Understanding Binding of Quaternary Ammonium Compounds with Cellulose-Based Fibers and Wipes for Renewable and Sustainable Hygiene Options
Abstract
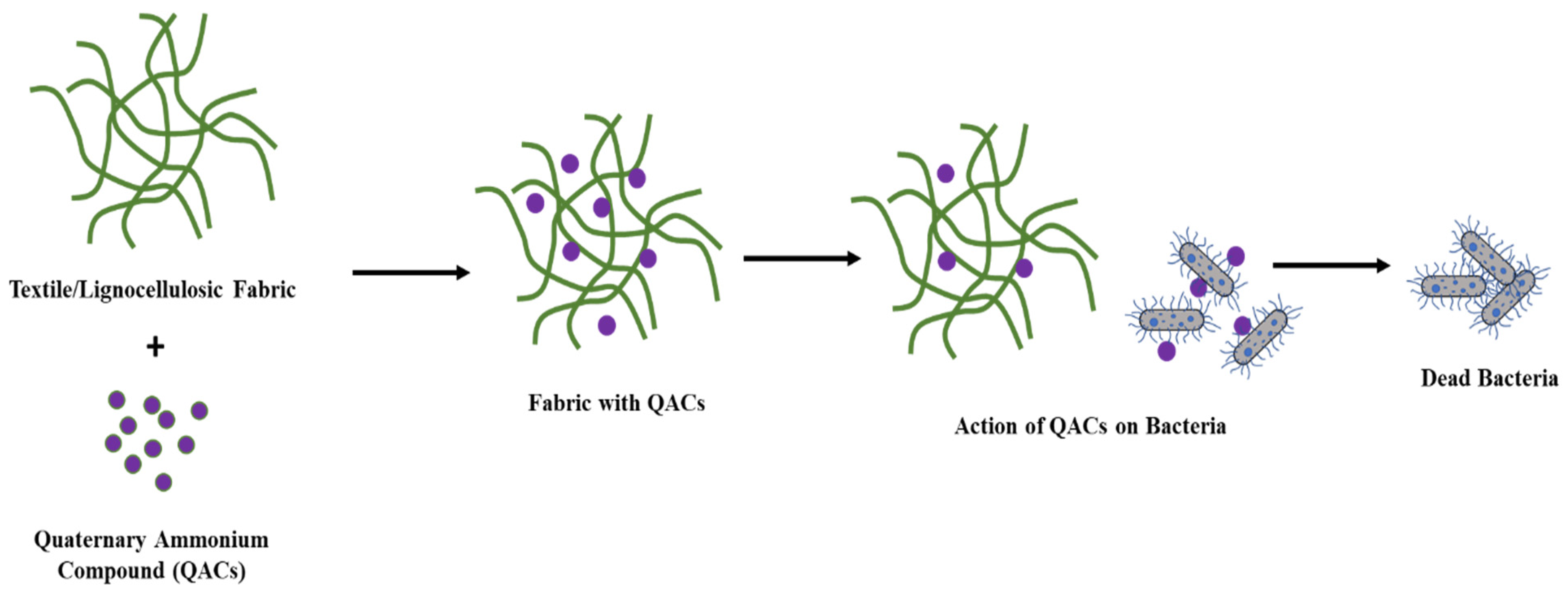
:1. Introduction
2. Experimental Section
2.1. Materials
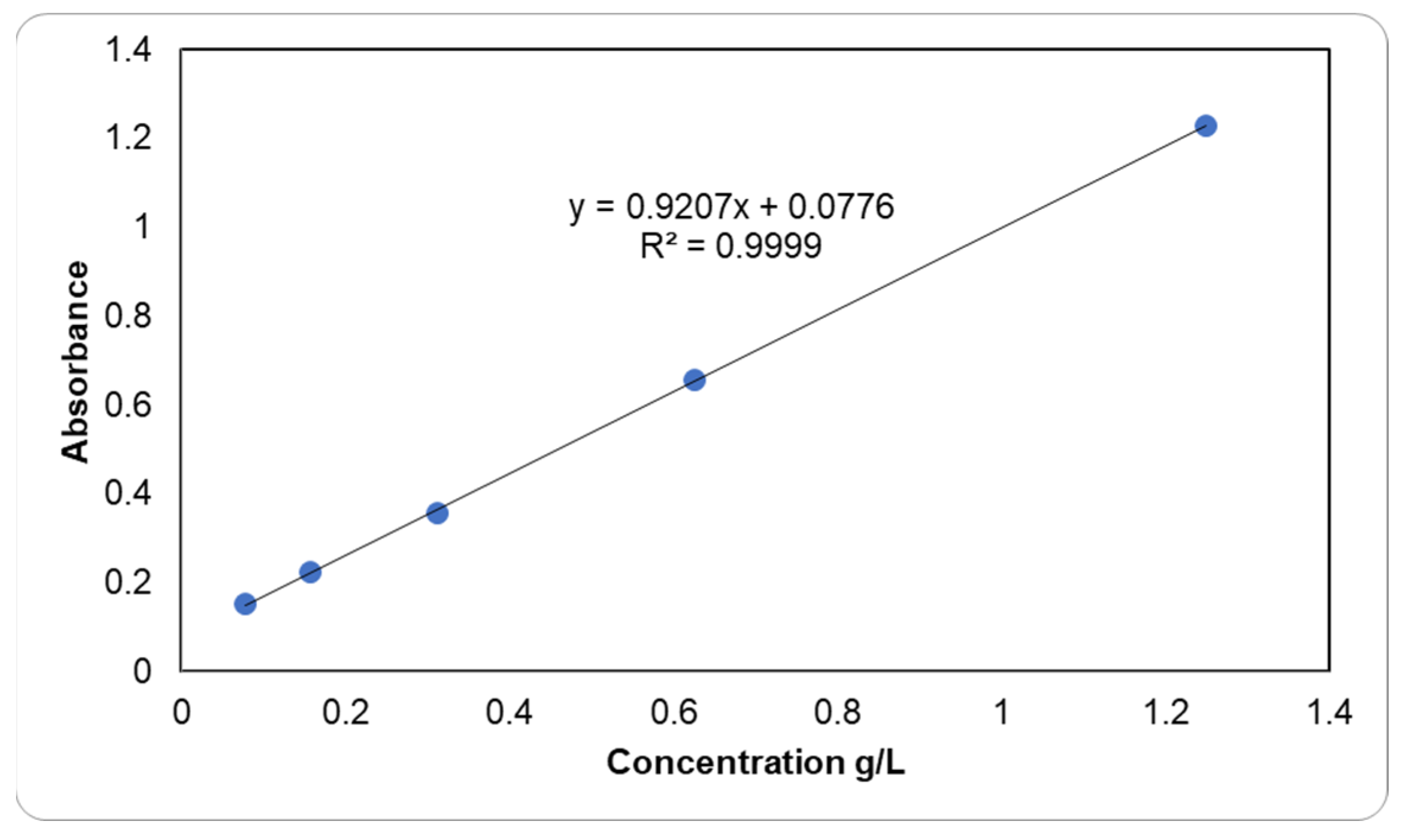
2.2. Methodology
- Characterization of Fibers: The fiber length (lw), fine contents, and other physical properties of the different wood and non-wood fibers were determined using a high-resolution fiber quality analyzer: HiRes FQA, OpTest Equipment Inc (Hawkesbury, ON, Canada). Before testing, the fiber quality analysis was calibrated and used according to the manufacturer’s specifications [25]. The textile fiber’s staple length was determined as per ASTS D5103 (performed manually), and its linear density was determined as per ASTM standard test method D1577 using a vibroscope.
- Characterization of Wipes: The basis weight of the wipes (in GSM) was calculated according to TAPPI standard T 410, and the thickness was measured using a caliper T 411. The absorbent capacity of the wipes was taken from the datasheet provided by the manufacturer (tested as per IEST-RP-CC004.3 sec.8.1).
2.3. Statistical Analysis
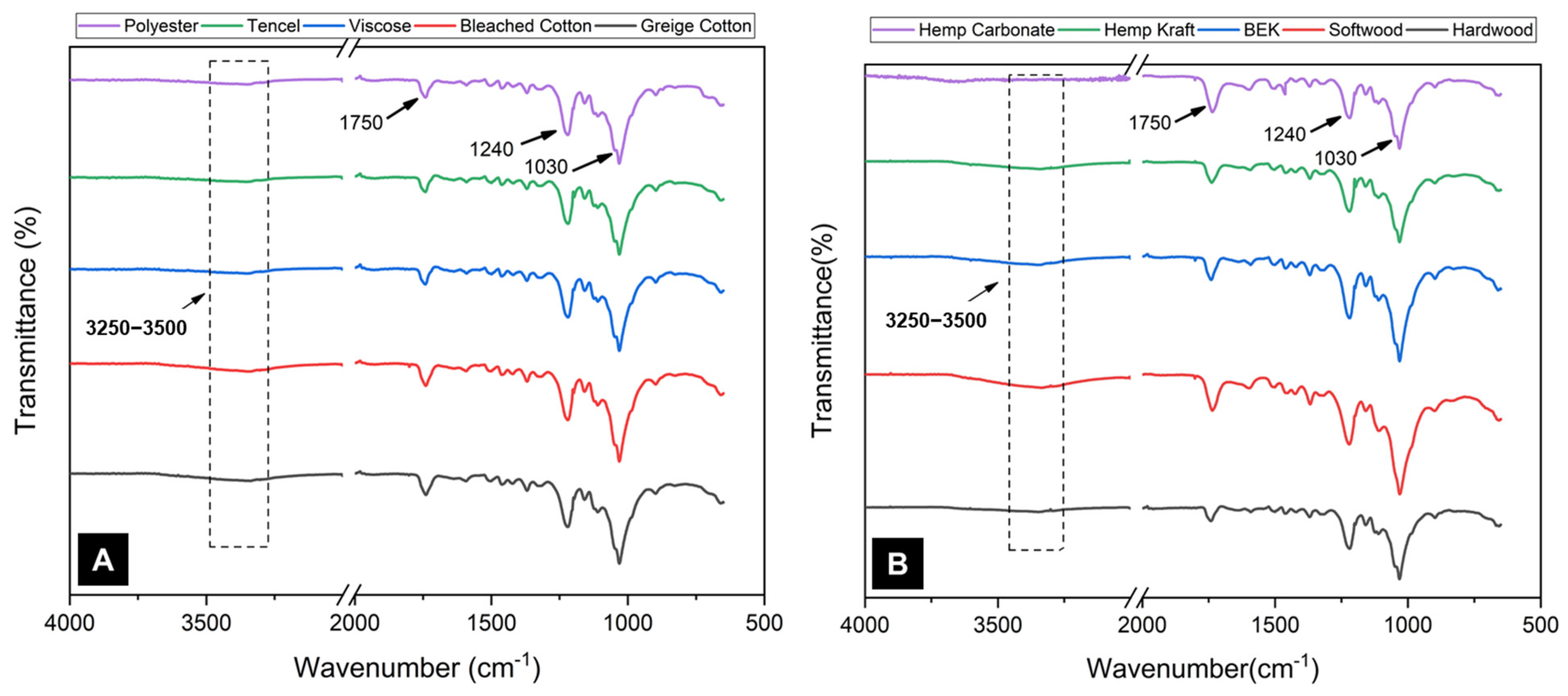
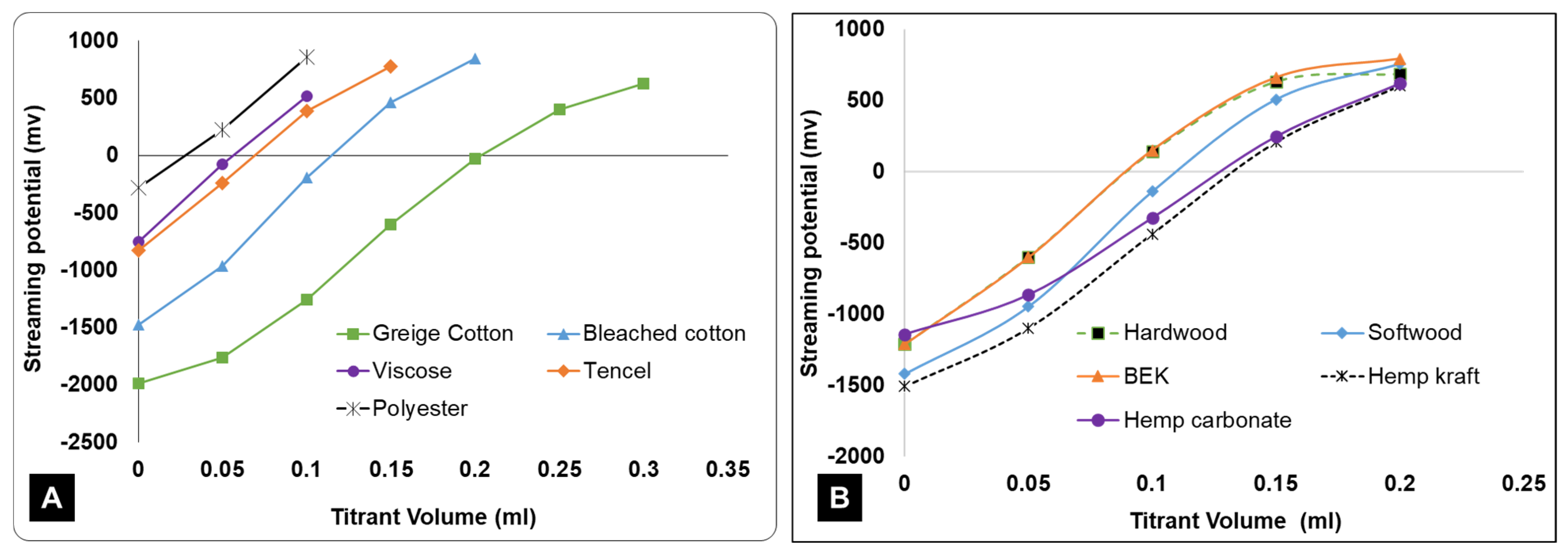
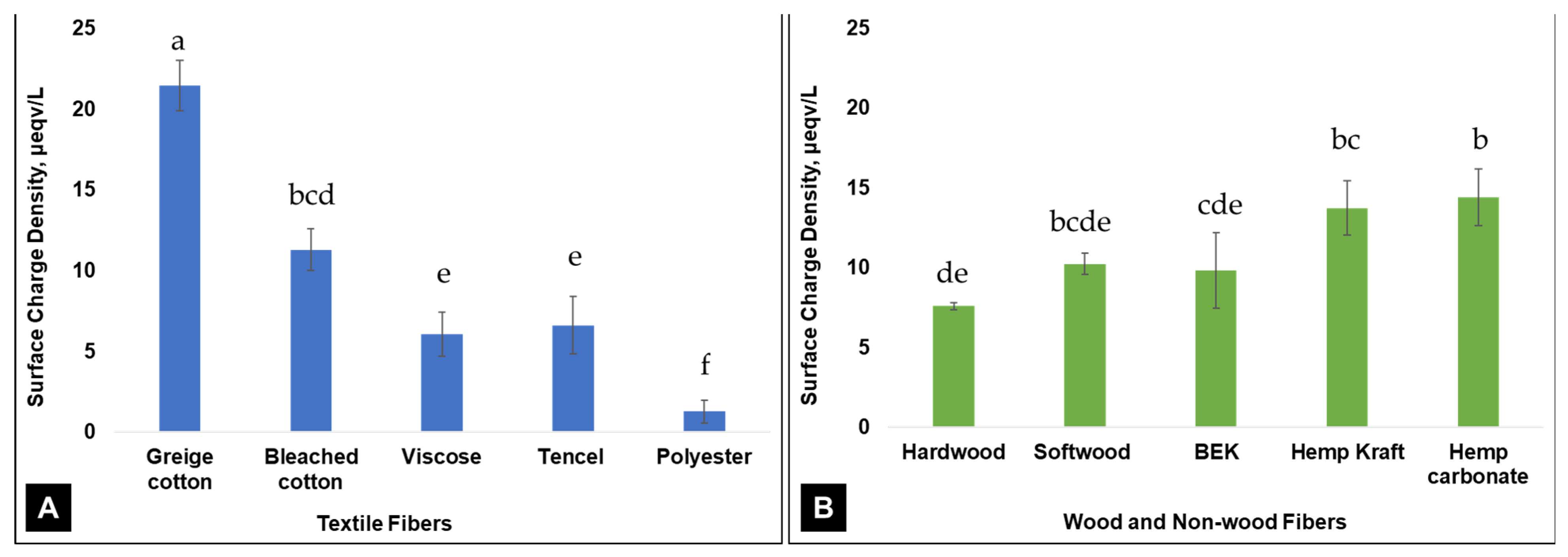
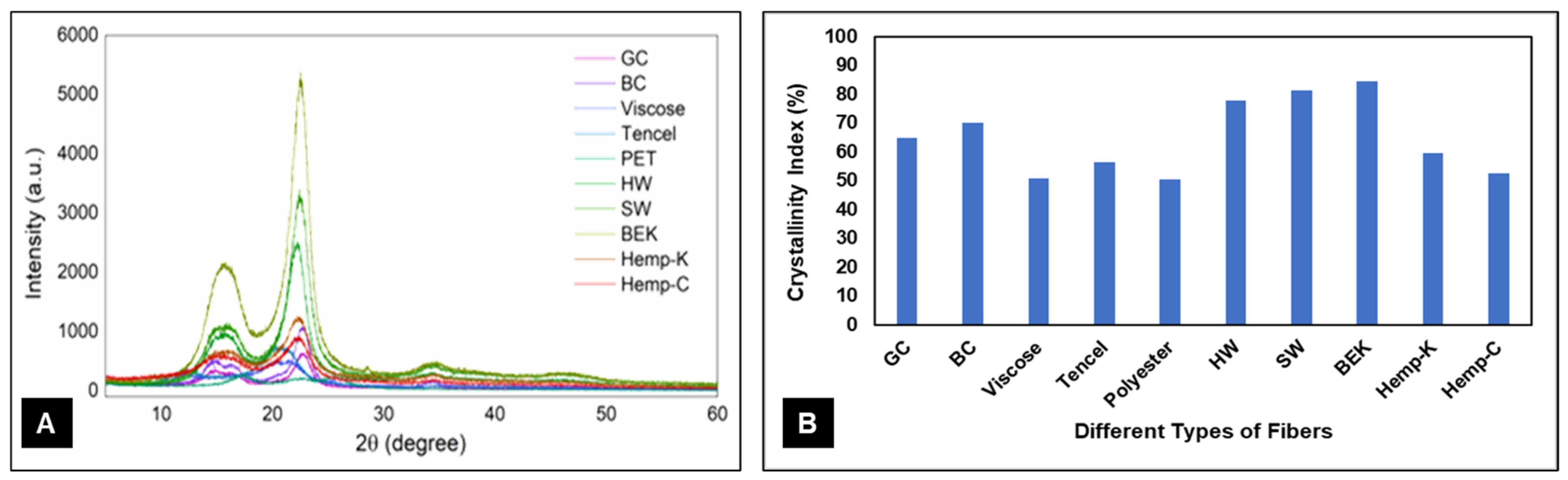
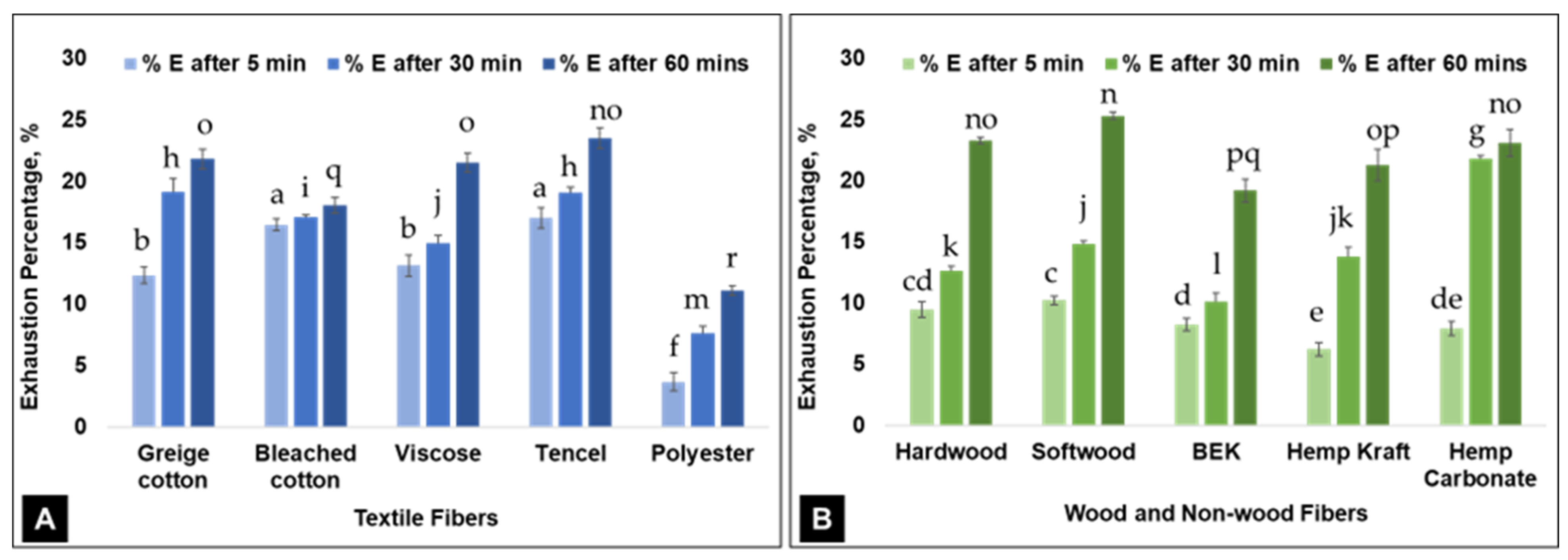
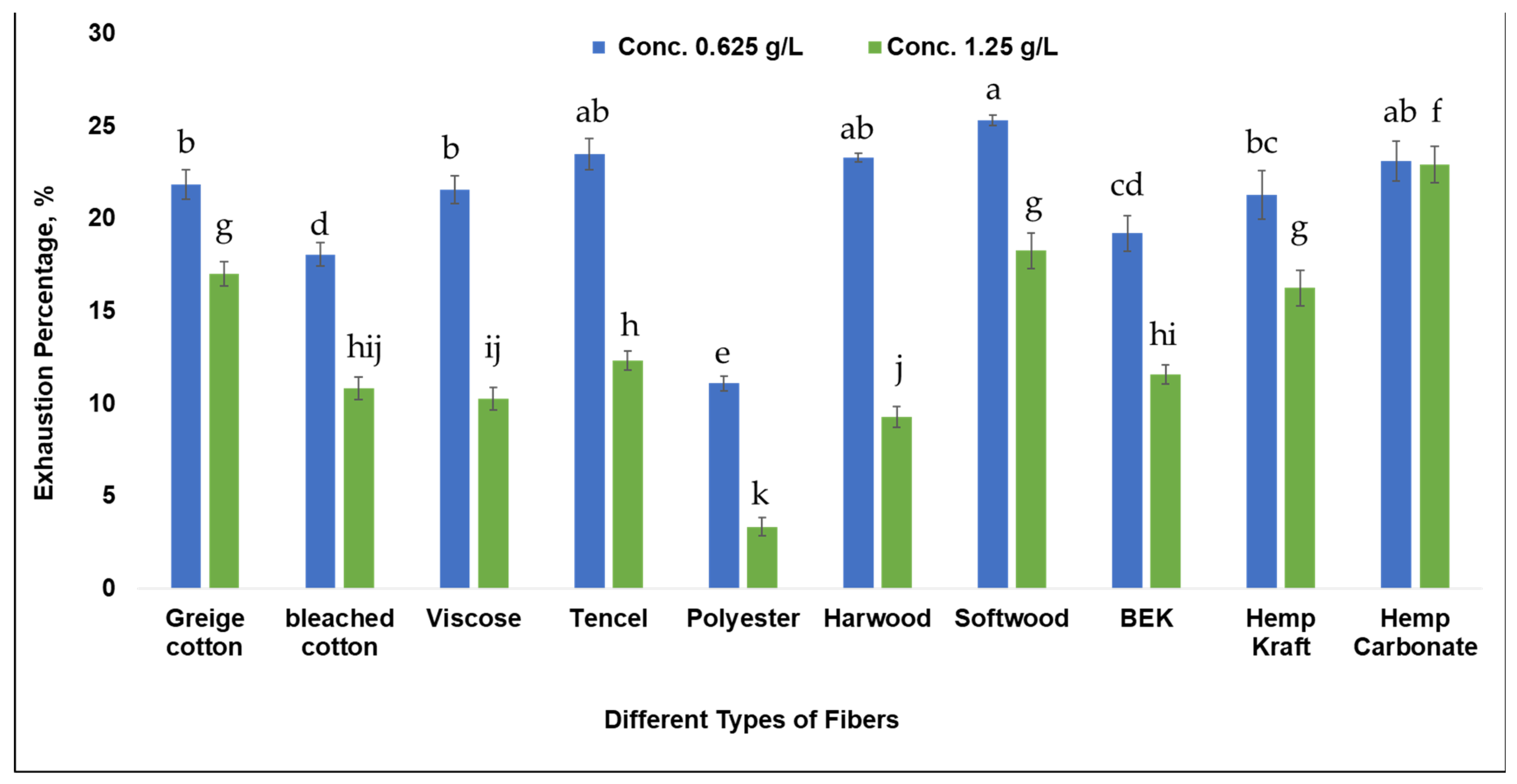
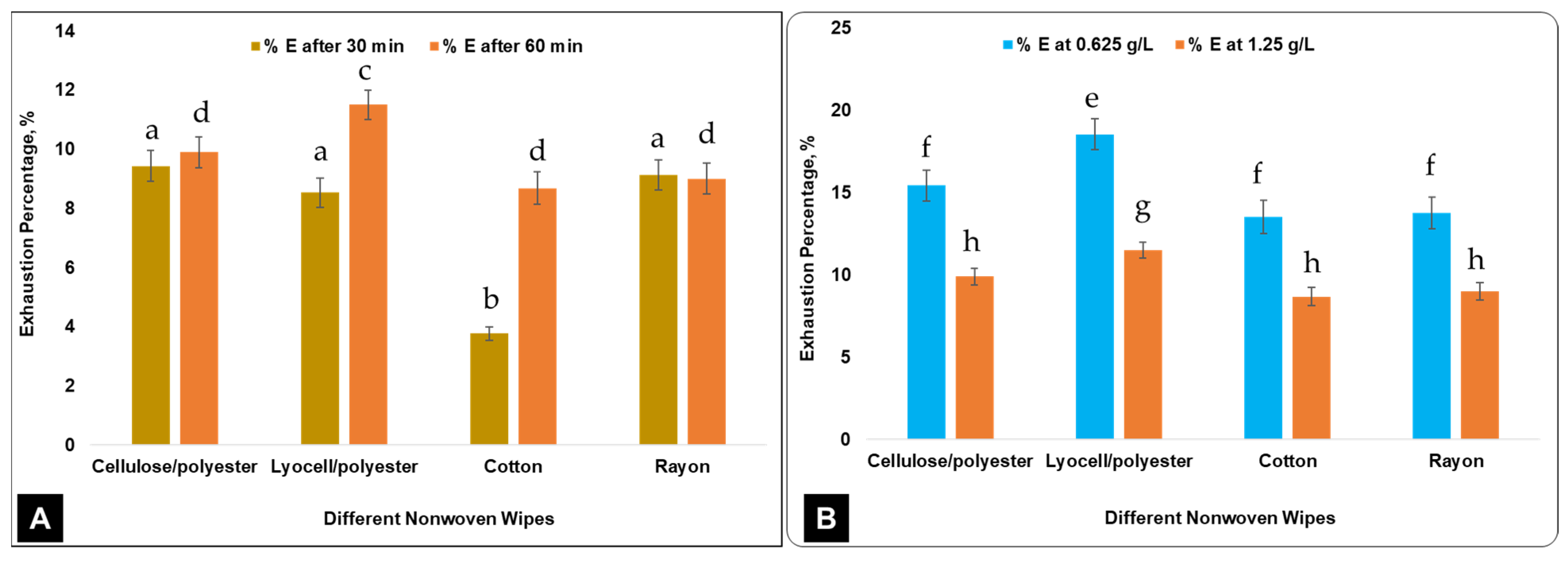
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D. 6—Nonwovens for Consumer and Industrial Wipes. In Applications of Nonwovens in Technical Textiles; Chapman, R.A., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2010; pp. 103–119. ISBN 978-1-84569-437-1. [Google Scholar]
- Baccouch, W.; Ghith, A.; Yalcin-Enis, I.; Sezgin, H.; Miled, W.; Legrand, X.; Faten, F. Investigation of the Mechanical, Thermal, and Acoustical Behaviors of Cotton, Polyester, and Cotton/Polyester Nonwoven Wastes Reinforced Epoxy Composites. J. Ind. Text. 2022, 51, 876–899. [Google Scholar] [CrossRef]
- Beesoon, S.; Behary, N.; Perwuelz, A. Universal Masking during COVID-19 Pandemic: Can Textile Engineering Help Public Health? Narrative Review of the Evidence. Prev. Med. 2020, 139, 106236. [Google Scholar] [CrossRef]
- Smithers Report Tracks Market Boom for Nonwoven Wipes. Available online: https://www.nonwovens-industry.com/contents/view_breaking-news/2020-12-01/smithers-report-tracks-market-boom-for-nonwoven-wipes (accessed on 1 September 2023).
- Nam, S.; Slopek, R.; Wolf, D.; Warnock, M.; Condon, B.D.; Sawhney, P.; Gbur, E.; Reynolds, M.; Allen, C. Comparison of Biodegradation of Low-Weight Hydroentangled Raw Cotton Nonwoven Fabric and That of Commonly Used Disposable Nonwoven Fabrics in Aerobic Captina Silt Loam Soil. Text. Res. J. 2016, 86, 155–166. [Google Scholar] [CrossRef]
- Goswami, P.; O’Haire, T. 3—Developments in the Use of Green (Biodegradable), Recycled and Biopolymer Materials in Technical Nonwovens. In Advances in Technical Nonwovens; Kellie, G., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2016; pp. 97–114. ISBN 978-0-08-100575-0. [Google Scholar]
- Salem, K.S.; Clayson, K.; Salas, M.; Haque, N.; Rao, R.; Agate, S.; Singh, A.; Levis, J.W.; Mittal, A.; Yarbrough, J.M.; et al. A Critical Review of Existing and Emerging Technologies and Systems to Optimize Solid Waste Management for Feedstocks and Energy Conversion. Matter 2023, 6, 3348–3377. [Google Scholar] [CrossRef]
- Ichim, M.; Filip, I.; Stelea, L.; Lisa, G.; Muresan, E.I. Recycling of Nonwoven Waste Resulting from the Manufacturing Process of Hemp Fiber-Reinforced Recycled Polypropylene Composites for Upholstered Furniture Products. Sustainability 2023, 15, 3635. [Google Scholar] [CrossRef]
- Cao, H.; Cobb, K.; Yatvitskiy, M.; Wolfe, M.; Shen, H. Textile and Product Development from End-of-Use Cotton Apparel: A Study to Reclaim Value from Waste. Sustainability 2022, 14, 8553. [Google Scholar] [CrossRef]
- Santos, A.S.; Ferreira, P.J.T.; Maloney, T. Bio-Based Materials for Nonwovens. Cellulose 2021, 28, 8939–8969. [Google Scholar] [CrossRef]
- Liu, M.; Ma, C.; Zhou, D.; Chen, S.; Zou, L.; Wang, H.; Wu, J. Hydrophobic, Breathable Cellulose Nonwoven Fabrics for Disposable Hygiene Applications. Carbohydr. Polym. 2022, 288, 119367. [Google Scholar] [CrossRef]
- Salem, K.S.; Jameel, H.; Lucia, L.; Pal, L. Sustainable High-Yield Lignocellulosic Fibers and Modification Technologies Educing Softness and Strength for Tissues and Hygiene Products for Global Health. Mater. Today Sustain. 2023, 22, 100342. [Google Scholar] [CrossRef]
- Divya, R.; Indumathi, T.R.; Venugopal, T.; Prakash, C. Investigation on Potential Application of Non-Wood Pulp for Hygiene Products as Absorbent Core Substantial. Biomass Conv. Bioref. 2023, 1–6. [Google Scholar] [CrossRef]
- Hamouda, T.; Ibrahim, H.M.; Kafafy, H.H.; Mashaly, H.M.; Mohamed, N.H.; Aly, N.M. Preparation of Cellulose-Based Wipes Treated with Antimicrobial and Antiviral Silver Nanoparticles as Novel Effective High-Performance Coronavirus Fighter. Int. J. Biol. Macromol. 2021, 181, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Slopek, R.; Condon, B.; Sawhney, P.; Reynolds, M.; Allen, C. Effect of Cotton Pectin Content and Bioscouring on Alkyl-Dimethyl-Benzyl-Ammonium Chloride Adsorption. Text. Res. J. 2012, 82, 1743–1750. [Google Scholar] [CrossRef]
- Song, X.; Vossebein, L.; Zille, A. Efficacy of Disinfectant-Impregnated Wipes Used for Surface Disinfection in Hospitals: A Review. Antimicrob. Resist. Infect. Control 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Cagide, F.; Fernandes, C.; Borges, A.; Borges, F.; Simões, M. Efficacy of Novel Quaternary Ammonium and Phosphonium Salts Differing in Cation Type and Alkyl Chain Length against Antibiotic-Resistant Staphylococcus Aureus. Int. J. Mol. Sci. 2024, 25, 504. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.A.; Jamil, S.N.A.M. Application of Quaternary Ammonium Compounds as Compatibilizers for Polymer Blends and Polymer Composites—A Concise Review. Appl. Sci. 2021, 11, 3167. [Google Scholar] [CrossRef]
- Huang, K.; Fan, X.; Ashby, R.; Ngo, H. Structure-Activity Relationship of Antibacterial Bio-Based Epoxy Polymers Made from Phenolic Branched Fatty Acids. Prog. Org. Coat. 2021, 155, 106228. [Google Scholar] [CrossRef]
- Wu, X.; Viner-Mozzini, Y.; Jia, Y.; Song, L.; Sukenik, A. Alkyltrimethylammonium (ATMA) Surfactants as Cyanocides—Effects on Photosynthesis and Growth of Cyanobacteria. Chemosphere 2021, 274, 129778. [Google Scholar] [CrossRef]
- Frolov, N.A.; Fedoseeva, K.A.; Hansford, K.A.; Vereshchagin, A.N. Novel Phenyl-Based Bis-Quaternary Ammonium Compounds as Broad-Spectrum Biocides. ChemMedChem 2021, 16, 2954–2959. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Salem, K.S.; Naithani, V.; Jameel, H.; Lucia, L.A.; Pal, L. Lignocellulosic Fibers from Renewable Resources Using Green Chemistry for a Circular Economy. Glob. Chall. 2020, 5, 2000065. [Google Scholar] [CrossRef]
- Pascoe, M.J.; Mandal, S.; Williams, O.A.; Maillard, J.-Y. Impact of Material Properties in Determining Quaternary Ammonium Compound Adsorption and Wipe Product Efficacy against Biofilms. J. Hosp. Infect. 2022, 126, 37–43. [Google Scholar] [CrossRef]
- Bloß, R.; Meyer, S.; Kampf, G. Adsorption of Active Ingredients of Surface Disinfectants Depends on the Type of Fabric Used for Surface Treatment. J. Hosp. Infect. 2010, 75, 56–61. [Google Scholar] [CrossRef]
- Slopek, R.; Condon, B.; Sawhney, P.; Reynolds, M.; Allen, C. Adsorption of Alkyl-Dimethyl-Benzyl-Ammonium Chloride on Differently Pretreated Non-Woven Cotton Substrates. Text. Res. J. 2011, 81, 1617–1624. [Google Scholar] [CrossRef]
- Engelbrecht, K.; Ambrose, D.; Sifuentes, L.; Gerba, C.; Weart, I.; Koenig, D. Decreased Activity of Commercially Available Disinfectants Containing Quaternary Ammonium Compounds When Exposed to Cotton Towels. Am. J. Infect. Control 2013, 41, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.S.; Dhamarikar, M.; Dharkar, A.; Chaturvedi, S.; Tiwari, S.; Namdeo, R. Surface Modification of Banana Fiber: A Review. Mater. Today Proc. 2021, 43, 904–915. [Google Scholar] [CrossRef]
- Yokota, S.; Tagawa, S.; Kondo, T. Facile Surface Modification of Amphiphilic Cellulose Nanofibrils Prepared by Aqueous Counter Collision. Carbohydr. Polym. 2021, 255, 117342. [Google Scholar] [CrossRef] [PubMed]
- Samaher Salem, K.; Kumar Kasera, N.; Ashiqur Rahman, M.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, D.; Condon, B.; Madison, C.; Reynolds, M.; Hron, R. An Optimized Co-Formulation Minimized Quaternary Ammonium Compounds Adsorption onto Raw Cotton Disposable Disinfecting Wipes and Maintained Efficacy against Methicillin-Resistant Staphylococcus Aureus, Vancomycin-Resistant Enterococcus Faecalis, and Pseudomonas Aeruginosa. Text. Res. J. 2018, 88, 2329–2338. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Goller, C.C.; Venditti, R.A. Aerobic Biodegradation in Freshwater and Marine Environments of Textile Microfibers Generated in Clothes Laundering: Effects of Cellulose and Polyester-Based Microfibers on the Microbiome. Mar. Pollut. Bull. 2020, 151, 110826. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Ansell, M.P.; Mwaikambo, L.Y. 2—The Structure of Cotton and Other Plant Fibres. In Handbook of Textile Fibre Structure; Eichhorn, S.J., Hearle, J.W.S., Jaffe, M., Kikutani, T., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2009; Volume 2, pp. 62–94. ISBN 978-1-84569-730-3. [Google Scholar]


| Wood and Non-Wood Fibers | Textile Fibers | |||||
|---|---|---|---|---|---|---|
| Fiber Type | Fiber Length, lw (mm) | Coarseness (mg.m−1) | Fines (%) | Fiber Type | Staple Length (mm) | Linear Density (Denier) |
| Hardwood | 1.15 ± 0.081 | 0.10 ± 0.009 | 20.58 ± 1.85 | Greige Cotton | 30.25 ± 2.72 | 1.75 ± 0.16 |
| Softwood | 2.24 ± 0.180 | 0.17 ± 0.015 | 21.97 ± 1.76 | Bleached Cotton | 23.21 ± 1.86 | 1.08 ± 0.68 |
| BEK | 0.79 ± 0.047 | 0.08 ± 0.005 | 9.50 ± 0.57 | Viscose | 26.45 ± 1.59 | 2.21 ± 0.14 |
| Hemp Kraft | 0.74 ± 0.052 | 0.15 ± 0.011 | 17.20 ± 1.20 | Tencel | 42.54 ± 2.97 | 1.42 ± 0.10 |
| Hemp Carbonate | 0.67 ± 0.054 | 0.22 ± 0.018 | 11.20 ± 0.89 | Polyester | 51.00 ± 4.08 | 0.95 ± 0.67 |
| Material | Construction | Thickness (μm) | GSM (g/m2) | Absorbent Capacity (mL/m2) |
|---|---|---|---|---|
| 50% Cellulose/50% Polyester blend | Hydroentangled, nonwoven | 575 ± 23.81 | 56.9 ± 2.5 | 344 ± 8.26 |
| 58% Polyester/42% Lyocell | Hydroentangled, apertured | 769.5 ± 38.47 | 74.4 ± 4.83 | 395 ± 17.77 |
| 100% Cotton | 2 × 1 twill woven | 435.25 ± 26.11 | 183.1 ± 5.29 | 300 ± 10.8 |
| 100% Rayon | Spunbond, nonwoven | 748.75 ± 26.21 | 71.99 ± 3.59 | 328 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mali, M.; Salem, K.S.; Sarder, R.; Agate, S.; Mathur, K.; Pal, L. Understanding Binding of Quaternary Ammonium Compounds with Cellulose-Based Fibers and Wipes for Renewable and Sustainable Hygiene Options. Sustainability 2024, 16, 1586. https://doi.org/10.3390/su16041586
Mali M, Salem KS, Sarder R, Agate S, Mathur K, Pal L. Understanding Binding of Quaternary Ammonium Compounds with Cellulose-Based Fibers and Wipes for Renewable and Sustainable Hygiene Options. Sustainability. 2024; 16(4):1586. https://doi.org/10.3390/su16041586
Chicago/Turabian StyleMali, Monika, Khandoker Samaher Salem, Roman Sarder, Sachin Agate, Kavita Mathur, and Lokendra Pal. 2024. "Understanding Binding of Quaternary Ammonium Compounds with Cellulose-Based Fibers and Wipes for Renewable and Sustainable Hygiene Options" Sustainability 16, no. 4: 1586. https://doi.org/10.3390/su16041586





