Greenshell Mussel Products: A Comprehensive Review of Sustainability, Traditional Use, and Efficacy
Abstract
:1. Introduction
2. GreenshellTM Mussel Industry, Traditional Use, Sustainability and Products
2.1. Traditional Use of GreenshellTM Mussels ‘Ka Whakangotea Ki Te Wai o Te Kākahi…’ (It Was Suckled on the Juice of the Freshwater Shellfish...)
Toroi–The Traditional Method of Fermentative Preservation
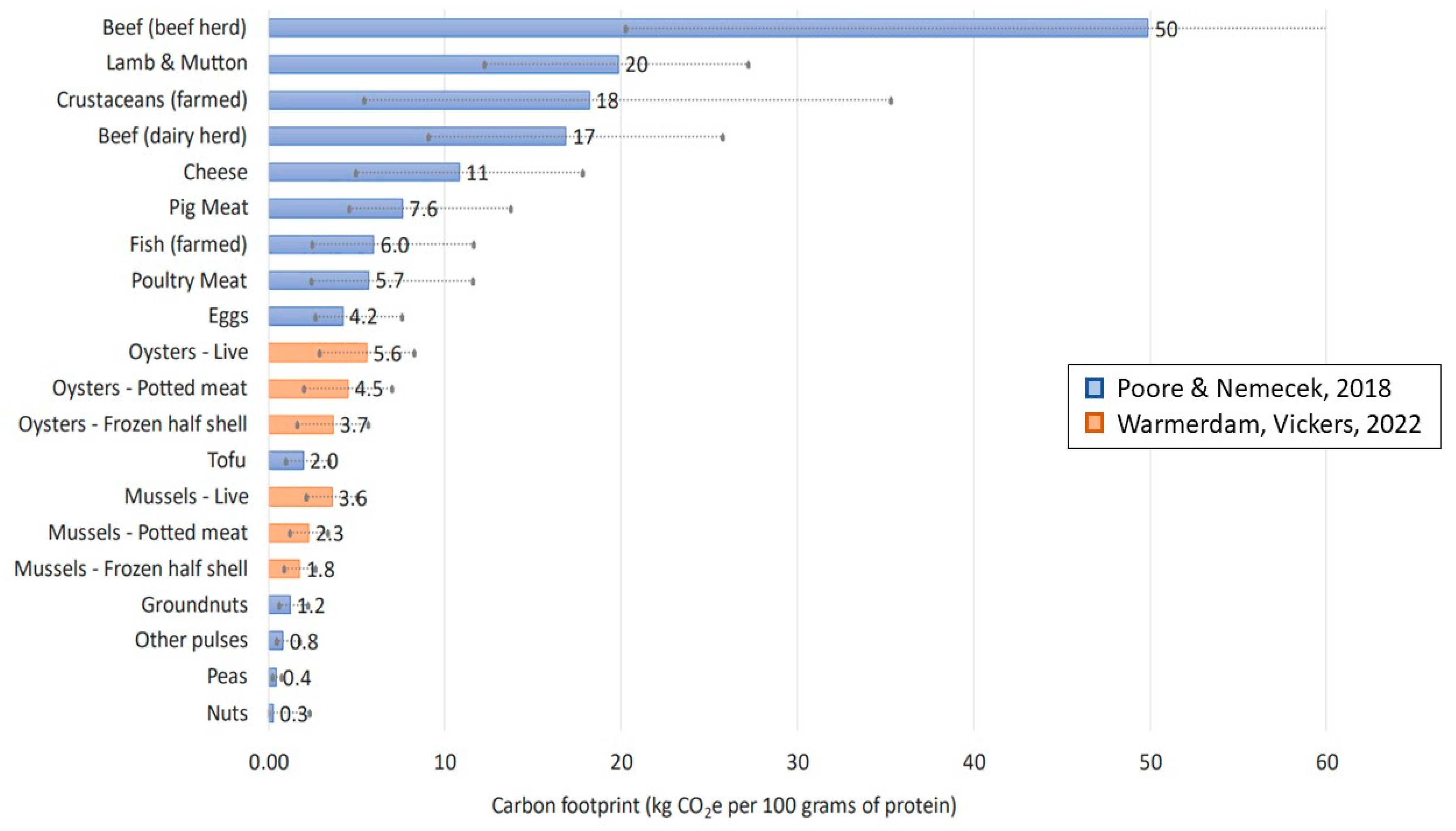
2.2. Sustainability of GSM Production
2.3. Nutritional Composition of GSM
2.4. Bioavailability of Bioactive Components in GSM Products
3. The GSM Health Benefits: Evidence from Clinical Studies
3.1. Osteoarthritis, Rheumatoid Arthritis and Osteoporosis
3.2. Metabolism, Chronic Inflammation and Cardiovascular Disease
3.3. Asthma and Airway Inflammation
3.4. Exercise-Induced Muscle Damage and Inflammation
3.5. Dysbiosis and Colorectal Cancer
3.6. Attention Deficit Hyperactivity Disorder (ADHD)
4. Potential Mechanisms and Molecular Pathways of GSM Components
4.1. Cellular and Molecular Mechanisms of Lipid Components of GSM
4.2. Cellular and Molecular Mechanisms of Glucosamine and Chondroitin Sulphate and Bioactive Peptides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Aquaculture, N.Z. Aquaculture Export Stats; Aquaculture New Zealand: Nelson, New Zealand, 2022. [Google Scholar]
- Coulson, S.; Palacios, T.; Vitetta, L. Perna canaliculus (green-lipped mussel): Bioactive components and therapeutic evaluation for chronic health conditions. In Novel Natural Products: Therapeutic Effects in Pain, Arthritis and Gastro-Intestinal Diseases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 91–132. [Google Scholar]
- Paul, L.J. A History of the Firth of Thames Dredge Fishery for Mussels: Use and Abuse of a Coastal Resource; Ministry of Agriculture and Forestry: Wellington, New Zealand, 2012; p. 27. [Google Scholar]
- Eason, C.T.; Adams, S.L.; Puddick, J.; Romanazzi, D.; Miller, M.R.; King, N.; Johns, S.; Forbes-Blom, E.; Hessian, P.A.; Stamp, L.K.; et al. Greenshell Mussels: A Review of Veterinary Trials and Future Research Directions. Vet. Sci. 2018, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Highton, J. Pilot study on the effect of New Zealand green mussel on rheumatoid arthritis. N. Z. Med. J. 1975, 81, 261–262. [Google Scholar]
- Huskisson, E.; Scott, J.; Bryans, R. Seatone is ineffective in rheumatoid arthritis. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 1358. [Google Scholar] [CrossRef] [Green Version]
- Gibson; Conway, V.; Chappell, D. Perna Canaliculus Treat. Arthritis. Pract. 1980, 224, 955–960. [Google Scholar]
- Miller, T.E.; Ormrod, D. Anti-Inflamm. Act. Perna Canaliculus (NZ Green Lipp. Mussel). N. Z. Med. J. 1980, 92, 187–193. [Google Scholar]
- Miller, T.E. Anti-inflammatory effects of mussel extracts. N. Z. Med. J. 1981, 93, 23–24. [Google Scholar]
- Couch, R.A.F.; Ormrod, D.J.; Miller, T.E.; Watkins, W.B. The Anti-inflammatory activity of Perna canaliculus (NZ green lipped mussel). N. Z. Med. J. 1982, 95, 803–806. [Google Scholar]
- Thien, F.; Hallsworth, M.P.; Soh, C.; Lee, T.H. Effects of exogenous eicosapentaenoic acid on generation of leukotriene C4 and leukotriene C5 by calcium ionophore-activated human eosinophils in vitro. J. Immunol. 1993, 150, 3546–3552. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Wakimoto, T.; Kondo, H.; Nii, H.; Kimura, K.; Egami, Y.; Oka, Y.; Yoshida, M.; Kida, E.; Ye, Y.; Akahoshi, S. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA 2011, 108, 17533–17537. [Google Scholar] [CrossRef] [Green Version]
- Treschow, A.; Hodges, L.; Wright, P.; Wynne, P.; Kalafatis, N.; Macrides, T. Novel anti-inflammatory ω-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 645–656. [Google Scholar] [CrossRef]
- Singh, M.; Hodges, L.; Wright, P.; Cheah, D.; Wynne, P.; Kalafatis, N.; Macrides, T. The CO2-SFE crude lipid extract and the free fatty acid extract from Perna canaliculus have anti-inflammatory effects on adjuvant-induced arthritis in rats. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 251–258. [Google Scholar] [CrossRef]
- Nagaoka, I.; Igarashi, M.; Sakamoto, K. Biological activities of glucosamine and its related substances. Adv. Food Nutr. Res. 2012, 65, 337–352. [Google Scholar]
- Varghese, S.; Theprungsirikul, P.; Sahani, S.; Hwang, N.; Yarema, K.; Elisseeff, J. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthr. Cartil. 2007, 15, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Gruenwald, J.; Petzold, E.; Busch, R.; Petzold, H.-P.; Graubaum, H.-J. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv. Ther. 2009, 26, 858–871. [Google Scholar] [CrossRef]
- Miller, M.R.; Tian, H. Changes in proximate composition, lipid class and fatty acid profile in Greenshell™ mussels (Perna canaliculus) over an annual cycle. Aquac. Res. 2018, 49, 1153–1165. [Google Scholar] [CrossRef]
- Taylor, M.C.; Roberts, R.; Miller, M. A Lipidomic exploration of Greenshell mussels™, Perna canaliculus. In Preparation.
- Sivakumaran, S. The Concise New Zealand Food Composition Tables 12th Edition 2016; Huffman, L., Sivakumaran, S., Eds.; The New Zealand Institute for Plant & Food Research Limited and Ministry of Health: Palmerston North, New Zealand, 2017. [Google Scholar]
- Whaanga, H.; Wehi, P.; Cox, M.; Roa, T.; Kusabs, I. Māori oral traditions record and convey indigenous knowledge of marine and freshwater resources. N. Z. J. Mar. Freshw. Res. 2018, 52, 487–496. [Google Scholar] [CrossRef]
- Best, E. Fishing Methods and Devices of the Maori; Dominion Museum: Wellington, New Zealand, 1929; Volume 12. [Google Scholar]
- Titcomb, M.; Fellows, D.B.; Pukui, M.K.; Devaney, D.M. Native use of marine invertebrates in old Hawaii. Pac. Sci. 1978, 32, 325–386. [Google Scholar]
- Dixon, L.L. Microbiological Quality of Toroi: A Māori Food Delicacy; The University of Waikato: Hamilton, New Zealand, 2007. [Google Scholar]
- Dixon, L.; Donnison, A.; Harfoot, C.; McDonald, I.R. Survival of Escherichia coli in toroi: A traditional Māori food. N. Z. J. Mar. Freshw. Res. 2007, 41, 369–375. [Google Scholar] [CrossRef]
- Dixon, L.; Donnison, A.; Ross, C.; McDonald, I.R. Addition of bacteriocins to inhibitListeria monocytogenes in Toroi: A traditional food of New Zealand Mãori. Ann. Microbiol. 2008, 58, 207. [Google Scholar] [CrossRef]
- Hudson, J.A.; Hasell, S.; Whyte, R.; Monson, S. Preliminary microbiological investigation of the preparation of two traditional Maori foods (Kina and Tiroi). J. Appl. Microbiol. 2001, 91, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Stenton-Dozey, J.; Broekhuizen, N. Provision of Ecological and Ecosystem Services by Mussel Farming in the Marlborough Sounds: A Literature Review in Context of the State of the Environment Pre- and Post-Mussel Farming; National Institute of Water & Atmospheric Research Ltd. 2019020CH (project MFI19201); National Institute of Water & Atmospheric Research Ltd.: Auckland, New Zealand, 2019; p. 145. Available online: https://www.marinefarming.co.nz/site_files/24792/upload_files/Fullreport_28.07.2021update.pdf?dl=1 (accessed on 16 January 2023).
- Riisgård, H.U.; Funch, P.; Larsen, P.S. The mussel filter–pump–present understanding, with a re-examination of gill preparations. Acta Zool. 2015, 96, 273–282. [Google Scholar] [CrossRef]
- Azra, M.N.; Okomoda, V.T.; Tabatabaei, M.; Hassan, M.; Ikhwanuddin, M. The Contributions of Shellfish Aquaculture to Global Food Security: Assessing Its Characteristics From a Future Food Perspective. Front. Mar. Sci. 2021, 8, 654897. [Google Scholar] [CrossRef]
- Warmerdam, S.; Vickers, J.; Palairet, N. Life Cycle Assessment of New Zealand Mussels and Oysters; Thinkstep Ltd. for Aquaculture New Zealand & Minstery of Primary Industries: Wellington, New Zealand, 2021. [Google Scholar]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar]
- Lingayat, A.; Balijepalli, R.; Chandramohan, V.P. Applications of solar energy based drying technologies in various industries–A review. Sol. Energy 2021, 229, 52–68. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I.; De Marco, I. Optimization of freeze-drying using a Life Cycle Assessment approach: Strawberries’ case study. J. Clean. Prod. 2017, 168, 1171–1179. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent drying of food products: A critical review. J. Food Eng. 2014, 121, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Strumillo, C.; Adamiec, J. Energy and Quality Aspects of Food Drying. Dry. Technol. 1996, 14, 423–448. [Google Scholar] [CrossRef]
- Ciesielski, K.; Zbicinski, I. Evaluation of Environmental Impact of the Spray-Drying Process. Dry. Technol. 2010, 28, 1091–1096. [Google Scholar] [CrossRef]
- White, M.T.; Bianchi, G.; Chai, L.; Tassou, S.A.; Sayma, A.I. Review of supercritical CO2 technologies and systems for power generation. Appl. Therm. Eng. 2021, 185, 116447. [Google Scholar] [CrossRef]
- Miller, M.R.; Araújo, B.C.; Casanovas, P. Composition of Greenshell™ Mussel Powders; Cawthron Isititute Prepared for Aquaculture New Zealand: Nelson, New Zealand, 2022; p. 24. [Google Scholar]
- Tanaka, T.; Takimoto, T.; Morishige, J.-I.; Kikuta, Y.; Sugiura, T.; Satouchi, K. Non-methylene-Interrupted Polyunsaturated Fatty Acids: Effective Substitute for Arachidonate of Phosphatidylinositol. Biochem. Biophys. Res. Commun. 1999, 264, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Perry, N.; Burgess, E.; Marshall, S. Regiospecific analyses of triacylglycerols of Hoki (Macruronus novaezelandiae) and Greenshell™ mussel (Perna canaliculus). J. Am. Oil Chemists’ Soc. 2011, 88, 509–515. [Google Scholar] [CrossRef]
- Smital, T.; Kurelec, B. The chemosensitizers of multixenobiotic resistance mechanism in aquatic invertebrates: A new class of pollutants. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 399, 43–53. [Google Scholar] [CrossRef]
- Chourasia, M.; Jain, S. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.; Gilzad-Kohan, M.H.; Aghazadeh-Habashi, A.; Jamali, F. Absorption and bioavailability of glucosamine in the rat. J. Pharm. Sci. 2012, 101, 2574–2583. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Miller, M.R.; Kruger, M.C.; Wynne, C.; Waaka, D.; Li, W.; Frampton, C.; Wolber, F.M.; Eason, C. Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats. Mar. Drugs 2020, 18, 524. [Google Scholar] [CrossRef]
- Gibson and Gibson, The treatment of arthritis with a lipid extract of Perna canaliculus: A randomized trial. Complement. Ther. Med. 1998, 6, 122–126. [CrossRef]
- Audeval, B.; Bouchacourt, P. Double-blind trial against placebo of extract of Perna canaliculus (green-lipped mussel) in osteoarthritis of the knee. Gaz. Med. 1986, 93, 111–116. [Google Scholar]
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jung, Y.B.; Seong, S.C.; Park, H.B.; Byun, K.Y.; Lee, D.C.; Song, E.K.; Son, J.H. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna Canaliculus) in patients with osteoarthritis of the hip and knee: A multicenter 2-month clinical trial. Eur. Ann. Allergy Clin. Immunol. 2003, 35, 212–216. [Google Scholar] [PubMed]
- Lau, C.S.; Chiu, P.K.; Chu, E.M.; Cheng, I.Y.; Tang, W.M.; Man, R.Y.; Halpern, G. Treatment of knee osteoarthritis with Lyprinol®, lipid extract of the green-lipped mussel-A double-blind placebo-controlled study. Prog. Nutr. 2004, 6, 17–31. [Google Scholar]
- Zawadzki, M.; Janosch, C.; Szechinski, J. Perna canaliculus Lipid Complex PCSO-524™ Demonstrated Pain Relief for Osteoarthritis Patients Benchmarked against Fish Oil, a Randomized Trial, without Placebo Control. Mar. Drugs 2013, 11, 1920–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stebbings, S.; Gray, A.; Schneiders, A.G.; Sansom, A. A randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of a novel green-lipped mussel extract-BioLex®-for managing pain in moderate to severe osteoarthritis of the hip and knee. BMC Complement. Altern. Med. 2017, 17, 416. [Google Scholar] [CrossRef]
- Abshirini, M.; Coad, J.; Wolber, F.M.; von Hurst, P.; Miller, M.R.; Tian, H.S.; Kruger, M.C. Green-lipped (greenshell™) mussel (Perna canaliculus) extract supplementation in treatment of osteoarthritis: A systematic review. Inflammopharmacology 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Caughey, D.; Grigor, R.; Caughey, E.; Young, P.; Gow, P.; Stewart, A. Perna canaliculus in the treatment of rheumatoid arthritis. Eur. J. Rheumatol. Inflamm. 1983, 6, 197–200. [Google Scholar]
- Larkin, J.; Capell, H.; Sturrock, R. Seatone in rheumatoid arthritis: A six-month placebo-controlled study. Ann. Rheum. Dis. 1985, 44, 199–201. [Google Scholar] [CrossRef]
- Gruenwald, J.; Graubaum, H.-J.; Hansen, K.; Grube, B. Efficacy and tolerability of a combination of Lyprinol® and high concentrations of EPA and DHA in inflammatory rheumatoid disorders. Adv. Ther. 2004, 21, 197–201. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107, S171–S184. [Google Scholar] [CrossRef] [Green Version]
- Soontornvipart, K.; Mongkhon, N.; Nganvongpanit, K.; Kongtawelert, P. Effect of PCSO-524 on OA biomarkers and weight-bearing properties in canine shoulder and coxofemeral osteoarthritis. Thai J. Vet. Med. 2015, 45, 157. [Google Scholar]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.S.; Wolber, F.M. The preventive effects of greenshell mussel (Perna canaliculus) on early-stage metabolic osteoarthritis in rats with diet-induced obesity. Nutrients 2019, 11, 1601. [Google Scholar] [CrossRef] [Green Version]
- Abshirini, M.; Coad, J.; Wolber, F.M.; Von Hurst, P.; Miller, M.R.; Tian, H.S.; Kruger, M.C. Effects of GreenshellTM mussel intervention on biomarkers of cartilage metabolism, inflammatory markers and joint symptoms in overweight/obese postmenopausal women: A randomized, double-blind, placebo-controlled trial. Front. Med. 2022, 9, 3587. [Google Scholar] [CrossRef]
- Dong, H.; Hutchins-Wiese, H.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.; Durham, H.; Feinn, R.; Kenny, A.M. Effects of omega-3 polyunsaturated fatty acid supplementation on bone turnover in older women. Int. J. Vitam. Nutr. Res. 2014, 84, 124–132. [Google Scholar] [CrossRef]
- Hutchins-Wiese, H.L.; Picho, K.; Watkins, B.A.; Li, Y.; Tannenbaum, S.; Claffey, K.; Kenny, A.M. High-dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: A pilot study. Nutr. Cancer 2014, 66, 68–76. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, Y.; Chen, Z.; Yu, X.; Ma, D. Effect of n-3 polyunsaturated fatty acid on bone health: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2022, 10, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Li, Y.; Allen, K.G.; Hoffmann, W.E.; Seifert, M.F. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J. Nutr. 2000, 130, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.J.; Gregoire, B.R.; Michelsen, K.G.; Picklo, M.J. Increasing dietary fish oil reduces adiposity and mitigates bone deterioration in growing C57BL/6 mice fed a high-fat diet. J. Nutr. 2020, 150, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, E.B.; Alderghaffar, M.; Wauquier, F.; Coxam, V.; Demontiero, O.; Vogrin, S.; Wittrant, Y.; Duque, G. The effects of dietary fatty acids on bone, hematopoietic marrow and marrow adipose tissue in a murine model of senile osteoporosis. Aging 2019, 11, 7938. [Google Scholar] [CrossRef] [PubMed]
- Momomura, R.; Naito, K.; Igarashi, M.; Watari, T.; Terakado, A.; Oike, S.; Sakamoto, K.; Nagaoka, I.; Kaneko, K. Evaluation of the effect of glucosamine administration on biomarkers of cartilage and bone metabolism in bicycle racers. Mol. Med. Rep. 2013, 7, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Sakamoto, K.; Yamamoto, T.; Ishida, K.; Yamaguchi, H.; Nagaoka, I. Evaluation of the effect of glucosamine administration on biomarkers for cartilage and bone metabolism in soccer players. Int. J. Mol. Med. 2009, 24, 487–494. [Google Scholar]
- Torrent, A.; Montell, E.; Vergés, J.; Carceller, M.; Blanco, A.; Terencio, M.; Ferrándiz, M.; Alcaraz, M. Effect of chondroitin sulphate and glucosamine in combination in an animal model of osteoarthritis and osteoporosis. Osteoarthr. Cartil. 2014, 22, S351. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Loehfelm, A.; Rizwan, M.Z.; Tups, A. A New Zealand green-lipped mussel oil-enriched high-fat diet exhibits beneficial effects on body weight and metabolism in mice. Br. J. Nutr. 2021, 125, 972–982. [Google Scholar] [CrossRef]
- Vaidya, H.B.; Gangadaran, S.; Cheema, S. A high fat-high sucrose diet enriched in blue mussels protects against systemic inflammation, metabolic dysregulation and weight gain in C57BL/6 mice. Food Res. Int. 2017, 100, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Aldairi, A.F.; Alyamani, R.A.; Al-Hazmi, A.; Halawani, I.F.; Alsubaihi, A.A.; Idris, S.; Fallatah, N.A.; Gassas, A.; Almalki, A.A.; Qasem, A. Antioxidant and antithrombotic effects of green mussels (perna canaliculus) in rats. J. Food Biochem. 2021, 45, e13865. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xiong, Q.; Yin, Y.; Ling, Z.; Chen, S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Front. Cardiovasc. Med. 2021, 8, 802306. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, H.M.; Gjertsson, I.; Andersson, S.; Calder, P.C.; Bärebring, L. Influence of blue mussel (Mytilus edulis) intake on fatty acid composition in erythrocytes and plasma phospholipids and serum metabolites in women with rheumatoid arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2019, 150, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.B.; Hopkins, S.E.; Wiener, H.W.; Purnell, J.Q.; O’Brien, D.M.; Zhang, C.X.; Aslan, J.E.; Aliwarga, T.; Pomeroy, J.J.; Thummel, K.E. Habitual Intake of Marine-Derived n-3 PUFAs is Inversely Associated with a Cardiometabolic Inflammatory Profile in Yup’ik Alaska Native People. J. Nutr. 2022, 152, 844–855. [Google Scholar] [CrossRef]
- Minihane, A.M.; Armah, C.K.; Miles, E.A.; Madden, J.M.; Clark, A.B.; Caslake, M.J.; Packard, C.J.; Kofler, B.M.; Lietz, G.; Curtis, P.J. Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: A retrospective analysis. J. Nutr. 2016, 146, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-H.; Gao, X.; Chung, V.C.; Zhong, W.-F.; Fu, Q.; Lv, Y.-B.; Wang, Z.-H.; Shen, D.; Zhang, X.-R.; Zhang, P.-D. Associations of regular glucosamine use with all-cause and cause-specific mortality: A large prospective cohort study. Ann. Rheum. Dis. 2020, 79, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, E.D.; Lampe, J.W.; Vaughan, T.L.; Peters, U.; Rehm, C.D.; White, E. Association between use of specialty dietary supplements and C-reactive protein concentrations. Am. J. Epidemiol. 2012, 176, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Barnes, P. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Mickleborough, T.D.; Vaughn, C.L.; Shei, R.-J.; Davis, E.M.; Wilhite, D.P. Marine lipid fraction PCSO-524™(lyprinol®/omega XL®) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013, 107, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Lello, J.; Liang, A.; Robinson, E.; Leutenegger, D.; Wheat, A. Treatment of children’s asthma with a lipid extract of the New Zealand green lipped mussel (Perna canaliculus)(Lyprinol®)—A double blind, randomized controlled trial in children with moderate to serve chronic obstructive asthma. Internet J. Asthma Allergy Immunol. 2012, 8, 1–12. [Google Scholar]
- Shei, R.-J.; Adamic, E.M.; Chapman, R.F.; Mickleborough, T.D. The Effects of PCSO-524®, a Patented Marine Oil Lipid derived from the New Zealand Green Lipped Mussel (Perna canaliculus), on Pulmonary and Respiratory Muscle Function in Non-asthmatic Elite Runners. Int. J. Exerc. Sci. 2018, 11, 669. [Google Scholar]
- Baum, K.; Telford, R.D.; Cunningham, R.B. Marine oil dietary supplementation reduces delayed onset muscle soreness after a 30 km run. Open Access J. Sport. Med. 2013, 4, 109. [Google Scholar] [CrossRef] [Green Version]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524®, a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sport. Nutr. 2015, 12, 1–17. [Google Scholar]
- Barenie, M.J.; Freemas, J.A.; Baranauskas, M.N.; Goss, C.S.; Freeman, K.L.; Chen, X.; Dickinson, S.L.; Fly, A.D.; Kawata, K.; Chapman, R.F. Effectiveness of a combined New Zealand green-lipped mussel and Antarctic krill oil supplement on markers of exercise-induced muscle damage and inflammation in untrained men. J. Diet. Suppl. 2020, 19, 184–211. [Google Scholar] [CrossRef]
- Pumpa, K.L.; Fallon, K.E.; Bensoussan, A.; Papalia, S. The effects of Lyprinol® on delayed onset muscle soreness and muscle damage in well trained athletes: A double-blind randomised controlled trial. Complement. Ther. Med. 2011, 19, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contraction. J. Int. Soc. Sport. Nutr. 2017, 14, 23. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in muscle damage and function. Nutrients 2018, 10, 552. [Google Scholar] [CrossRef] [Green Version]
- Braun, W.; Flynn, M.; Armstrong, W.; Jacks, D. The effects of chondroitin sulfate supplementation on indices of muscle damage induced by eccentric arm exercise. J. Sport. Med. Phys. Fit. 2005, 45, 553. [Google Scholar]
- Arendt-Nielsen, L.; Weidner, M.; Bartholin, D.; Rosetzsky, A. A double-blind randomized placebo controlled parallel group study evaluating the effects of ibuprofen and glucosamine sulfate on exercise induced muscle soreness. J. Musculoskelet. Pain 2007, 15, 21–28. [Google Scholar] [CrossRef]
- Gudis, K.; Sakamoto, C. The role of cyclooxygenase in gastric mucosal protection. Dig. Dis. Sci. 2005, 50, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Seibert, K.; Masferrer, J. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor 1994, 4, 17–23. [Google Scholar] [PubMed]
- Sinha, M.; Gautam, L.; Shukla, P.K.; Kaur, P.; Sharma, S.; Singh, T.P. Current perspectives in NSAID-induced gastropathy. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rainsford, K.; Whitehouse, M. Gastroprotective and anti-inflammatory properties of green lipped mussel (Perna canaliculus) preparation. Arzneimittel-forschung 1980, 30, 2128–2132. [Google Scholar]
- McPhee, S.; Hodges, L.D.; Wright, P.F.A.; Wynne, P.M.; Kalafatis, N.; Harney, D.W.; Macrides, T.A. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 346–356. [Google Scholar] [CrossRef]
- Coulson, S.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel (Perna canaliculus) extract efficacy in knee osteoarthritis and improvement in gastrointestinal dysfunction: A pilot study. Inflammopharmacology 2012, 20, 71–76. [Google Scholar] [CrossRef]
- Shang, Q.; Yin, Y.; Zhu, L.; Li, G.; Yu, G.; Wang, X. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int. J. Biol. Macromol. 2016, 86, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.E.; Vilén, E.M.; Namburi, R.B.; Benjdia, A.; Beneteau, J.; Malleron, A.; Bonnaffé, D.; Driguez, P.-A.; Descroix, K.; Lassalle, G. Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J. Biol. Chem. 2014, 289, 24289–24303. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.; Stolarczyk, E.; Mouni, F.; Brassart, C.; Vidal, O.; Aïssi, E.; Bouquelet, S.; Krzewinski, F. Characterisation of glutamine fructose-6-phosphate amidotransferase (EC 2.6. 1.16) and N-acetylglucosamine metabolism in Bifidobacterium. Arch. Microbiol. 2008, 189, 157–167. [Google Scholar] [CrossRef]
- Yu, H.-N.; Zhu, J.; Pan, W.-s.; Shen, S.-R.; Shan, W.-G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C. L A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.; Lampe, J.; Peters, U.; Shen, D.; Vaughan, T.; White, E. Use of glucosamine and chondroitin supplements and risk of colorectal cancer. Cancer Causes Control. 2013, 24, 1137–1146. [Google Scholar] [CrossRef]
- Wu, R.; Li, P.; Wang, Y.; Su, N.; Xiao, M.; Li, X.; Shang, N. Structural analysis and anti-cancer activity of low-molecular-weight chondroitin sulfate from hybrid sturgeon cartilage. Carbohydr. Polym. 2022, 275, 118700. [Google Scholar] [CrossRef]
- Kean, J.D.; Sarris, J.; Scholey, A.; Silberstein, R.; Downey, L.A.; Stough, C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524®) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology 2017, 234, 403–420. [Google Scholar] [PubMed] [Green Version]
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchén, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.; Chen, H.; Shen, S.; Xu, F.; Wang, R.; Yin, J.; Chen, X.; Guan, M.; Shen, L.; Pan, H. Highly Purified Eicosapentaenoic Acid Alleviates the Inflammatory Response and Oxidative Stress in Macrophages during Atherosclerosis via the miR-1a-3p/sFRP1/Wnt/PCP-JNK Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Scorletti, E.; West, A.L.; Bhatia, L.; Hoile, S.P.; McCormick, K.G.; Burdge, G.C.; Lillycrop, K.A.; Clough, G.F.; Calder, P.C.; Byrne, C.D. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: Results from the WELCOME trial. J. Hepatol. 2015, 63, 1476–1483. [Google Scholar] [CrossRef] [Green Version]
- Cartolano, F.D.C.; Dias, G.D.; Miyamoto, S.; Damasceno, N.R.T. Omega-3 Fatty Acids Improve Functionality of High-Density Lipoprotein in Individuals With High Cardiovascular Risk: A Randomized, Parallel, Controlled and Double-Blind Clinical Trial. Front. Nutr. 2021, 8, 767535. [Google Scholar]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Yang, B.; Ren, X.-l.; Li, Z.-h.; Shi, M.-q.; Ding, F.; Su, K.-P.; Guo, X.-j.; Li, D. Lowering effects of fish oil supplementation on proinflammatory markers in hypertension: Results from a randomized controlled trial. Food Funct. 2020, 11, 1779–1789. [Google Scholar] [CrossRef]
- Joy, J.M.; Gundermann, D.M.; Lowery, R.P.; Jäger, R.; McCleary, S.A.; Purpura, M.; Roberts, M.D.; Wilson, S.; Hornberger, T.A.; Wilson, J.M. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr. Metab. 2014, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sorgi, P.J.; Hallowell, E.M.; Hutchins, H.L.; Sears, B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [PubMed]
- Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M. Specialized pro-resolving lipid mediators: The future of chronic pain therapy? Int. J. Mol. Sci. 2021, 22, 10370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, C.; Cho, S.H.; Lee, H.J. Green lipped mussel oil complex suppresses lipopolysaccharide stimulated inflammation via regulating nuclear factor-κB and mitogen activated protein kinases signaling in RAW264. 7 murine macrophages. Food Sci. Biotechnol. 2017, 26, 815–822. [Google Scholar] [CrossRef]
- Ostojic, M.; Zevrnja, A.; Vukojevic, K.; Soljic, V. Immunofluorescence analysis of NF-kB and iNOS expression in different cell populations during early and advanced knee osteoarthritis. Int. J. Mol. Sci. 2021, 22, 6461. [Google Scholar] [CrossRef]
- Miller, M.R.; Pearce, L.; Bettjeman, B.I. Detailed distribution of lipids in Greenshell™ Mussel (Perna canaliculus). Nutrients 2014, 6, 1454–1474. [Google Scholar] [CrossRef] [Green Version]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Fuchs, B. Analytical methods for (oxidized) plasmalogens: Methodological aspects and applications. Free Radic. Res. 2015, 49, 599–617. [Google Scholar] [CrossRef]
- André, A.; Juaneda, P.; Sébédio, J.-L.; Chardigny, J.-M. Plasmalogen metabolism-related enzymes in rat brain during aging: Influence of n-3 fatty acid intake. Biochimie 2006, 88, 103–111. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddhachat, K.; Siengdee, P.; Chomdej, S.; Soontornvipart, K.; Nganvongpanit, K. Effects of different omega-3 sources, fish oil, krill oil, and green-lipped mussel against cytokine-mediated canine cartilage degradation. In Vitro Cell. Dev. Biol. -Anim. 2017, 53, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.; Wolber, F.M. Non-polar lipid from greenshell mussel (Perna canaliculus) inhibits osteoclast differentiation. Bone Rep. 2021, 15, 101132. [Google Scholar] [CrossRef]
- Zhan, Q.; Tian, Y.; Han, L.; Wang, K.; Wang, J.; Xue, C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020, 11, 7048–7060. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, F.; Li, Y.; Dai, Y.; Liu, Y.; Wang, J.; Xue, C. Oil from Antarctic krill (Euphausia superba) facilitates bone formation in dexamethasone-treated mice. Food Sci. Biotechnol. 2019, 28, 539–545. [Google Scholar] [CrossRef]
- Kasonga, A.; Kruger, M.C.; Coetzee, M. Activation of PPARs modulates signalling pathways and expression of regulatory genes in osteoclasts derived from human CD14+ monocytes. Int. J. Mol. Sci. 2019, 20, 1798. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.; Dodd, J.; Ormrod, D.; Geddes, R. Anti-inflammatory activity of glycogen extracted fromPerna canaliculus (NZ green-lipped mussel). Agents Actions 1993, 38, C139–C142. [Google Scholar] [CrossRef]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; Lane, N.E. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann. Rheum. Dis. 2010, 69, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Chiusaroli, R.; Piepoli, T.; Zanelli, T.; Ballanti, P.; Lanza, M.; Rovati, L.C.; Caselli, G. Experimental pharmacology of glucosamine sulfate. Int. J. Rheumatol. 2011, 2011, 939265. [Google Scholar] [CrossRef] [Green Version]
- Cheleschi, S.; Tenti, S.; Giannotti, S.; Veronese, N.; Reginster, J.-Y.; Fioravanti, A. A combination of celecoxib and glucosamine sulfate has anti-inflammatory and chondroprotective effects: Results from an in vitro study on human osteoarthritic chondrocytes. Int. J. Mol. Sci. 2021, 22, 8980. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, W.; Chen, H.; Shao, X.; Lin, P.; Liu, X.; Li, X.; Ye, H. Glucosamine promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2018, 42, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Lambert, C. Chondroitin and glucosamine in the management of osteoarthritis: An update. Curr. Rheumatol. Rep. 2013, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Byron, C.R.; Stewart, M.C.; Stewart, A.A.; Pondenis, H.C. Effects of clinically relevant concentrations of glucosamine on equine chondrocytes and synoviocytes in vitro. Am. J. Vet. Res. 2008, 69, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, E.; Penolazzi, L.; Pandolfi, A.; Mandatori, D.; Sollazzo, V.; Piva, R. Human osteoclasts/osteoblasts 3D dynamic co-culture system to study the beneficial effects of glucosamine on bone microenvironment. Int. J. Mol. Med. 2021, 47, 1–9. [Google Scholar] [CrossRef]
- Kantor, E.D.; Lampe, J.W.; Navarro, S.L.; Song, X.; Milne, G.L.; White, E. Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. J. Altern. Complement. Med. 2014, 20, 479–485. [Google Scholar] [CrossRef]
- Misra, D.; Fielding, R.A.; Felson, D.T.; Niu, J.; Brown, C.; Nevitt, M.; Lewis, C.E.; Torner, J.; Neogi, T. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. 2019, 71, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.R.; Abdulla, J.; Andersen, H.E.; Schwarz, P.; Suetta, C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur. Geriatr. Med. 2018, 9, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Gromova, O.; Torshin, I.Y.; Lila, A.; Shostak, N.; Rudakov, K. Molecular mechanisms of myoprotective action of chondroitin sulfate and glucosamine sulfate in sarcopenia. Neurol. Neuropsychiatry Psychosom. 2019, 11, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2021, 119, 348–370. [Google Scholar] [CrossRef]
- Sabroe, R.; Black, A.K. Angiotensin–converting enzyme (ACE) inhibitors and angio–oedema. Br. J. Dermatol. 1997, 136, 153–158. [Google Scholar] [PubMed]
- Jayaprakash, R.; Perera, C.O. Partial purification and characterization of bioactive peptides from cooked New Zealand green-lipped mussel (Perna canaliculus) protein hydrolyzates. Foods 2020, 9, 879. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, M.R.; Abshirini, M.; Wolber, F.M.; Tuterangiwhiu, T.R.; Kruger, M.C. Greenshell Mussel Products: A Comprehensive Review of Sustainability, Traditional Use, and Efficacy. Sustainability 2023, 15, 3912. https://doi.org/10.3390/su15053912
Miller MR, Abshirini M, Wolber FM, Tuterangiwhiu TR, Kruger MC. Greenshell Mussel Products: A Comprehensive Review of Sustainability, Traditional Use, and Efficacy. Sustainability. 2023; 15(5):3912. https://doi.org/10.3390/su15053912
Chicago/Turabian StyleMiller, Matthew R., Maryam Abshirini, Frances M. Wolber, Te Rerekohu Tuterangiwhiu, and Marlena C. Kruger. 2023. "Greenshell Mussel Products: A Comprehensive Review of Sustainability, Traditional Use, and Efficacy" Sustainability 15, no. 5: 3912. https://doi.org/10.3390/su15053912





