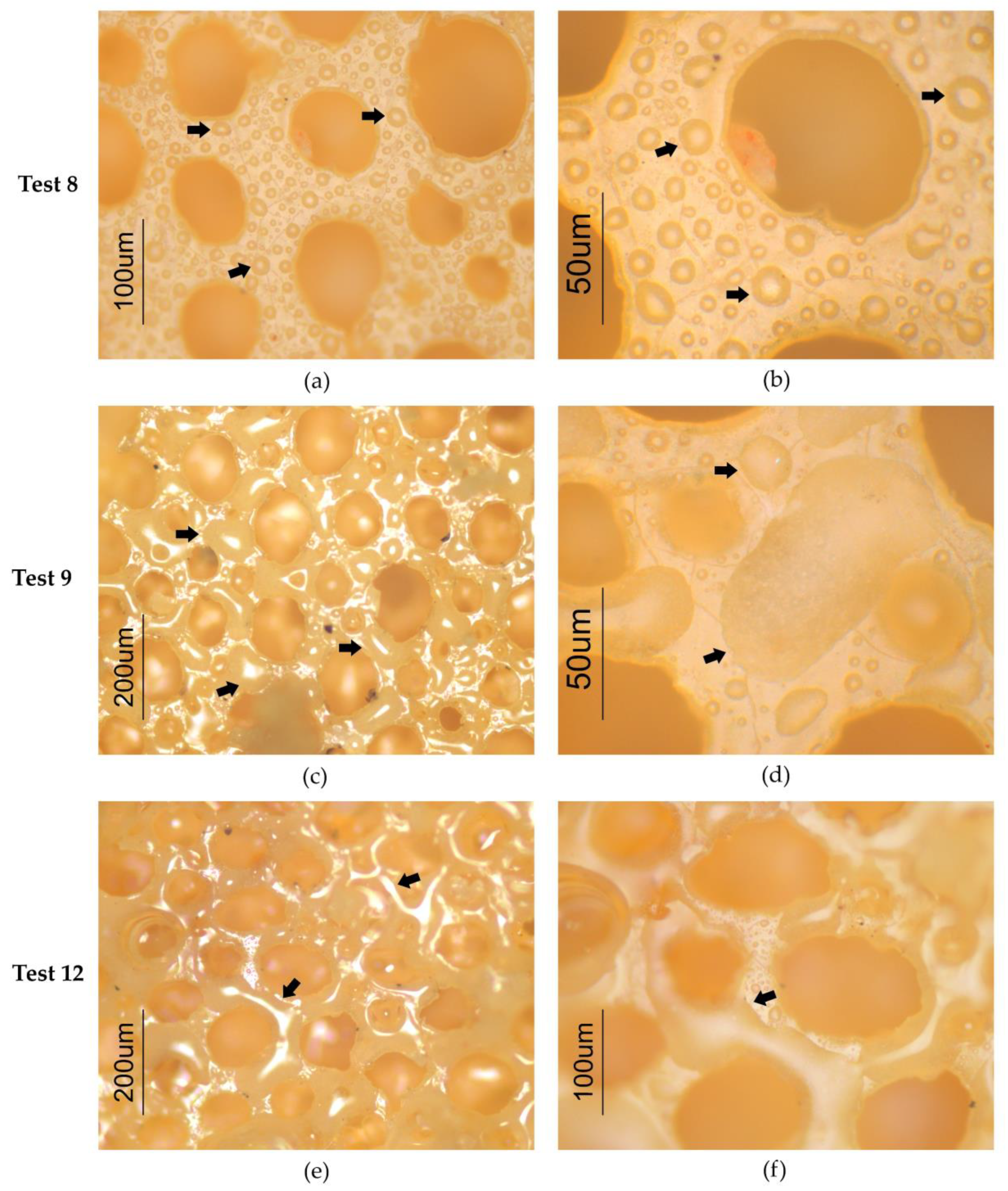
2.4.3. Imaging
2.4.4. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy
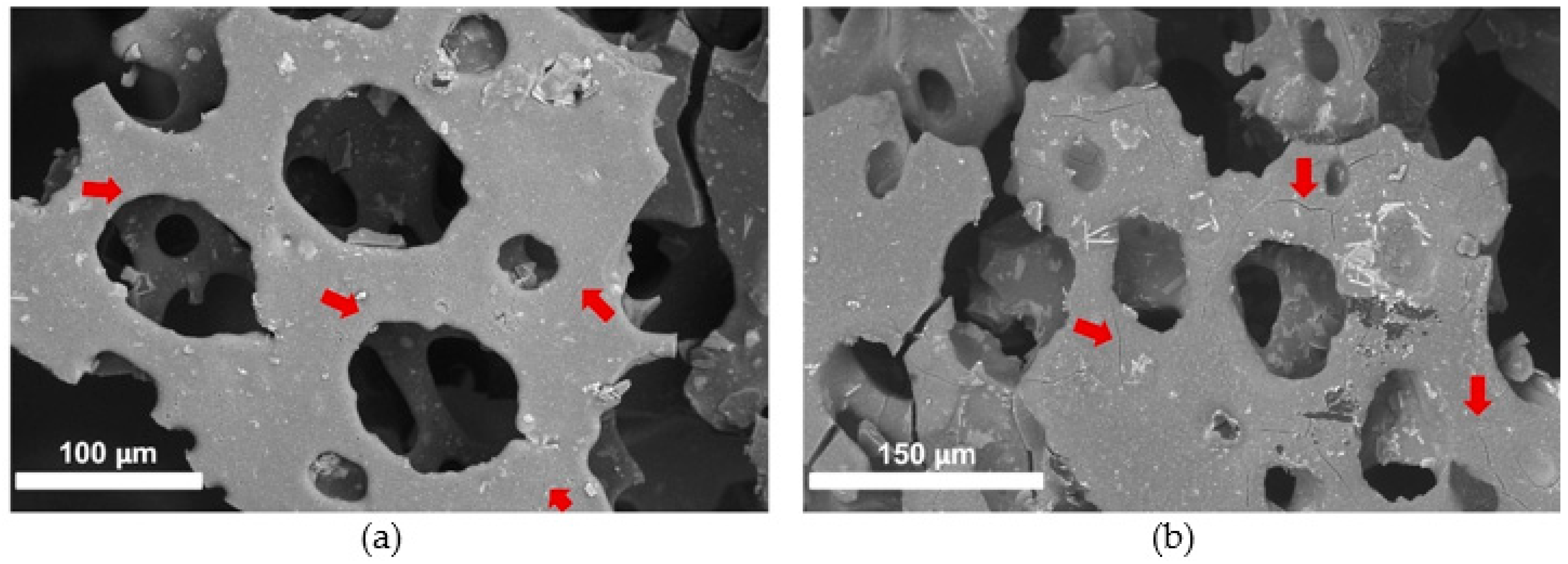
2.4.5. Scanning Electron Microscopy (SEM) Imaging
3. Results and Discussion
3.1. Safety Assessment: Impact of scCO2 and Selected Experimental Conditions on Mock-Ups
3.2. Supercritical Carbon Dioxide (CO2)-Assisted Impregnation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flexer, G.; Spring, K. Approaching the Preservation of Polyurethane Soles on Football Boots. In Plastics in Peril: Focus on Conservation of Polymeric Materials in Cultural Heritage, Virtual Conference; Available online: https://www.youtube.com/watch?v=Nz_zbg_Zq4o&list=PLDhExi_byiwnJwb4Nx3Z3Xk5SefFvBCdx&index=9&pp=iAQB (accessed on 6 February 2024).
- Ferreira, J.T. Supercritical CO2 Based Green Technologies for the Consolidation of Foams in Cultural Heritage. The Case Study of Robert Enke’s Pair of Gloves. Master’s Thesis, NOVA School of Science and Technology, Universidade NOVA de Lisboa, Almada, Portugal, 2020. Available online: http://hdl.handle.net/10362/113703 (accessed on 6 February 2024).
- Bartoletti, A.; França de Sá, S.; Ferreira, J.T.; Ferreira, J.L. Exploring Conservation Options for a Pair of Foam-Based Goalkeeper Gloves Belonging to Museu Benfica—Cosme Damião. In Future Talks 021—Smart Solutions in the Conservation of the Modern; Bechthold, T., Ed.; Die Neue Sammlung—The Design Museum: Munich, Germany, 2023; pp. 60–69. [Google Scholar]
- van Oosten, T. PUR Facts: Conservation of Polyurethane Foam in Art and Design; Amsterdam University Press—RCE Publications: Amsterdam, The Netherlands, 2011; ISBN 978-90-485-1207-2. [Google Scholar]
- Pellizzi, E.; Lattuati-Derieux, A.; de Lacaillerie, J.-B.E.; Lavédrine, B.; Cheradame, H. Reinforcement Properties of 3-Aminopropylmethyldiethoxysilane and N-(2-Aminoethyl)-3-Aminopropylmethyldimethoxysilane on Polyurethane Ester Foam. Polym. Degrad. Stab. 2012, 97, 2340–2346. [Google Scholar] [CrossRef]
- Pellizzi, E.; Lattuati-Derieux, A.; de Lacaillerie, J.-B.E.; Lavédrine, B.; Cheradame, H. Consolidation of Artificially Degraded Polyurethane Ester Foam with Aminoalkylalkoxysilanes. Polym. Degrad. Stab. 2016, 129, 106–113. [Google Scholar] [CrossRef]
- Daher, C.; Fabre-Francke, I.; Balcar, N.; Barabant, G.; Cantin, S.; Fichet, O.; Chéradame, H.; Lavédrine, B.; Lattuati Derieux, A. Consolidation of Degraded Polyurethane Foams by Means of Polysiloxane Mixtures: Polycondensation Study and Application Treatment. Polym. Degrad. Stab. 2018, 158, 92–101. [Google Scholar] [CrossRef]
- van Aubel, C.; de Groot, S.; van Keulen, H.; Snijders, E. Digging into the Past of Nature Carpets: The Evaluation of Treatments on Artworks by Piero Gilardi Made from Polyurethane Ether Foam. J. Cult. Herit. 2019, 35, 271–278. [Google Scholar] [CrossRef]
- Chaumat, G.; Tran, K.; Dekkers, J.M.; Pellizzi, E.; Lattuati-Derieux, A. On-Going Studies in Consolidation of Polyurethane (PUR) Foams. In Preservation of Plastic Artefacts in Museum Collections (POPART); Lavédrine, B., Fournier, A., Martin, G., Eds.; Comité des Travaux Historiques et Scientifiques (CTHS): Paris, France, 2012; pp. 271–293. [Google Scholar]
- Köppen, J.; Brunner, S.; Gómez-Sánchez, E. Shoemaker’s Nightmare: Deterioration of Shoe Soles and Tests for the Conservation of Degraded Closed-Cell Polyester Urethane Museum Objects. In Future Talks 019—Surfaces. Lectures and Workshops on the Conservation of the Modern; Bechthold, T., Ed.; Die Neue Sammlung—The Design Museum: Munich, Germany, 2021; pp. 95–102. [Google Scholar]
- Weidner, E. Impregnation via Supercritical CO2–What We Know and What We Need to Know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction—An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Baumgärtel, H., Franck, E.U., Grünbein, W., Eds.; Topics in Physical Chemistry; Steinkopff Heidelberg: Heidelberg, Germany, 1994; Volume 4, ISBN 978-3-662-07382-7. [Google Scholar]
- Clifford, T. Fundamentals of Supercritical Fluids; Oxford University Press: Oxford, UK, 1998; ISBN 978-0-19-850137-4. [Google Scholar]
- Belinsky, M.R. (Ed.) Supercritical Fluids; Nova Science Publishers, Incorporated: New York, NY, USA, 2010; ISBN 978-1-60741-930-3. [Google Scholar]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Weingärtner, H.; Franck, E.U. Supercritical Water as a Solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Kemmere, M.F.; Meyer, T. (Eds.) Supercritical Carbon Dioxide: In Polymer Reaction Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 978-3-527-31092-0. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Hayden, E.P. (Ed.) Supercritical Carbon Dioxide. In Functions and Applications; Nova Science Publishers, Incorporated: New York, NY, USA, 2020; ISBN 978-1-5361-7404-5. [Google Scholar]
- Hrnčič, M.K.; Cör, D.; Verboten, M.T.; Knez, Ž. Application of Supercritical and Subcritical Fluids in Food Processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef]
- McHugh, M.A. Solubility of Polymers in Supercritical Carbon Dioxide. In Green Chemistry Using Liquid and Supercritical Carbon Dioxide; DeSimone, J., Tumas, W., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 125–133. ISBN 978-0-19-515483-2. [Google Scholar]
- Sadowski, G. Phase Behavior of Polymer Systems in High-Pressure Carbon Dioxide. In Supercritical Carbon Dioxide: In Polymer Reaction Engineering; Kemmere, M.F., Thierry, M., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 15–35. [Google Scholar]
- Škerget, M.; Knez, Ž.; Knez-Hrnčič, M. Solubility of Solids in Sub- and Supercritical Fluids: A Review. J. Chem. Eng. Data 2011, 56, 694–719. [Google Scholar] [CrossRef]
- Shen, Z.; McHugh, M.A.; Xu, J.; Belardi, J.; Kilic, S.; Mesiano, A.; Bane, S.; Karnikas, C.; Beckman, E.; Enick, R. CO2-Solubility of Oligomers and Polymers That Contain the Carbonyl Group. Polymer 2023, 44, 1491–1498. [Google Scholar] [CrossRef]
- Zhu, T.; Gong, H.; Dong, M.; Yang, Z.; Guo, C.; Liu, M. Phase Equilibrium of PVAc + CO2 Binary Systems and PVAc + CO2 + Ethanol Ternary Systems. Fluid Phase Equilib. 2018, 458, 264–271. [Google Scholar] [CrossRef]
- Bach, E.; Cleve, E.; Schollmeyer, E. Past, Present and Future of Supercritical Fluid Dyeing Technology—An Overview. Rev. Prog. Color. Relat. Top. 2002, 32, 88–102. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical Fluid Dyeing of Synthetic and Natural Textiles—A Review. Color. Technol. 2013, 129, 2–17. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Simplicio, A.L.; Vega-González, A.; Subra-Paternault, P.; Coimbra, P.; Gil, M.H.; de Sousa, H.C.; Duarte, C.M.M. Supercritical Fluid Impregnation of a Biocompatible Polymer for Ophthalmic Drug Delivery. J. Supercrit. Fluids 2007, 42, 373–377. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Trindade Coutinho, I.; Champeau, M. Synergistic Effects in the Simultaneous Supercritical CO2 Impregnation of Two Compounds into Poly(L- Lactic Acid) and Polyethylene. J. Supercrit. Fluids 2020, 166, 105019. [Google Scholar] [CrossRef]
- Gurina, D.L.; Budkov, Y.A.; Kiselev, M.G. Impregnation of Poly(Methyl Methacrylate) with Carbamazepine in Supercritical Carbon Dioxide: Molecular Dynamics Simulation. J. Phys. Chem. B 2020, 124, 8410–8417. [Google Scholar] [CrossRef]
- Meneses, L.; Craveiro, R.; Jesus, A.R.; Reis, M.A.M.; Freitas, F.; Paiva, A. Supercritical CO2 Assisted Impregnation of Ibuprofen on Medium-Chain-Length Polyhydroxyalkanoates (Mcl-PHA). Molecules 2021, 26, 4772. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Long, J.; Shi, M. Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation. Materials 2022, 15, 5552. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-Assisted Impregnation/Deposition of Polymeric Materials with Pharmaceutical, Nutraceutical, and Biomedical Applications: A Review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-Assisted Impregnation of Cellulose Microparticles with R-Carvone: Effect of Process Variables on Impregnation Yield. J. Supercrit. Fluids 2022, 188, 105671. [Google Scholar] [CrossRef]
- Acda, M.N.; Morrell, J.J.; Levien, K.L. Supercritical Fluid Impregnation of Selected Wood Species with Tebuconazole. Wood Sci. Technol. 2001, 35, 127–136. [Google Scholar] [CrossRef]
- Muin, M.; Adachi, A.; Inoue, M.; Yoshimura, T.; Tsunoda, K. Feasibility of Supercritical Carbon Dioxide as a Carrier Solvent for Preservative Treatment of Wood-Based Composites. J. Wood Sci. 2003, 49, 65–72. [Google Scholar] [CrossRef]
- Kang, S.-M.; Levien, K.L.; Morrell, J.J. Supercritical Fluid Impregnation of Wood with Biocides Using Temperature Reduction to Induce Deposition. Wood Sci. Technol. 2005, 39, 328–338. [Google Scholar] [CrossRef]
- Aroso, I.M.; Duarte, A.R.C.; Pires, R.R.; Mano, J.F.; Reis, R.L. Cork Processing with Supercritical Carbon Dioxide: Impregnation and Sorption Studies. J. Supercrit. Fluids 2015, 104, 251–258. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Liu, H. Green and Efficient Processing of Wood with Supercritical CO2: A Review. Appl. Sci. 2021, 11, 3929. [Google Scholar] [CrossRef]
- Yang, L.; Xu, W.Z.; Zomaya, D.; Charpentier, P.A. Softwood Impregnation by MMA Monomer Using Supercritical CO2. J. Supercrit. Fluids 2022, 189, 105712. [Google Scholar] [CrossRef]
- Singh, M.; Dey, E.S.; Bhand, S.; Dicko, C. Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials. Insects 2021, 13, 18. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; José Galotto, M.; Guarda, A.; Julio, R. Supercritical Impregnation for Food Applications: A Review of the Effect of the Operational Variables on the Active Compound Loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Lucic Skoric, M.; Milovanovic, S.; Zizovic, I.; Ortega-Toro, R.; Santagata, G.; Malinconico, M.; Kalagasidis Krusic, M. Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics. Polymers 2022, 14, 4360. [Google Scholar] [CrossRef] [PubMed]
- Von Ulmann, A. Non-Polluting Removal of Pesticides from Historic Textiles—A Project at the Germanishes Nationalmuseum Nurnberg and the Deutsh Bundesstiftung Umwelt (1999–2001). In Proceedings of the Cultural Heritage Research: A Pan-European Challenge, Proceedings of the 5th EC Conference, Cracow, Poland, 16–18 May 2002; Kozłowski, R., Ed.; Institute of Catalysis and Surface Chemistry, Polish Academy of Sciences: Cracow, Poland, 2002; pp. 334–336. [Google Scholar]
- Sousa, M.; Melo, M.J.; Casimiro, T.; Aguiar-Ricardo, A. The Art of CO2 for Art Conservation: A Green Approach to Antique Textile Cleaning. Green Chem. 2007, 9, 943. [Google Scholar] [CrossRef]
- Frade, C.S.C.; Cruz, P.; Lopes, E.; Sousa, M.M.; Hallett, J.; Santos, R.; Aguiar-Ricardo, A.; Casimiro, T. Cleaning Classical Persian Carpets with Silk and Precious Metal Thread: Conservation and Ethical Considerations. In Proceedings of the ICOM Committee for Conservation 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011; Critério Artes Gráficas, Lda; ICOM Committee for Conservation: Almada, Portugal, 2011. [Google Scholar]
- Aslanidou, D.; Tsioptsias, C.; Panayiotou, C. A Novel Approach for Textile Cleaning Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2013, 76, 83–93. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuneable Textile Cleaning and Disinfection Process Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2016, 118, 128–139. [Google Scholar] [CrossRef]
- Selli, E.; Langè, E.; Mossa, A.; Testa, G.; Seves, A. Preservation Treatments of Aged Papers by Supercritical Carbon Dioxide. Macromol. Mater. Eng. 2000, 280–281, 71–75. [Google Scholar] [CrossRef]
- Dobrodskaya, T.V.; Egoyants, P.A.; Ikonnikov, V.K.; Romashenkova, N.D.; Sirotin, S.A.; Dobrusina, S.A.; Podgornaya, N.I. Treatment of Paper with Basic Agents in Alcohols and Supercritical Carbon Dioxide to Neutralize Acid and Prolong Storage Time. Russ. J. Appl. Chem. 2004, 77, 2017–2021. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tan, W.; Liu, C.Y.; Fang, Y.X. Deacidification of Paper in Supercritical Carbon Dioxide (CO2SCF) Solvent System with Magnesium Acetate and Calcium Hydroxide. Adv. Mater. Res. 2011, 347–353, 504–507. [Google Scholar] [CrossRef]
- Tan, W.; Cheng, L.F.; Fang, Y.X. Deacidification of Paper Using Supercritical Carbon Dioxide Containing Calcium Propionate or Magnesium Bicarbonate. Adv. Mater. Res. 2013, 781–784, 2637–2640. [Google Scholar] [CrossRef]
- Yanjuan, W.; Yanxiong, F.; Wei, T.; Chunying, L. Preservation of Aged Paper Using Borax in Alcohols and the Supercritical Carbon Dioxide System. J. Cult. Herit. 2013, 14, 16–22. [Google Scholar] [CrossRef]
- Kang, S.M.; Unger, A.; Morrell, J.J. The Effect of Supercritical Carbon Dioxide Extraction on Color Retention and Pesticide Reduction of Wooden Artifacts. J. Am. Inst. Conserv. 2004, 43, 151–160. [Google Scholar] [CrossRef]
- Tello, H.; Unger, A.; Gockel, F.; Jelen, E. Decontamination of Ethnological Objects with Supercritical Carbon Dioxide. In Proceedings of the ICOM Committee for Conservation 14th Triennial Meeting, The Hague, The Netherlands, 12–16 September 2005; James & James/Earthscan: London, UK, 2005; pp. 110–119. [Google Scholar]
- Tello, H.; Unger, A. Liquid and Supercritical Carbon Dioxide as a Cleaning and Decontamination Agent for Ethnographic Materials and Objects. In Proceedings of the Pesticide Mitigation in Museum Collections: Science in Conservation, Washington, DC, USA; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2010; pp. 35–50, (23–24 April 2007). [Google Scholar]
- Tuminello, W.H.; Bracci, S.; Piacenti, F. New Developments in Fluorinated Materials for Stone Preservation. APT Bull. J. Preserv. Technol. 2002, 33, 19–22. [Google Scholar] [CrossRef]
- Balcar, N.; Barabant, G.; Bollard, C.; Kuperholc, S.; Keneghan, B.; Laganà, A.; van Oosten, T.; Segel, K.; Shashoua, Y. Studies in Cleaning Plastics. In Preservation of Plastic Artefacts in Museum Collections (POPART); Lavédrine, B., Fournier, A., Martin, G., Eds.; Comité des Travaux Historiques et Scientifiques (CTHS): Paris, France, 2012; pp. 225–269. Available online: https://popart-highlights.mnhn.fr/active-conservation-of-plastic-artefacts/studies-in-cleaning-plastics/index.html (accessed on 6 February 2024).
- Bartoletti, A.; Soares, I.; Ramos, A.M.; Shashoua, Y.; Quye, A.; Casimiro, T.; Ferreira, J.L. Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value. Polymers 2023, 15, 566. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Brantley, N.H.; West, B.L.; Vincent, M.F.; Eckert, C.A. In Situ Spectroscopy of Polymers Subjected to Supercritical CO2 : Plasticization and Dye Impregnation. Appl. Spectrosc. 1997, 51, 491–494. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of Supercritical Carbon Dioxide with Polymers. I. Crystalline Polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of Supercritical Carbon Dioxide with Polymers. II. Amorphous Polymers. J. Appl. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Alessi, P.; Cortesi, A.; Kikic, I.; Vecchione, F. Plasticization of Polymers with Supercritical Carbon Dioxide: Experimental Determination of Glass-transition Temperatures. J. Appl. Polym. Sci. 2003, 88, 2189–2193. [Google Scholar] [CrossRef]
- Watanabe, M.; Hashimoto, Y.; Kimura, T.; Kishida, A. Characterization of Engineering Plastics Plasticized Using Supercritical CO2. Polymers 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhang, H.; Xu, L.; Li, Y.; Dong, M. Effects of Cosolvent on Dissolution Behaviors of PVAc in Supercritical CO2: A Molecular Dynamics Study. Chem. Eng. Sci. 2019, 206, 22–30. [Google Scholar] [CrossRef]
- Viana, C. Are All Vinyl Paints the Same? The Impact of Paint Formulations on Their Stability and the State of Conservation of Ângelo De Sousa’s Paintings. Master’s Thesis, NOVA School of Science and Technology, Almada, Portugal, 2022. Available online: http://hdl.handle.net/10362/141569 (accessed on 6 February 2024).
- Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Poly(Vinyl Acetate) Paints in Works of Art: A Photochemical Approach. Part 1. Polym. Degrad. Stab. 2010, 95, 453–461. [Google Scholar] [CrossRef]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar] [CrossRef]
- Oleari, C. Standard Colorimetry: Definitions, Algorithms and Software; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- dos Santos, K.A.M.; Suarez, P.A.Z.; Rubim, J.C. Photo-Degradation of Synthetic and Natural Polyisoprenes at Specific UV Radiations. Polym. Degrad. Stab. 2005, 90, 34–43. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, X.; Huang, G.; Zheng, J.; Huang, J.; Li, G. Thermal Ageing Behavior of Styrene–Butadiene Random Copolymer: A Study on the Ageing Mechanism and Relaxation Properties. Polym. Degrad. Stab. 2012, 97, 1704–1715. [Google Scholar] [CrossRef]
- Zhang, P.; He, J.; Zhou, X. An FTIR Standard Addition Method for Quantification of Bound Styrene in Its Copolymers. Polym. Test 2008, 27, 153–157. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. Photochemical Degradation Study of Polyvinyl Acetate Paints Used in Artworks by Py–GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 158–163. [Google Scholar] [CrossRef] [PubMed]
- França De Sá, S.; Viana, C.; Ferreira, J.L. Tracing Poly(Vinyl Acetate) Emulsions by Infrared and Raman Spectroscopies: Identification of Spectral Markers. Polymers 2021, 13, 3609. [Google Scholar] [CrossRef] [PubMed]









| Test Type | Test No. | Temp. (°C) | Pressure (MPa) | CO2 Density (g/mL) | Pressurisation Time (min) | Exposure Time (min) | Depressurisation Time (min) | PVAc (MW) | Co-Solvent |
|---|---|---|---|---|---|---|---|---|---|
| Safety | 1 | 33 | 10 | 0.73834 | 40 | 30 | 120 | - | - |
| 2 | 40 | 36 | 0.93932 | 10 | 120 | 5 | - | - | |
| Consolidation | 3 | 40 | 28 | 0.89853 | 10 | 120 | 10 | 167,000 | - |
| 4 | 40 | 28 | 0.89853 | 10 | 120 | 11 | 167,000 | - | |
| 5 | 40 | 28 | 0.89853 | 8 | 120 | 9 | 167,000 | - | |
| 6 | 40 | 28 | 0.89853 | 7 | 120 | 7 | 167,000 | - | |
| 7 | 40 | 36 | 0.93932 | 8 | 120 | 2 | 167,000 | - | |
| 8 | 40 | 36 | 0.93932 | 6 | 120 | 10 | 83,000 | - | |
| 9 | 40 | 36 | 0.93932 | 5 | 120 | 5 | 83,000 | - | |
| 10 | 40 | 36 | 0.93932 | 7 | 120 | 5.5 | 83,000 | - | |
| 11 | 40 | 36 | 0.93932 | 4.5 | 120 | 5.5 | 83,000 | - | |
| 12 | 40 | 36 | 0.93932 | 5.5 | 120 | 2 | 83,000 | - | |
| 13 | 40 | 36 | 0.93932 | 5 | 120 | 4.5 | 83,000 | EtOH (1) | |
| 14 | 40 | 36 | 0.93932 | 4.5 | 120 | 3.5 | 83,000 | EtOH (1) | |
| 15 | 40 | 36 | 0.93932 | 10 | 120 | 1 | 83,000 | EtOH (1) | |
| 16 (2) | 40 | 36 | 0.93932 | 4 | 120 | 5.5 | 83,000 | - | |
| 17 (2) | 40 | 36 | 0.93932 | 8 | 120 | 5 | 83,000 | - | |
| 18 (2) | 40 | 36 | 0.93932 | 8 | 120 | 4.5 | 83,000 | - |
| Test No. | Area | Before | After | Variations | |||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | Δb* | ΔE*ab | ||
| Test 1 | α | 73.41 (±0.10) | 1.47 (±0.09) | 17.64 (±0.21) | 72.76 (±0.11) | 1.03 (±0.04) | 18.46 (±0.37) | 0.82 | 1.14 |
| β | 74.72 (±0.55) | 1.00 (±0.04) | 17.94 (±0.13) | 74.95 (±0.32) | 0.86 (±0.05) | 19.05 (±0.13) | 1.10 | 1.14 | |
| Test 2 | α | 67.88 (±0.13) | 13.41 (±0.03) | 41.94 (±0.04) | 67.94 (±0.07) | 13.40 (±0.02) | 42.15 (±0.66) | 0.21 | 0.22 |
| β | 71.33 (±0.03) | 13.95 (±0.03) | 46.38 (±0.02) | 71.40 (±0.07) | 13.64 (±0.02) | 46.23 (±0.02) | −0.14 | 0.35 | |
| Polyisoprene [74] | Poly(Butadiene-Styrene) [75] | ||
|---|---|---|---|
| Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment |
| 3036 | ν(=C–H) | 3077 | ν(C–H) |
| 2962 | νas(CH3) | 3025 | ν(C–H) |
| 2928 | νas(CH2) | 2919 | νas(CH3) |
| 2855 | νs(CH2) | 2845 | νs(CH3) |
| 1450 | δ(CH2) | 1493 | ν(aromatic ring) |
| 1377 | δas(CH3) | 1450 | δ(CH2) |
| 899 | δ(CH3) | 967 | δ(C–H) (1,4-trans) |
| 837 | δ(=C–H) | 910 | δ(C–H) (1,2 vinyl) |
| 758 | δ(C–H) (phenyl groups) | ||
| 700 | δ(C–H) (1,4-cis) | ||
| Test No. | Presence of Consolidant (via ATR-FTIR) | Surface Cohesion (Empirical Obs.) | ||
|---|---|---|---|---|
| Top Surface | Core | Lateral | ||
| 3 | Y | Y | Y | Y |
| 4 | N | N | N | N |
| 5 | N | N | N | N |
| 6 | M | N | N | N |
| 7 | Y | M | N | M |
| 8 | Y | M | M | N |
| 9 | Y | M | N | Y |
| 10 | Y | M | M | Y |
| 11 | Y | M | M | Y |
| 12 | Y | M | Y | Y * |
| 13 | M | M | M | N |
| 14 | Y | M | M | Y |
| 15 | Y | Y | M | Y * |
| 16 | Y | M | Y | M |
| 17 | Y | M | M | M |
| 18 | Y | M | M | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomás Ferreira, J.; Bartoletti, A.; França de Sá, S.; Quye, A.; Shashoua, Y.; Casimiro, T.; Ferreira, J.L. Proof-of-Concept Study on the Feasibility of Supercritical Carbon Dioxide-Assisted Consolidation Treatment for a Pair of Goalkeeper Gloves on Synthetic Latex-Based Foam Mock-Ups. Sustainability 2024, 16, 1562. https://doi.org/10.3390/su16041562
Tomás Ferreira J, Bartoletti A, França de Sá S, Quye A, Shashoua Y, Casimiro T, Ferreira JL. Proof-of-Concept Study on the Feasibility of Supercritical Carbon Dioxide-Assisted Consolidation Treatment for a Pair of Goalkeeper Gloves on Synthetic Latex-Based Foam Mock-Ups. Sustainability. 2024; 16(4):1562. https://doi.org/10.3390/su16041562
Chicago/Turabian StyleTomás Ferreira, Joana, Angelica Bartoletti, Susana França de Sá, Anita Quye, Yvonne Shashoua, Teresa Casimiro, and Joana Lia Ferreira. 2024. "Proof-of-Concept Study on the Feasibility of Supercritical Carbon Dioxide-Assisted Consolidation Treatment for a Pair of Goalkeeper Gloves on Synthetic Latex-Based Foam Mock-Ups" Sustainability 16, no. 4: 1562. https://doi.org/10.3390/su16041562








