A Study on the Sintering Mechanism of High-Strength Light Bricks Manufactured from Coal Gasification Slag
Abstract
:1. Introduction
2. Experiment
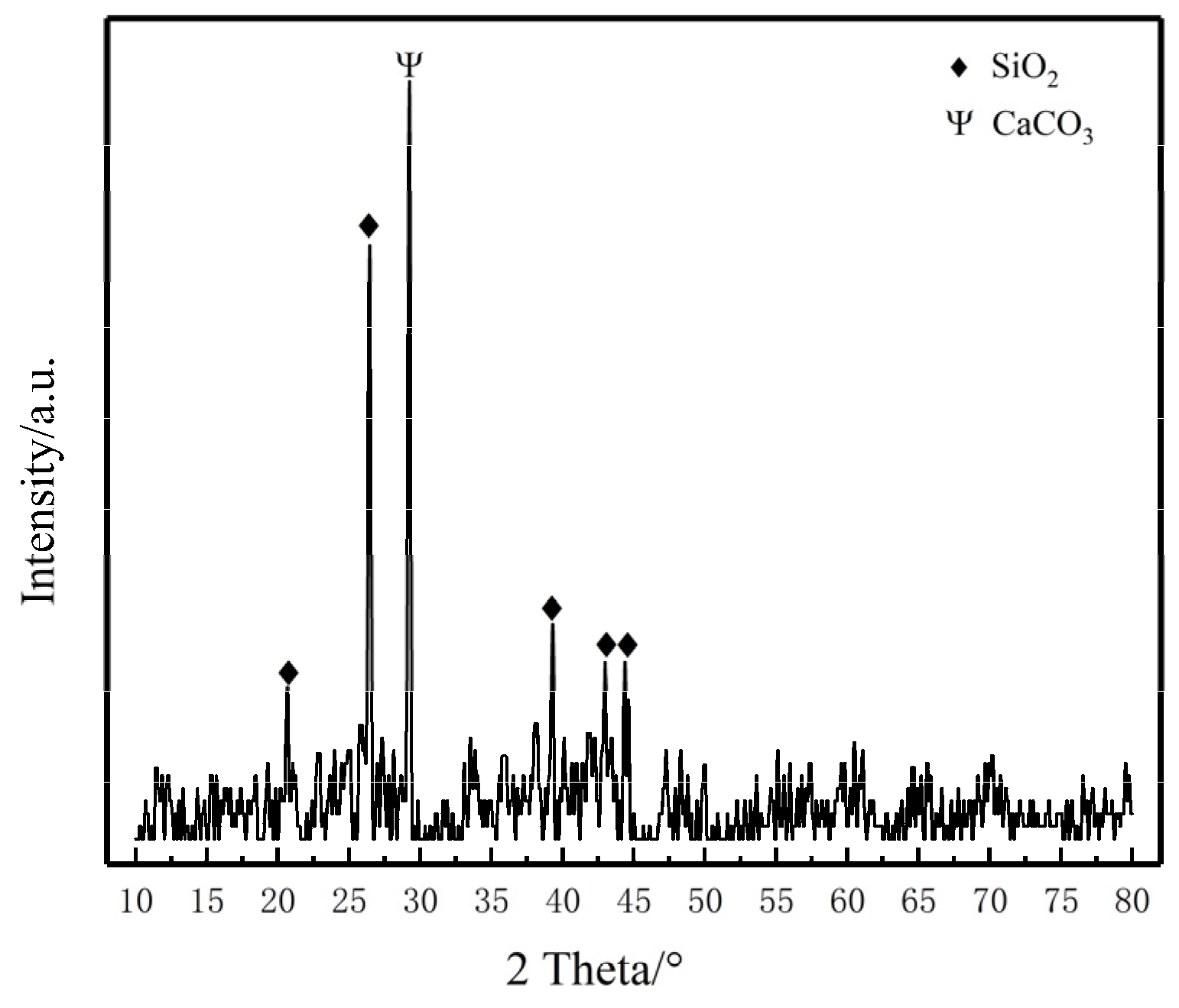
2.1. Materials
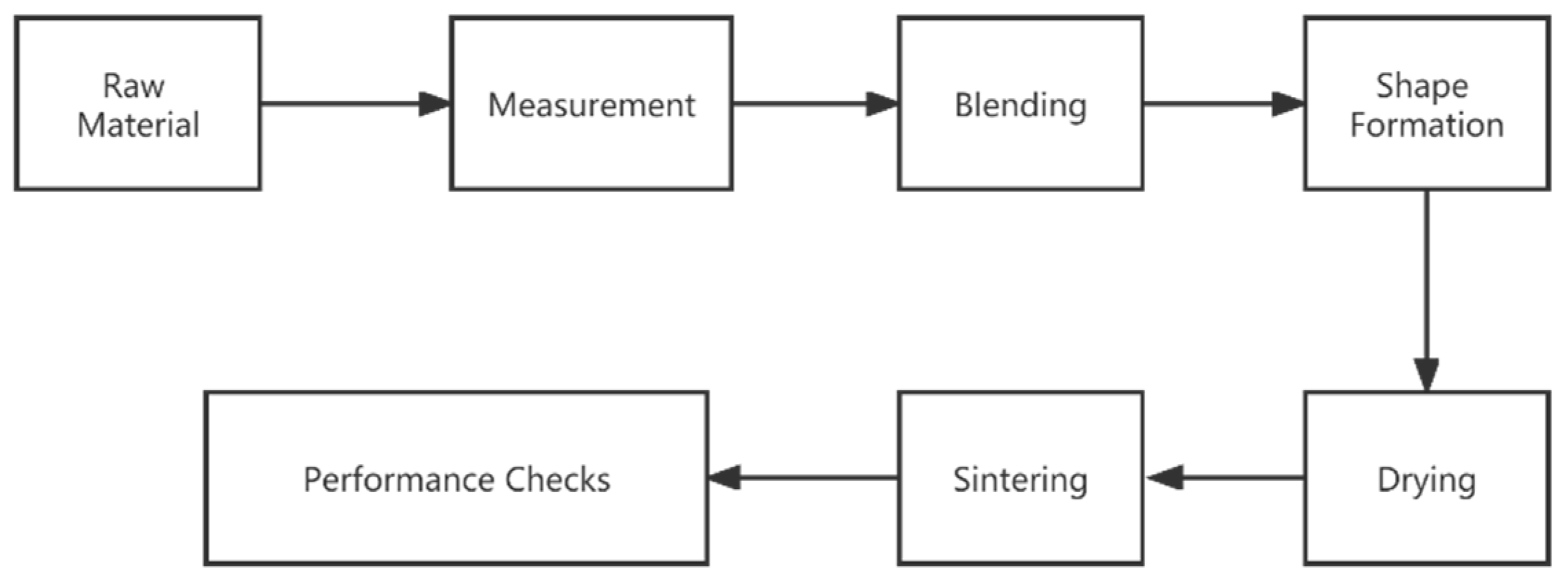
2.2. Experiment Workflow
- (1)
- Measurement: The measurement of the raw materials was based on the introduction amounts, designed in Table 1, using an 0.01 g electric balance.
- (2)
- Blending: Since the raw materials vary in their densities, in order to avoid stratification, they were placed in an NJ-160B stirrer and mixed thoroughly with an appropriate amount of water (10–20%) for a homogeneous mixture.
- (3)
- Shape formation: A YAW-1000 computer-controlled pressure tester and brick molds were used to press the homogeneous material mixture into several pieces of cylinder blank (Φ70 mm × 20 mm). The formation pressure was controlled at 10–20 MPa.
- (4)
- Drying: The pieces of blank were moved into a drying oven for drying at 105–110 °C for 24 h. This step stopped when the blank weight became constant.
- (5)
- Sintering: The dried sample pieces were sintered in a chamber electric furnace with predefined heat treatment approaches. The sintering temperatures were 1050 °C, 1100 °C, 1150 °C, 1200 °C, and 1250 °C. The introduction amounts of coal gasification slag in the samples are given in Table 2.
2.3. Performance Checks
3. Results and Discussion
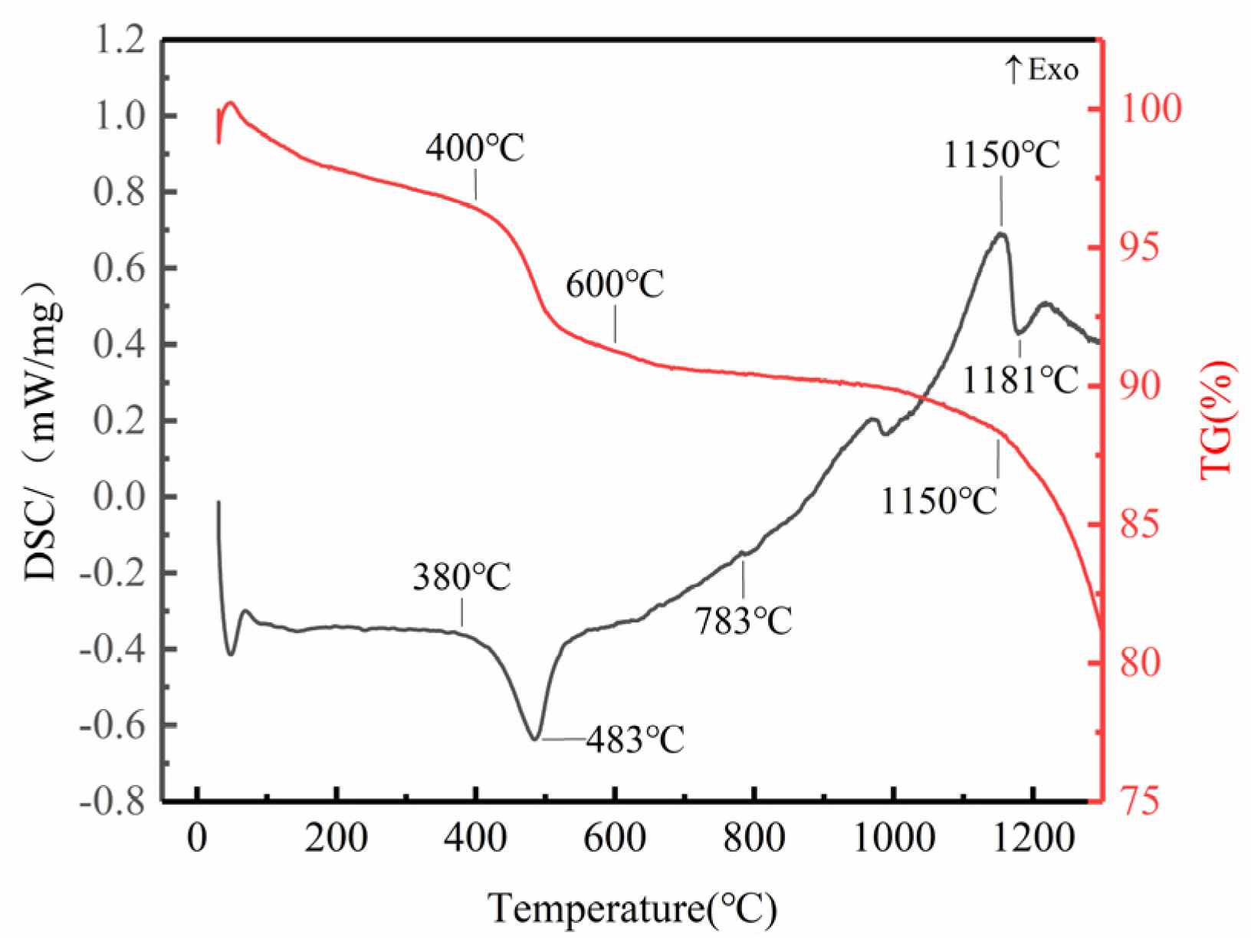
3.1. TG-DSC Analysis
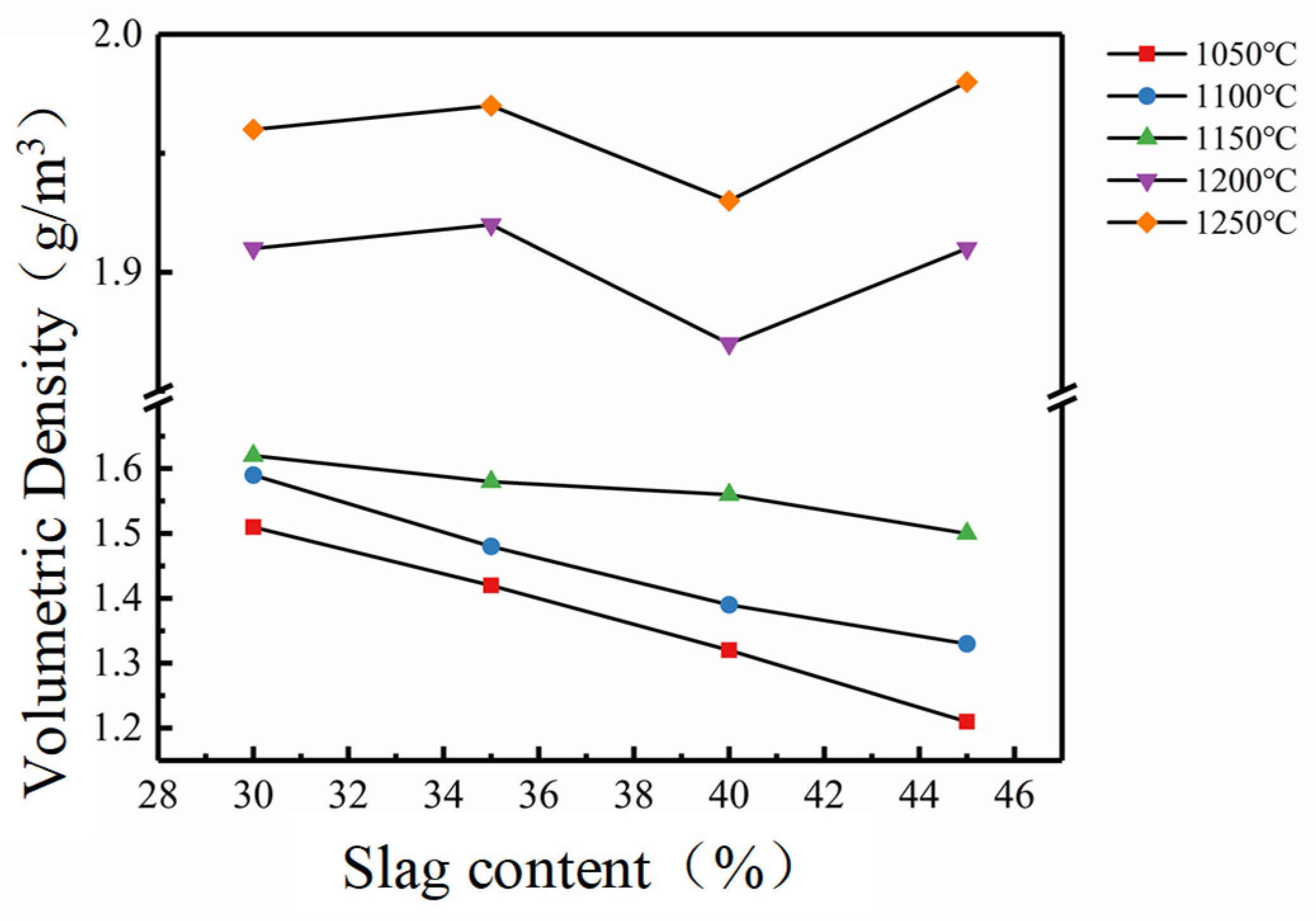
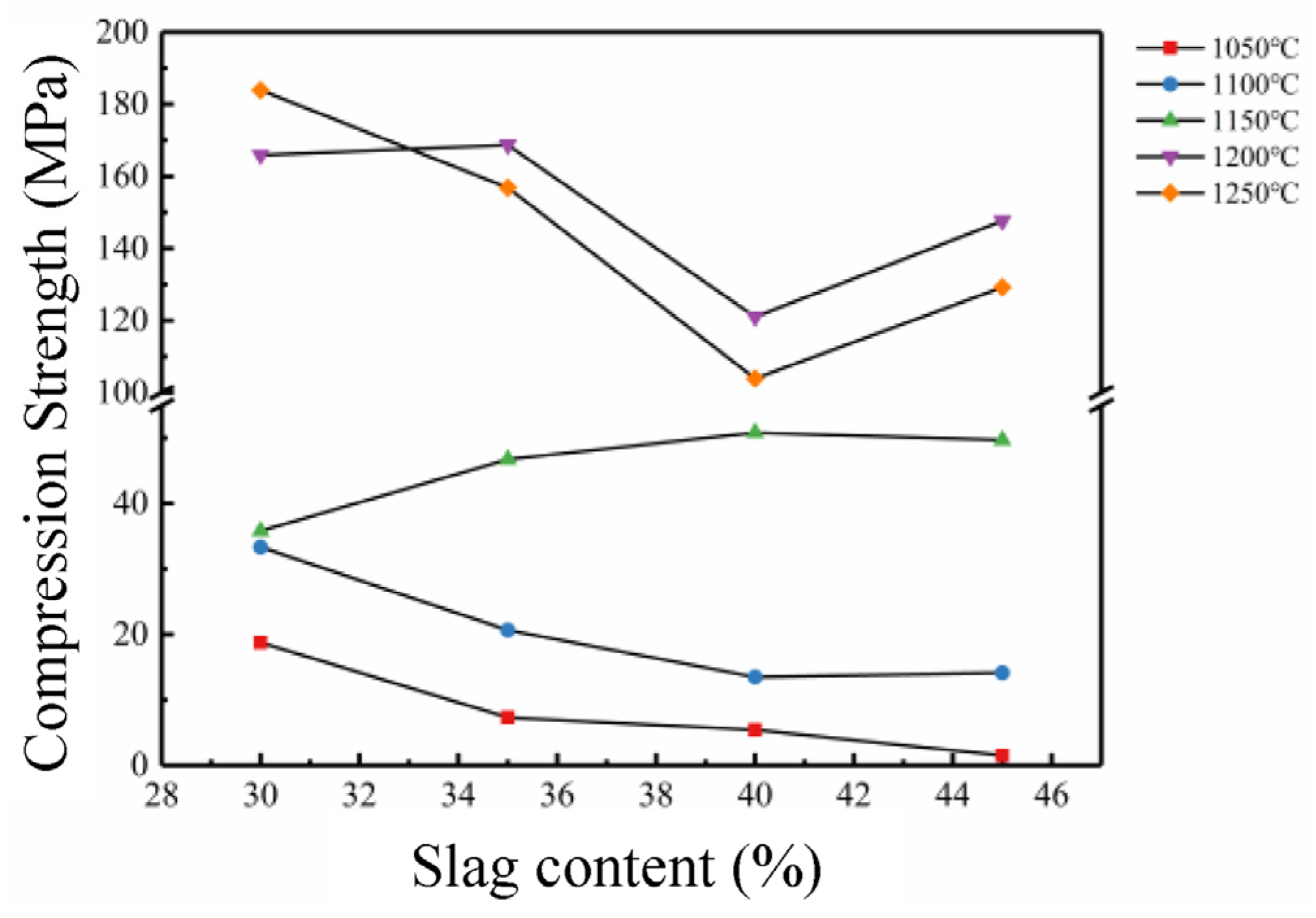
3.2. Performance Checks and Analysis
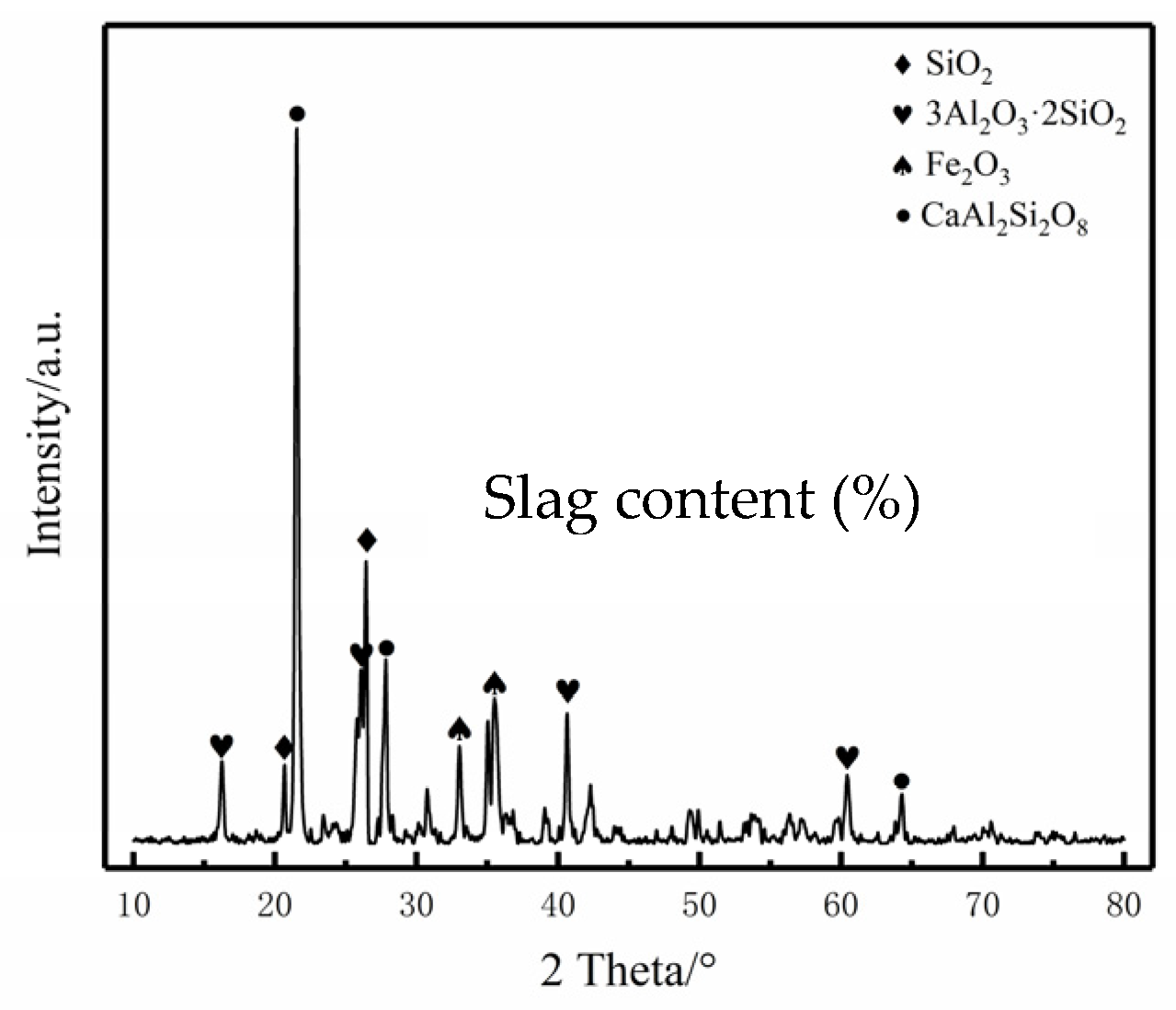
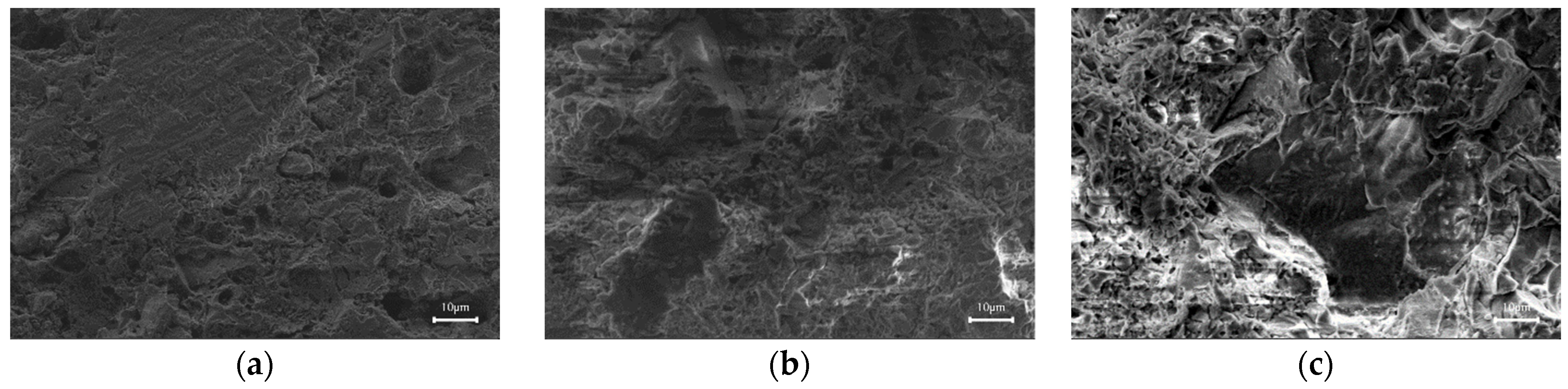
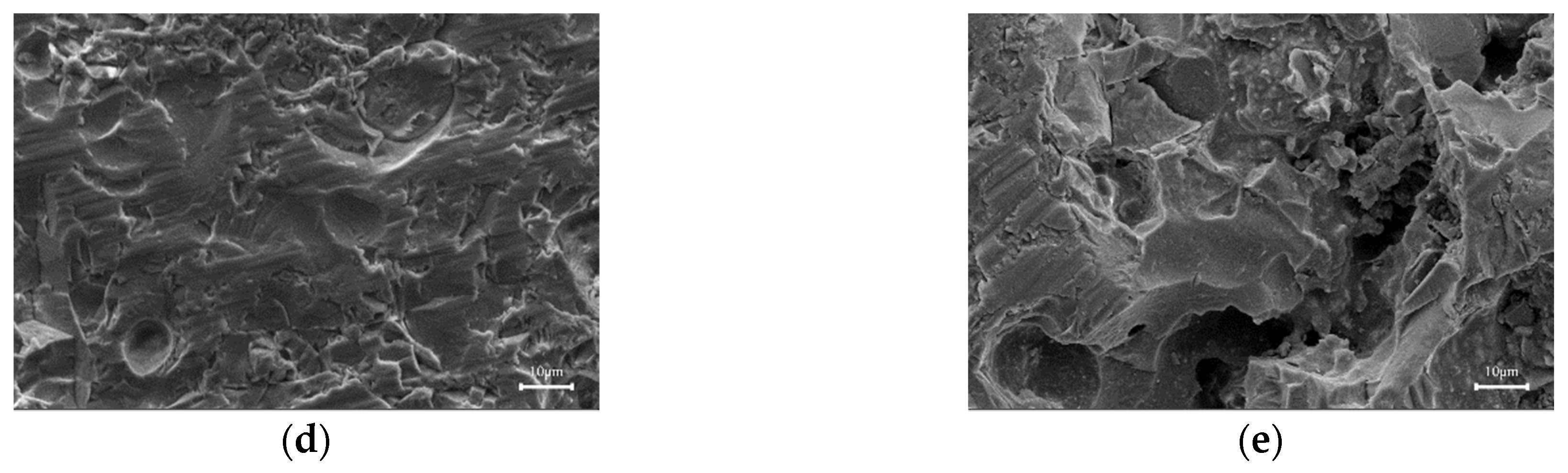
3.3. Microscopic Morphology of the Sintered Bricks
4. Conclusions
- (1)
- Coal gasification slag may be used as the main raw material in the manufacture of sintered bricks. The optimum sintering temperature of coal-gasification-slag-sintered brick is 1150 oC and its maximum addition amount could be as high as 45%. The volumetric density and compression strength of the sintered bricks were found to be negatively correlated to the introduction amount of the coal gasification slag, but positively correlated to the sintering temperature.
- (2)
- By adjusting the ratios of the coal gasification slag (main raw material), supplementary materials, and additives, sintered bricks with a density of 1.5 g/cm3 and a compression strength of 49.71 MPa may be manufactured under certain conditions. These bricks meet the requirements of MU30, the highest in the China National Standard GB/T 5101-2017.
- (3)
- The main crystalline phase in the sintered bricks manufactured from coal gasification slag is anorthite, and some hematite, mullite, and quartz crystals are also present. These crystals act as the fundamental skeleton of the sintered bricks. With the adhesion of amorphous glassy materials inside the brick blank, the skeleton crystals adhere to form sintered bricks with a certain mechanical strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.Y.; Huang, S.; Ji, L.Y.; Wu, Y.; Gao, J.S. Structure characteristics and gasification activity of residual carbon from entrained-flow coal gasification slag. Fuel 2014, 122, 67–75. [Google Scholar] [CrossRef]
- Li, Q. Application of fly ash in environmental engineering. Sci. Technol. Inf. 2008, 131–133. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.B.; Wu, H.; Cai, X.L.; Zhuo, J.D.; Lai, S.Y.; Liu, H.G.; Jing, Y.H.; Yuan, W. Basic characteristics of coal gasification residual. Clean Coal Technol. 2015, 21, 110–113. (In Chinese) [Google Scholar] [CrossRef]
- Matjie, R.H.; Alphen, C.V.; Pistorius, P.C. Mineralogical characterisation of secunda gasifier feedstock and coarse ash. Miner. Eng. 2006, 19, 256–261. [Google Scholar] [CrossRef]
- Matjie, R.H.; Li, Z.S.; Ward, C.R.; French, D. Chemical composition of glass and crystalline phases in coarse coal gasification ash. Fuel 2008, 87, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Wagner, N.J.; Matjie, R.H.; Slaghuis, J.H.; van Heerden, J. Characterization of unburned carbon present in coarse gasification ash. Fuel 2008, 87, 683–691. [Google Scholar] [CrossRef]
- Acosta, A.; Aineto, M.; Iglesias, I.; Romero, M.; Rincón, J.M. Physico-chemical characterization of slag waste coming from GICC thermal power plant. Mater. Lett. 2001, 50, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Acosta, A.; Iglesias, I.; Aineto, M.; Romero, M.; Rincón, J.M. Utilisation of IGCC slag and clay steriles in soft mud bricks (by pressing) for use in building bricks manufacturing. Waste Manag. 2002, 22, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Räsänen, A.; Huuhka, S.; Pakkala, T.; Lahdensivu, J. Methods for evaluating the technical performance of reclaimed bricks. Case Stud. Constr. Mater. 2022, 17, e01504. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Ahmed, S.M.; Hafez, R.D.A. Sugarcane Pulp Sand and Paper Grain Sand as Partial Fine Aggregate Replacement in Environment-friendly Concrete Bricks. Case Stud. Constr. Mater. 2023, 18, e01612. [Google Scholar] [CrossRef]
- Carvalho, A.; Xavier, G.C.; Alexandr, J.; Pedroti, L.G.; de Azevedo, A.R.G.; Vieira, C.M.F.; Monteiro, S.N. Environmental Durability of Soil-Cement Block Incorporated With Ornamental Stone Waste. Mater. Sci. Forum 2014, 798–199, 548–553. [Google Scholar] [CrossRef]
- Yin, H.F.; Tang, Y.; Ren, G.; Zhang, J.Z. Study on the characteristic and application of gasification slag from Texaco gasifier. Coal Convers. 2009, 32, 30–33. (In Chinese) [Google Scholar]
- Chen, Y.L.; Zhang, Y.M.; Chen, T.J.; Han, C. Firing process and mechanism of fired bricks prepared with iron tailings from western Hubei. J. Build. Mater. 2014, 17, 159–163. (In Chinese) [Google Scholar] [CrossRef]
- Li, D.W.; Zhang, L.Q.; Liu, X.F.; Du, Y.; Chen, Y.Q. Investigation of high red mud containing sintered brick. New Build. Mater. 2009, 36, 26–29. (In Chinese) [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.T.; Ma, Y.; Wang, Z.F.; Liu, H. Preparation of anorthite-mullite lightweight refractories by various starches in-situ consolidation. Refractories 2016, 50, 363–366. (In Chinese) [Google Scholar] [CrossRef]
- Sun, F.Y.; Lin, J.H.; Ren, K.F. Study on synthesis of mullite by micro-crystal muscovite and industrial alumina. Bull. Chin. Ceram. Soc. 2011, 29, 686–688+704. (In Chinese) [Google Scholar] [CrossRef]
- Li, R.F.; Zhou, Y.; Li, S.B.; Li, C.Y.; Huang, Z.Y. Sintering process and mechanism of fine-grained iron tailings from Beijing. J. Build. Mater. 2018, 21, 672–677. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, Y.L.; Qin, G.; Huo, J.C.; Shu, W. Technical research of titanium slag-shale sintered products. J. Wuhan Univ. Technol. 2012, 36, 11–16. (In Chinese) [Google Scholar] [CrossRef]
- Feng, Y.P.; Yin, H.F.; Yuan, H.D.; Zhang, L.; Cui, H. Study on the preparation of lightweight heat-insulation wall materials using gasification slag. Bull. Chin. Ceram. Soc. 2014, 33, 497–501+510. (In Chinese) [Google Scholar] [CrossRef]

| Component | SiO2 | Al2O3 | Fe2O3 | CaO | TFe | LOI | Other |
|---|---|---|---|---|---|---|---|
| Weight | 19.04 | 8.93 | 9.13 | 9 | 6.39 | 48.9 | 5 |
| Sample | A | B | C | D |
|---|---|---|---|---|
| Slag amount | 30 | 35 | 40 | 45 |
| Sample Name | Quartz | Kaolinite | Calcite | Muscovite | Diopside | Hematite | Anorthite | Mullite |
|---|---|---|---|---|---|---|---|---|
| Slag | √ | × | √ | × | × | √ | × | × |
| Supplementary Materials | √ | × | × | √ | √ | × | × | × |
| Additives | √ | √ | × | × | × | × | × | × |
| Sintered Samples | √ | × | × | × | × | × | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Niu, G.; Guo, W. A Study on the Sintering Mechanism of High-Strength Light Bricks Manufactured from Coal Gasification Slag. Sustainability 2023, 15, 4860. https://doi.org/10.3390/su15064860
Liu F, Niu G, Guo W. A Study on the Sintering Mechanism of High-Strength Light Bricks Manufactured from Coal Gasification Slag. Sustainability. 2023; 15(6):4860. https://doi.org/10.3390/su15064860
Chicago/Turabian StyleLiu, Fang, Guofeng Niu, and Wentao Guo. 2023. "A Study on the Sintering Mechanism of High-Strength Light Bricks Manufactured from Coal Gasification Slag" Sustainability 15, no. 6: 4860. https://doi.org/10.3390/su15064860





