The Thermophysical Aspects of the Transformation of Porous Structures in Versatile Nanostructured Materials
Abstract
:1. Introduction
2. Research Materials and Methods
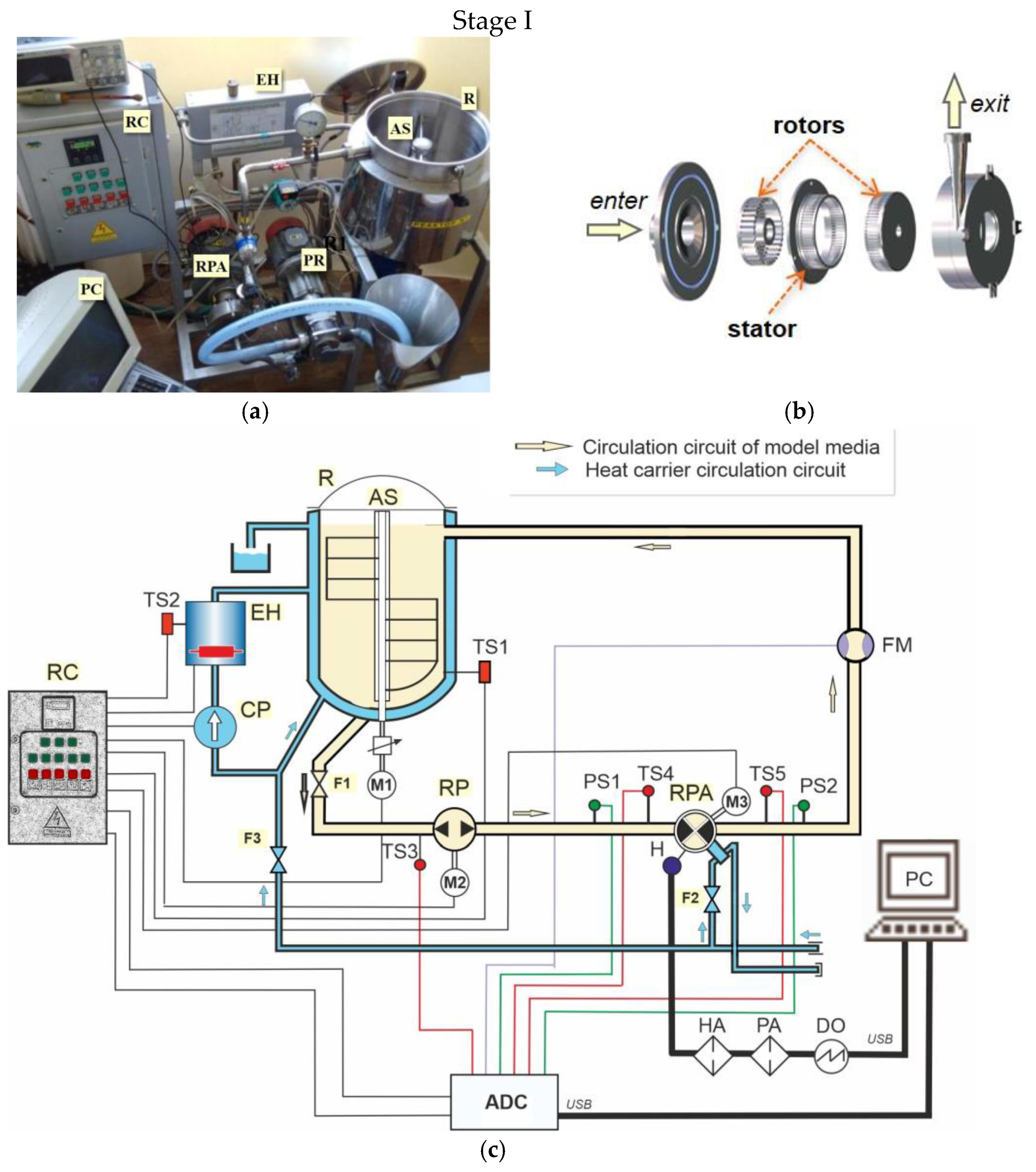
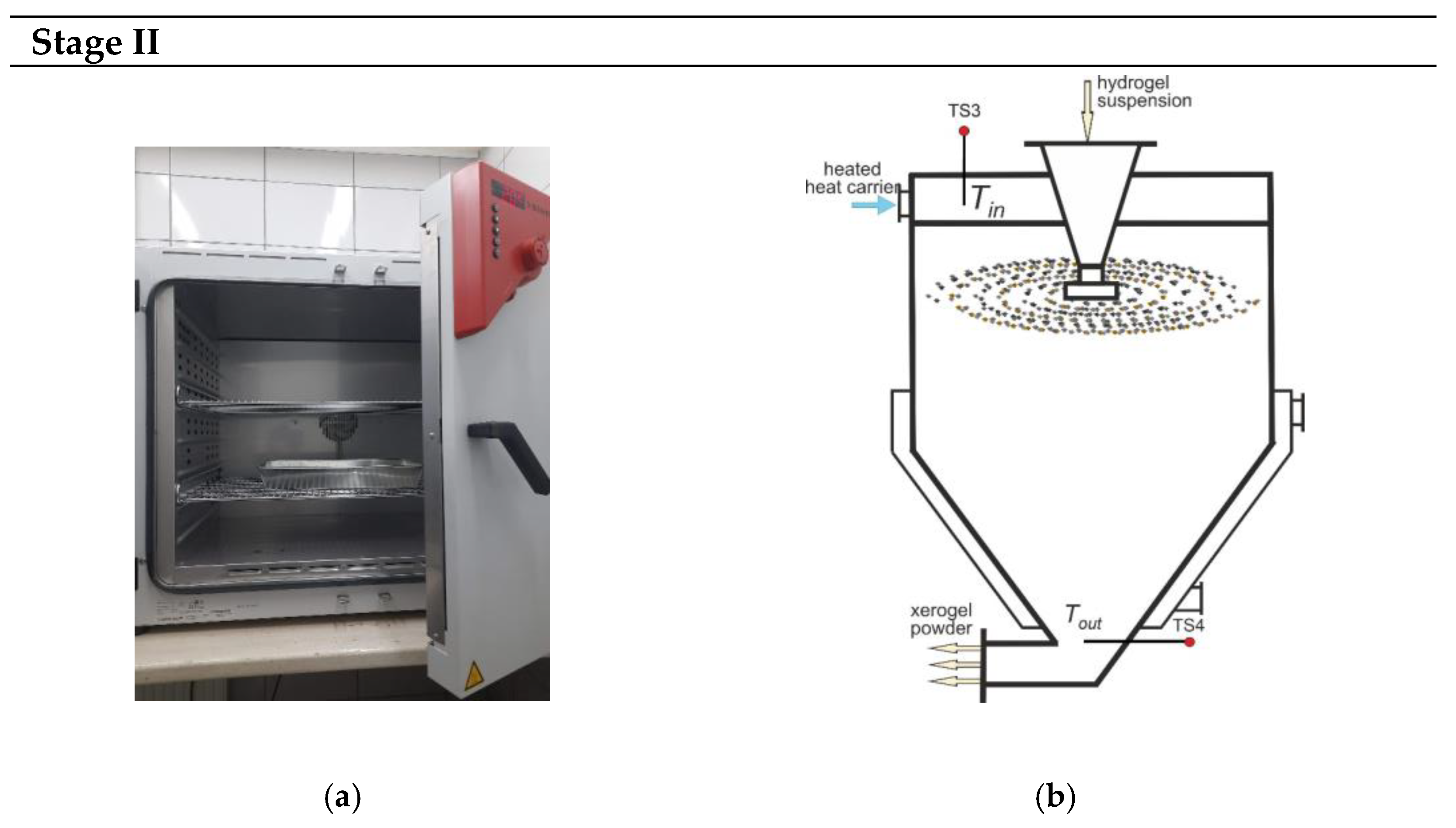
2.1. Methods and Equipment
2.2. Research Methods
3. Results and Discussion
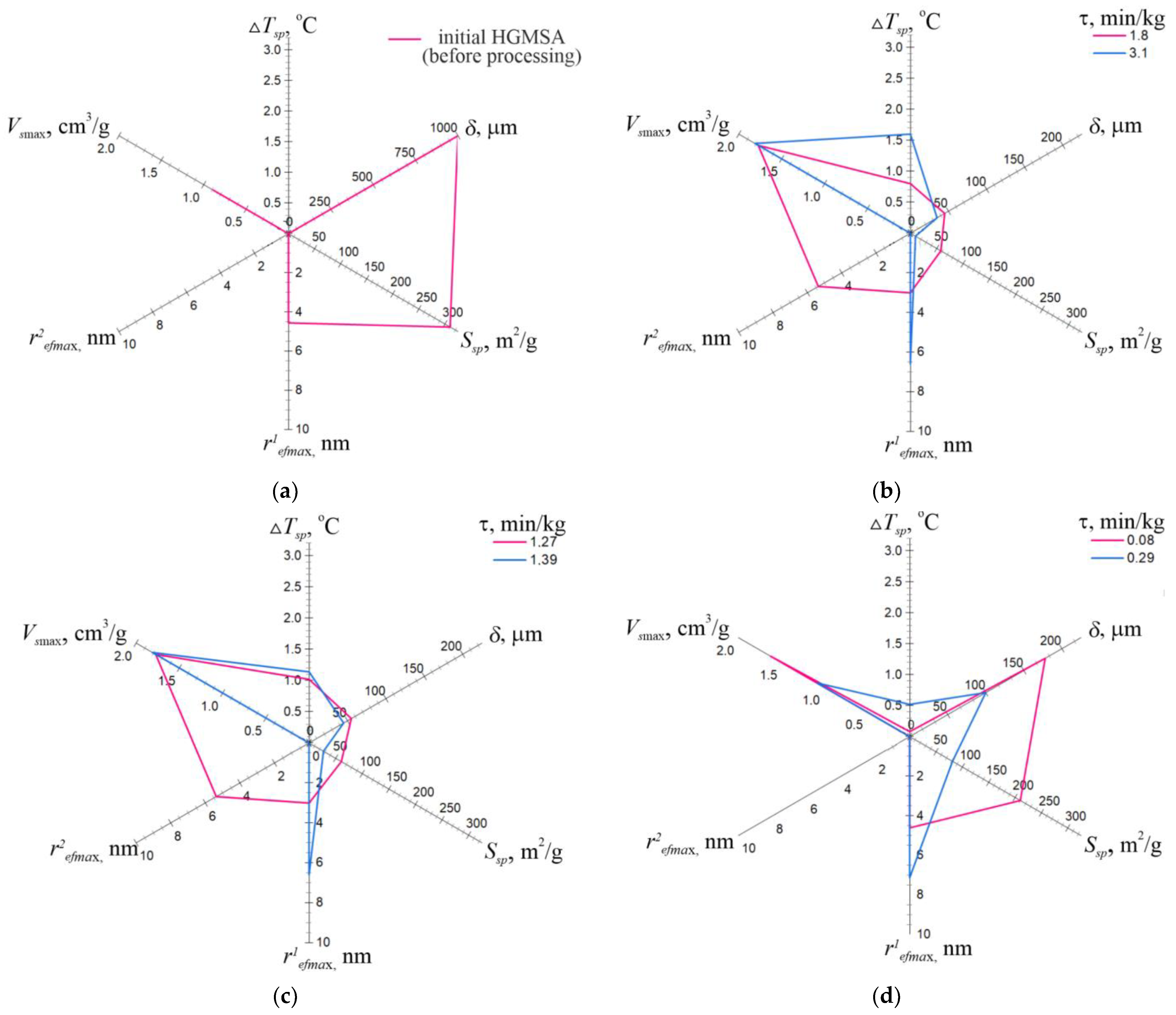
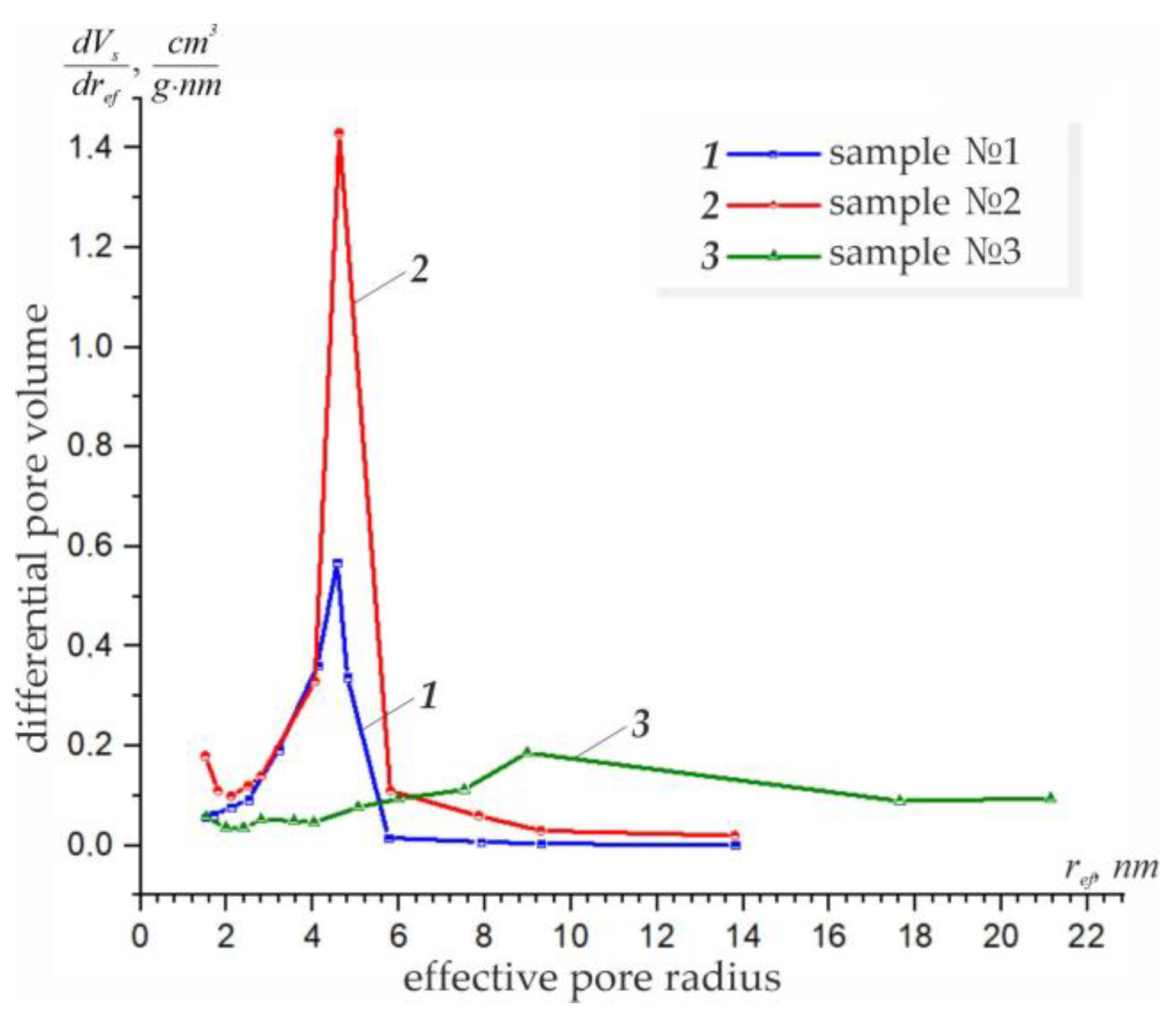
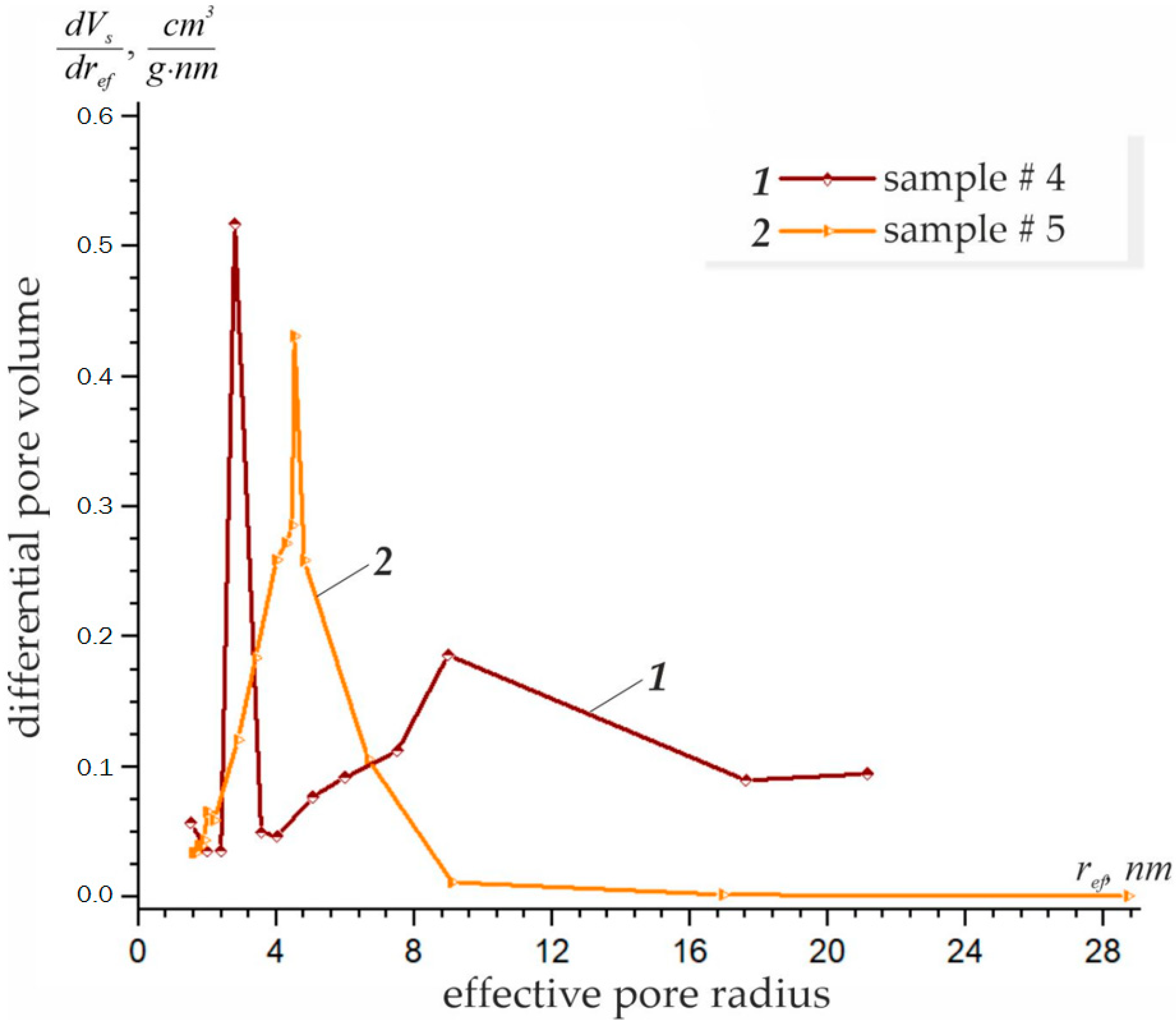
3.1. Effect of Concentration on the Structural–Adsorption Characteristics of Nanostructures
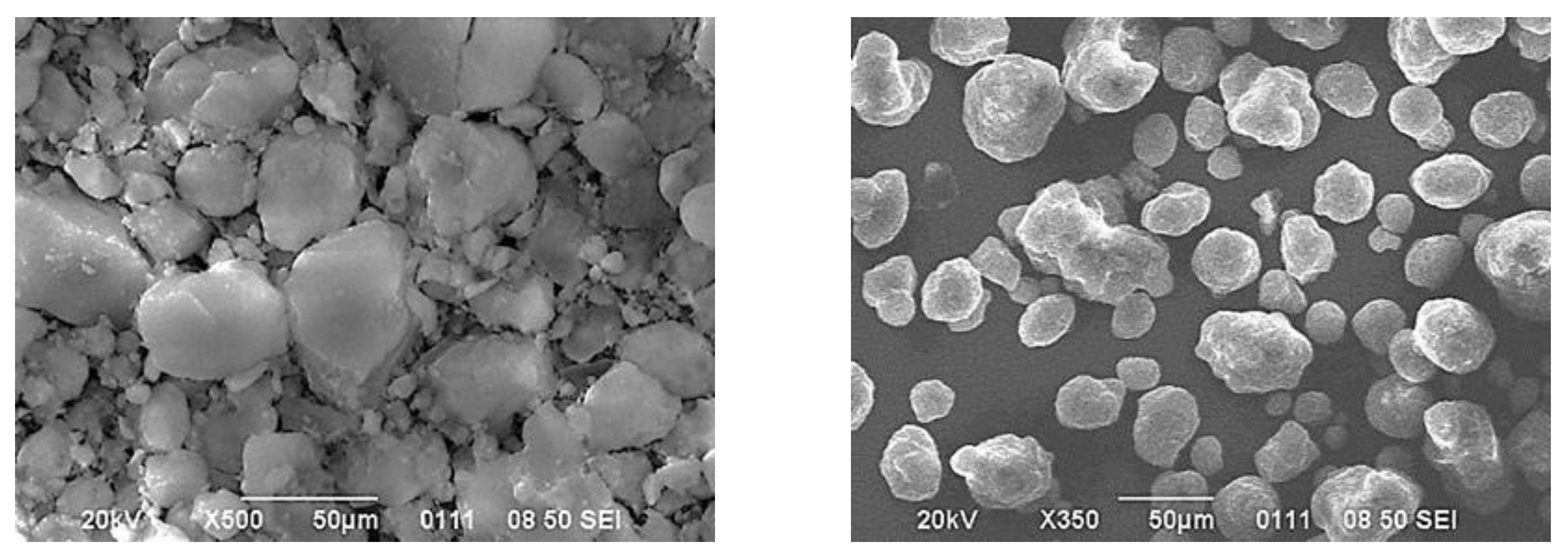
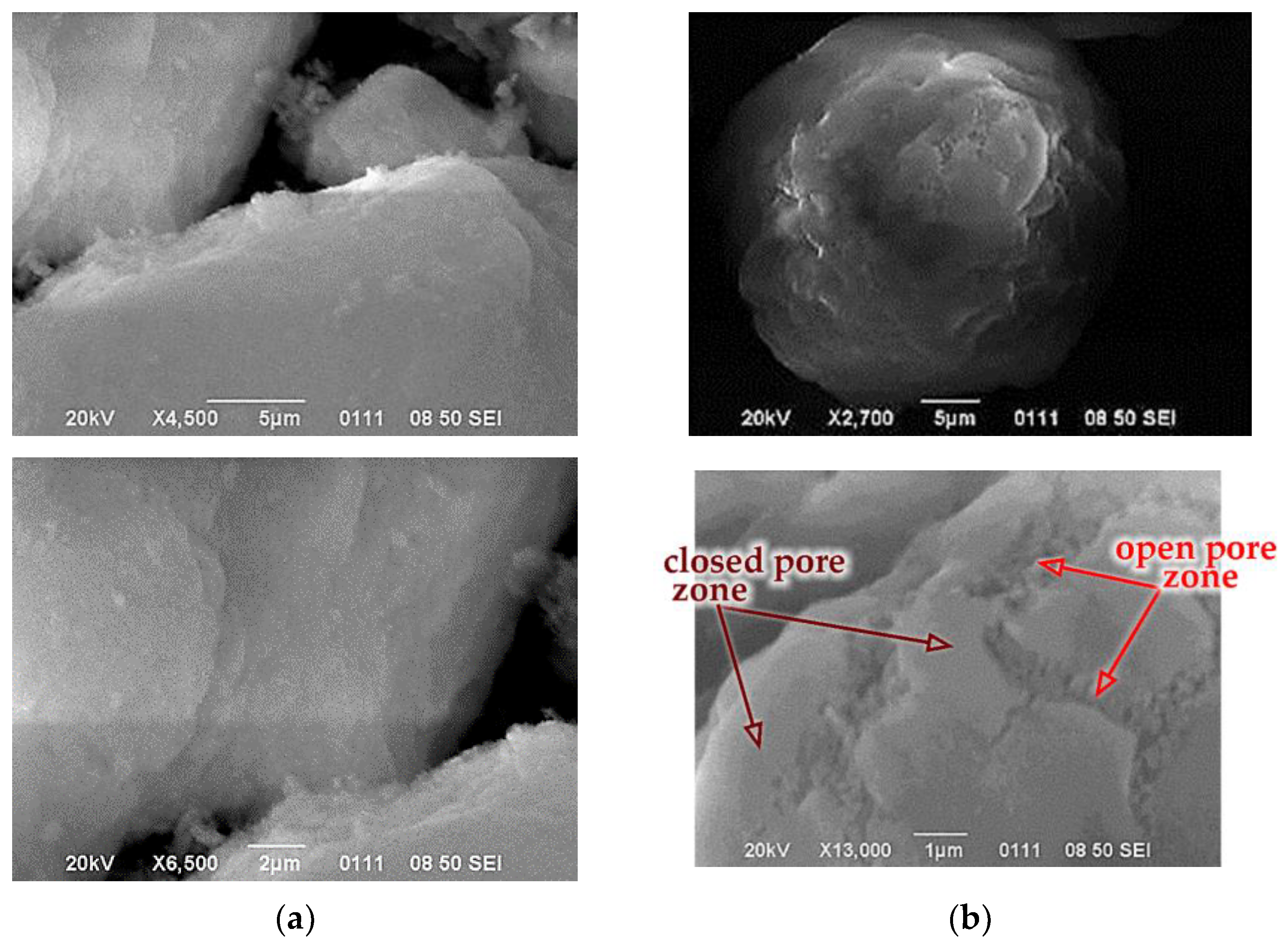
3.2. Peculiarities of Xerogel Formation by Convective Drying Methods
3.3. Rheological Behaviour of Hydrogel Suspensions
4. Conclusions
4.1. General Scientific Conclusions
4.2. Directions of Further Research and Their Preliminary Results
4.3. Financial Support
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Basok, B.; Davydenko, B.; Koshlak, H.; Novikov, V. Free Convection and Heat Transfer in Porous Ground Massif during Ground Heat Exchanger Operation. Materials 2022, 15, 4843. [Google Scholar] [CrossRef]
- Lobanov, L.M.; Chalaev, D.M.; Goncharov, P.V.; Grabova, T.L.; Pashchin, M.O.; Goncharova, O.M.; Sydorenko, V.V. Development of Equipment for Air Decontamination in the Ventilation and Air Conditioning Systems of Public Buildings with the Use of the Photocatalysis and Plasmochemistry Methods. Sci. Innov. 2023, 19, 57–71. [Google Scholar] [CrossRef]
- Mazzotta, E.; De Santo, M.; Lombardo, D.; Leggio, A.; Pasqua, L. Mesoporous silicas in materials engineering: Nanodevices for bionanotechnologies. Mater. Today Bio 2022, 17, 100472. [Google Scholar] [CrossRef]
- Gomez, G.E.; Hamer, M.; Regiart, M.D.; Tortella, G.R.; Seabra, A.B.; Soler Illia, G.J.A.A.; Fernández-Baldo, M.A. Advances in Nanomaterials and Composites Based on Mesoporous Materials as Antimicrobial Agents: Relevant Applications in Human Health. Antibiotics 2024, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, A.; Dell’Olio, G.; Spadafora, A.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. Hybrid Polymer-Silica Nanostructured Materials for Environmental Remediation. Molecules 2023, 28, 5105. [Google Scholar] [CrossRef] [PubMed]
- Jambhrunkar, M.; Maghrebi, S.; Doddakyathanahalli, D.; Wignall, A.; Prestidge, C.A.; Bremmell, K.E. Mesoporous Organosilica Nanoparticles to Fight Intracellular Staphylococcal Aureus Infections in Macrophages. Pharmaceutics 2023, 15, 1037. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Yu, A.; Dai, X.; Wang, Z.; Chen, H.; Guo, B.; Huang, L. Recent Advances of Mesoporous Silica as a Platform for Cancer Immunotherapy. Biosensors 2022, 12, 109. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, W.; Fang, J.; Yin, J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int. Mater. Rev. 2021, 66, 77–113. [Google Scholar] [CrossRef]
- El-Samak, A.A.; Ponnamma, D.; Hassan, M.K.; Ammar, A.; Adham, S.; Al-Maadeed, M.A.A.; Karim, A. Designing flexible and porous fibrous membranes for oil water separation—A review of recent developments. Polym. Rev. 2020, 60, 671–716. [Google Scholar] [CrossRef]
- Ye, S.; Wang, B.; Shi, Y.; Wang, B.; Zhang, Y.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Superhydrophobic and superelastic thermoplastic polyurethane/multiwalled carbon nanotubes porous monolith for durable oil/water separation. Compos. Commun. 2020, 21, 100378. [Google Scholar] [CrossRef]
- Kovylin, R.S.; Yudin, V.V.; Shurygina, M.P.; Fedoseev, V.B.; Chesnokov, S.A.; Fedushkin, I.L.; Piskunov, A.V. Porogen Concentration Effect on the Pore Structure and Properties Evolution of Polymer Monolith Based on Oligocarbonate Dimethacrylate OCM-2. Materials 2023, 16, 3177. [Google Scholar] [CrossRef]
- Haider, Z.; Haleem, A.; Farooq, U.; Shi, L.; Claver, U.P.; Memon, K.; Memon, K.; Fareed, A.; Khan, I.; Mbogba, M.K.; et al. Highly porous polymer cryogel based tribopositive material for high performance triboelectric nanogenerators. Nano Energy 2020, 68, 104294. [Google Scholar] [CrossRef]
- Irzhak, V.I. Percolation Threshold in Polymer Nanocomposites. Colloid J. 2021, 83, 64–69. [Google Scholar] [CrossRef]
- Ni, X.; Hui, C.; Su, N.; Cutler, R.; Liu, F. A 3D percolation model for multicomponent nanocarbon composites: The critical role of nematic transition. Nanotechnology 2019, 30, 185302. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; ISBN 0-12-300956-1. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Yang, S.B.; Karim, M.R.; Alnaser, I.A.; Alahmari, A.D.; Almubaddel, F.S.; Assaifan, A.K. Preparation and Characterization of Electrospun Poly(lactic acid)/Poly(ethylene glycol)–b–poly(propylene glycol)–b–poly(ethylene glycol)/Silicon Dioxide Nanofibrous Adsorbents for Selective Copper (II) Ions Removal from Wastewater. Membranes 2023, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Koshlak, H. Synthesis of zeolites from coal fly ash using alkaline fusion and its applications in removing heavy metals. Materials 2023, 16, 4837. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Homburg, S.V.; Patel, A.V. Silica Hydrogels as Entrapment Material for Microalgae. Polymers 2022, 14, 1391. [Google Scholar] [CrossRef]
- Al Soubaihi, R.M.; Saoud, K.M.; Ye, F.; Myint MT, Z.; Saeed, S.; Dutta, J. Synthesis of hierarchically porous silica aerogel supported Palladium catalyst for low-temperature CO oxidation under ignition/extinction conditions. Microporous Mesoporous Mater. 2020, 292, 109758. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. (Eds.) IUPAC Compendium of Chemical Terminology; Gold Book, Version 2.3.3; International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2014. [Google Scholar] [CrossRef]
- Guizard, C.G.; Julbe, A.; Ayral, A. Design of nanosized structures in sol-gel derived porous solids. Applications in catalyst and inorganic membrane preparation. J. Mater. Chem. 1999, 9, 55–65. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Yanovska, E.S.; Kołodyn’ska, D.; Sternik, D.; Pylypchuk, I.V.; Ischenko, M.V.; Tertyk, A.V. Preparation and properties of organomineral adsorbent obtained by sol–gel technology. J. Therm. Anal. Calorim. 2016, 125. [Google Scholar] [CrossRef]
- Turchina, T.; Zhukotsky, E.; Dekusha, G.; Makarenko, A. Influence of structuring additive on output mushrooms powder when drying by spraying method. Sci. Work. 2020, 84, 67–75. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ogi, T.; Wang, W.-N.; Gradon, L.; Okuyama, K. Template-assisted spray-drying method for the fabrication of porous particles with tunable structures. Adv. Powder Technol. 2019, 30, 2908–2924. [Google Scholar] [CrossRef]
- Pavlenko, A.M.; Basok, B.I. Kinetics of water evaporation from emulsions. Heat Transf. Res. 2005, 36, 425–430. [Google Scholar] [CrossRef]
- Basok, B.I.; Davydenko, B.V.; Avramenko, A.O.; Pirozhenko, I.O. Hydrodynamics, Heat Transfer and Crushing Effects in Rotationally Pulsating Flows; Monograph; Express: Kyiv, Ukraine, 2012; 298p, ISBN 978-966-02-6716-9. Available online: https://www.studmed.ru/basok-b-i-davydenko-b-v-avramenko-a-a-pirozhenko-i-a-gidrodinamika-teploobmen-i-effekty-drobleniya-vo-vraschatelno-pulsiruyuschih-potokah-rotorno-pulsacionnyy-apparat-rpa-_0ce44dd1f0f.html (accessed on 18 January 2024).
- Uriev, N.B. Technology of Dispersed Systems and Materials: Physicochemical Dynamics of Structure Formation and Rheology; Monograph; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; ISBN 978-3-527-80619-5. [Google Scholar]
- Dolinsky, A.A.; Avramenko, A.A.; Tyrinov, A.I.; Grabova, T.L. Study of the Dynamics of formation of Spatial Nanostructures. In Nanoplasmonics, Nano-Optics, Nanocomposites and Surface Studies: Springer Proceedings in Physics; Fesenko, O., Yatsenko, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 167, pp. 223–232. [Google Scholar] [CrossRef]
- Pavlenko, A.; Koshlak, H.; Basok, B. Application of thermal and cavitation effects for heat and mass transfer process intensification in multicomponent liquid media. In Proceedings of the 7th Thermal and fluids Engineering Conference (TfEC-2022), Las Vegas, NV, USA, 16–18 May 2022; pp. 1267–1284. [Google Scholar] [CrossRef]
- Delaplace, G.; Gu, Y.; Liu, M.; Jeantet, R.; Xiao, J.; Chen, X.D. Homogenization of liquids inside a new soft elastic reactor: Revealing mixing behavior through dimensional analysis. Chem. Eng. Sci. 2018, 192, 1071–1080. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.F.; Bosco, J.; Deterre, R.; Axelos, M.A.; Weiss, P. Injection of calcium phosphate pastes: Prediction of injection force and comparison with experiments. J. Mater. Sci. Mater. Med. 2012, 23, 1593–1603. [Google Scholar] [CrossRef]
- Both, E.M. Powder Morphology Development during Spray Drying. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019; 130p. [Google Scholar] [CrossRef]
- Siemons, I.; Vaessen EM, J.; Van Peski, S.O.; Boom, R.M.; Schutyser, M.A.I. Protective effect of carrier matrices on survival of Lactobacillus plantarum WCFS1 during single droplet drying explained by particle morphology development. J. Food Eng. 2021, 292, 110263. [Google Scholar] [CrossRef]
- Slinyakova, I.B.; Denisova, T.I. Silicone Adsorbents: Preparation, Properties, Application; Monograph; Naukova Dumka: Kyiv, Ukraine, 1988; 190p, ISBN 5-12-000224-2. [Google Scholar]
- Wu, T.; Ke, Q.; Lu, M.; Pan, P.; Zhou, Y.; Gu, Z.; Cui, G.; Lu, H. Recent Advances in Carbon-Silica Composites: Preparation, Properties, and Applications. Catalysts 2022, 12, 573. [Google Scholar] [CrossRef]
- Kraiwattanawong, K.; Sano, N.; Tamon, H. Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels. Polymers 2021, 13, 2631. [Google Scholar] [CrossRef]
- Kohns, R.; Torres-Rodríguez, J.; Euchler, D.; Seyffertitz, M.; Paris, O.; Reichenauer, G.; Enke, D.; Huesing, N. Drying of Hierarchically Organized Porous Silica Monoliths–Comparison of Evaporative and Supercritical Drying. Gels 2023, 9, 71. [Google Scholar] [CrossRef]
- Yashina, N.I.; Plygan, E.P.; Semenov, V.G. Sol-Gel Technology of the Mesoporous Methylsilicic Acid Hydrogel: Medicine Aspects of Globular Porous Organosilicon Materials Application. In Sol-Gel Methods for Material Processing; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2008; pp. 481–488. [Google Scholar] [CrossRef]
- Enterosgel Research. Innovative Entrosorption Method; EnteroMed Limited: London, UK, 2015; Available online: https://enterosgel.lt/upload/iblock/275/2750602317e6d4d475016895976e7aae.pdf (accessed on 5 January 2024).
- Shevchenko, Y.; Dushanin, B.; Polianskyi, O.; Yashina, N. Methyl-Silicic Acid Hydrogels as Adsorbing Agents of Middle Molecular Weight Metabolites and Process for the Preparation Thereof. UA Patent 7472A, 26 February 1999. Available online: https://uapatents.com/7-7472-gidrogeli-metilkremniehvo-kisloti-enterosgel-super-yak-adsorbenti-seredno-molekulyarnikh-metabolitiv-ta-sposib-kh-oderzhannya.html (accessed on 10 June 2023).
- Tolcheyev, Y.; Chygyryk, O.; Semenov, V. Method for Producing Asorbent Based on a Methyl-Silcic Acid Hydrogel. U.S. Patent 2010/0240532 A1, 23 September 2010. Available online: https://patentimages.storage.googleapis.com/88/e7/35/58538c52ebb7be/US20100240532A1.pdf (accessed on 10 June 2023).
- Obodovich, O.; Grabova, T.; Posunko, D.; Bazieiev, R. Implementation of the DPIE method in technologies of obtaining structured systems. Thermophys. Therm. Power Eng. 2021, 43, 13–20. [Google Scholar] [CrossRef]
- Hrabov, L.M.; Merschii, V.I.; Zhylieiev, V.T. Reactron Homogenizer. UA Patent 20698, 15 April 2002. Available online: https://sis.nipo.gov.ua/uk/search/detail/326568/ (accessed on 5 January 2024).
- Dolinsky, A.; Grabova, T.; Stepanova, O. Creation and introduction the effective technologies and equipment for the manufacture of pharmaceuticals. Part 1. Thermophys. Therm. Power Eng. 2017, 37, 31–43. [Google Scholar] [CrossRef]
- Dolinsky, A.A.; Avramenko, A.O.; Ivanytskyi, G.K. Use of DPIE Mechanisms and Methods to Control the Kinetics of Nanolevel Processes. Visnyk Natl. Acad. Sci. Ukr. 2013, 8, 47–57. Available online: http://dspace.nbuv.gov.ua/handle/123456789/67871 (accessed on 21 May 2023).
- Pavlenko, A.M.; Basok, B.I.; Avramenko, A.A. Heat conduction of a multi-layer disperse particle of emulsion. Heat Transf. Res. 2005, 36, 55–61. [Google Scholar] [CrossRef]
- Dro´zdziel, P.; Vitenko, T.; Voroshchuk, V.; Narizhnyy, S.; Snizhko, O. Discrete-Impulse Energy Supply in Milk and Dairy Product Processing. Materials 2021, 14, 4181. [Google Scholar] [CrossRef]
- Dolinsky, A.; Maletska, K. Spray drying. In Heat Technologies and Equipment for Obtaining Powder Materials; Monograph; Akademperiodika: Kyiv, Ukraine, 2015; Volume 2, 390p, ISBN 978-966-360-300-1. [Google Scholar]
- Jankowska, H.; Dzido, A.; Krawczyk, P. Determination of Rheological Parameters of Non-Newtonian Fluids on an Example of Biogas Plant Substrates. Energies 2023, 16, 1128. [Google Scholar] [CrossRef]
- Number of Novel Coronavirus (COVID-19) Confirmed Cases and Deaths as of April 26, 2023, by Region. Available online: https://www.statista.com/topics/6139/covid-19-impact-on-the-global-economy/#editorsPicks (accessed on 5 January 2024).
- Impact of the Coronavirus Pandemic on the Global Economy—Statistics & Facts. Available online: https://www.statista.com/topics/6139/covid-19-impact-on-the-global-economy/#topicOverview (accessed on 5 January 2024).
- Koshlak, H.; Basok, B.; Davydenko, B. Heat Transfer through Double-Chamber Glass Unit with Low-Emission Coating. Energies 2024, 17, 1100. [Google Scholar] [CrossRef]
- Basok, B.I.; Bazeev, Y.T.; Dubovsky, S.V. Energy and Global Warming; Scientific Thought: Kyiv, Ukraine, 2023; 172p, ISBN 978-966-00-1841-9. [Google Scholar] [CrossRef]


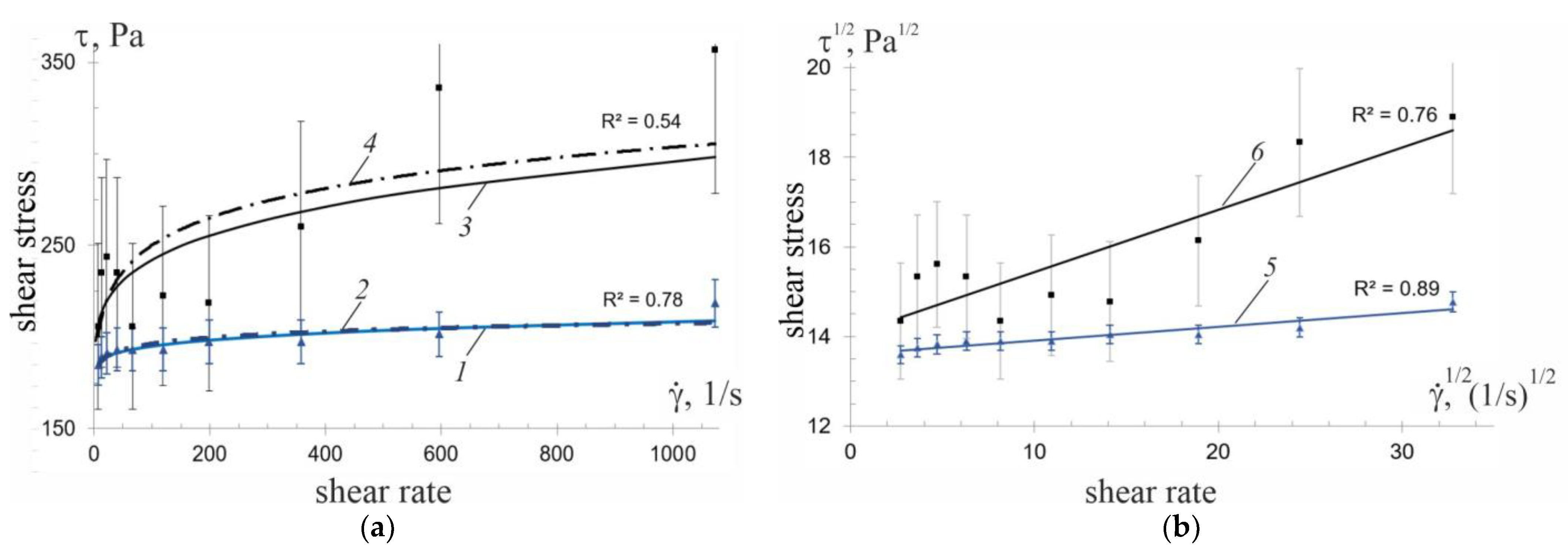
 —load mode;
—load mode;  —unload mode;
—unload mode;  —hysteresis area of suspension flow before treatment and after hydrodynamic treatment.
—hysteresis area of suspension flow before treatment and after hydrodynamic treatment.
 —load mode;
—load mode;  —unload mode;
—unload mode;  —hysteresis area of suspension flow before treatment and after hydrodynamic treatment.
—hysteresis area of suspension flow before treatment and after hydrodynamic treatment.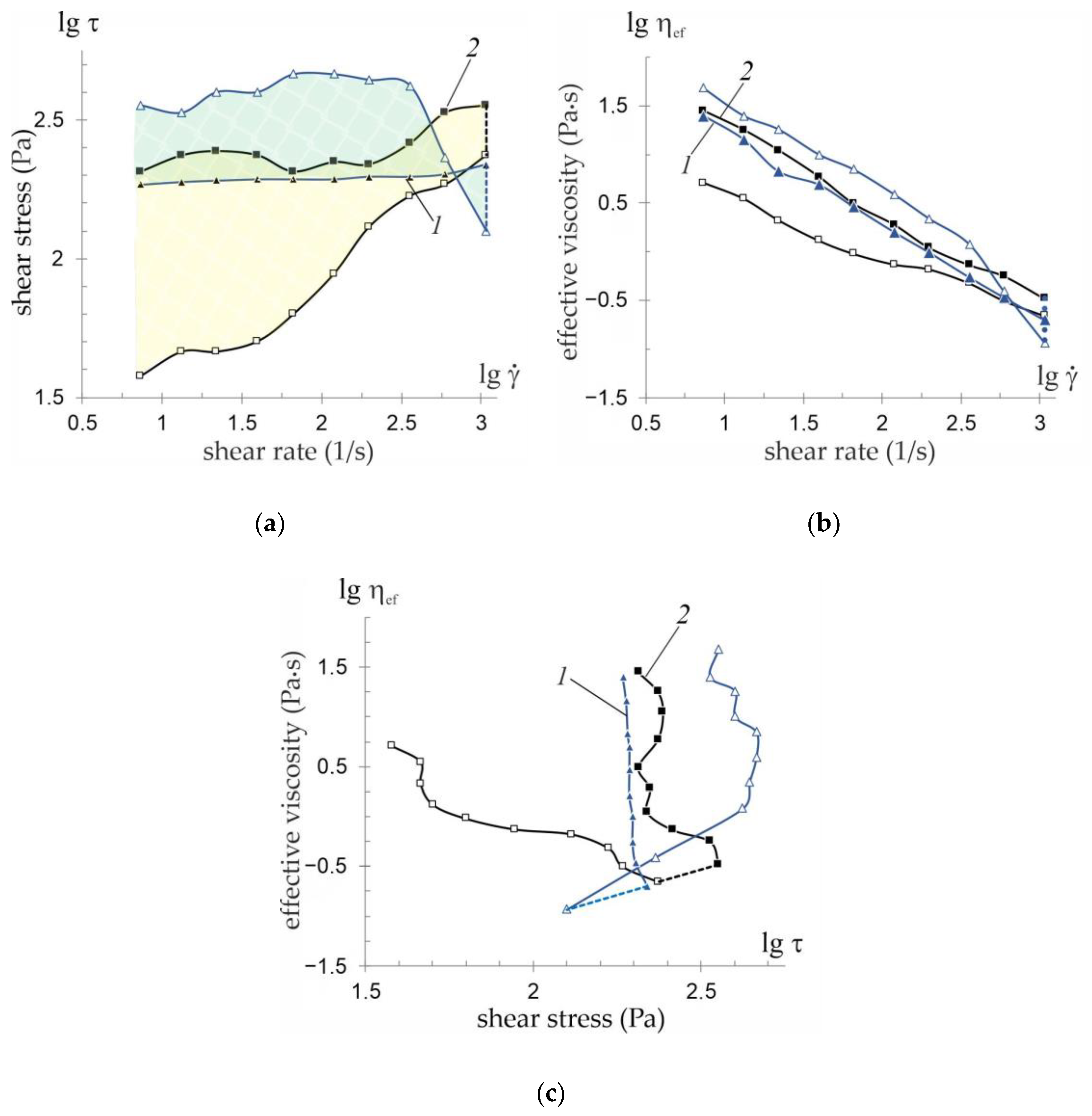
 —experimental data.
—experimental data.
 —experimental data.
—experimental data.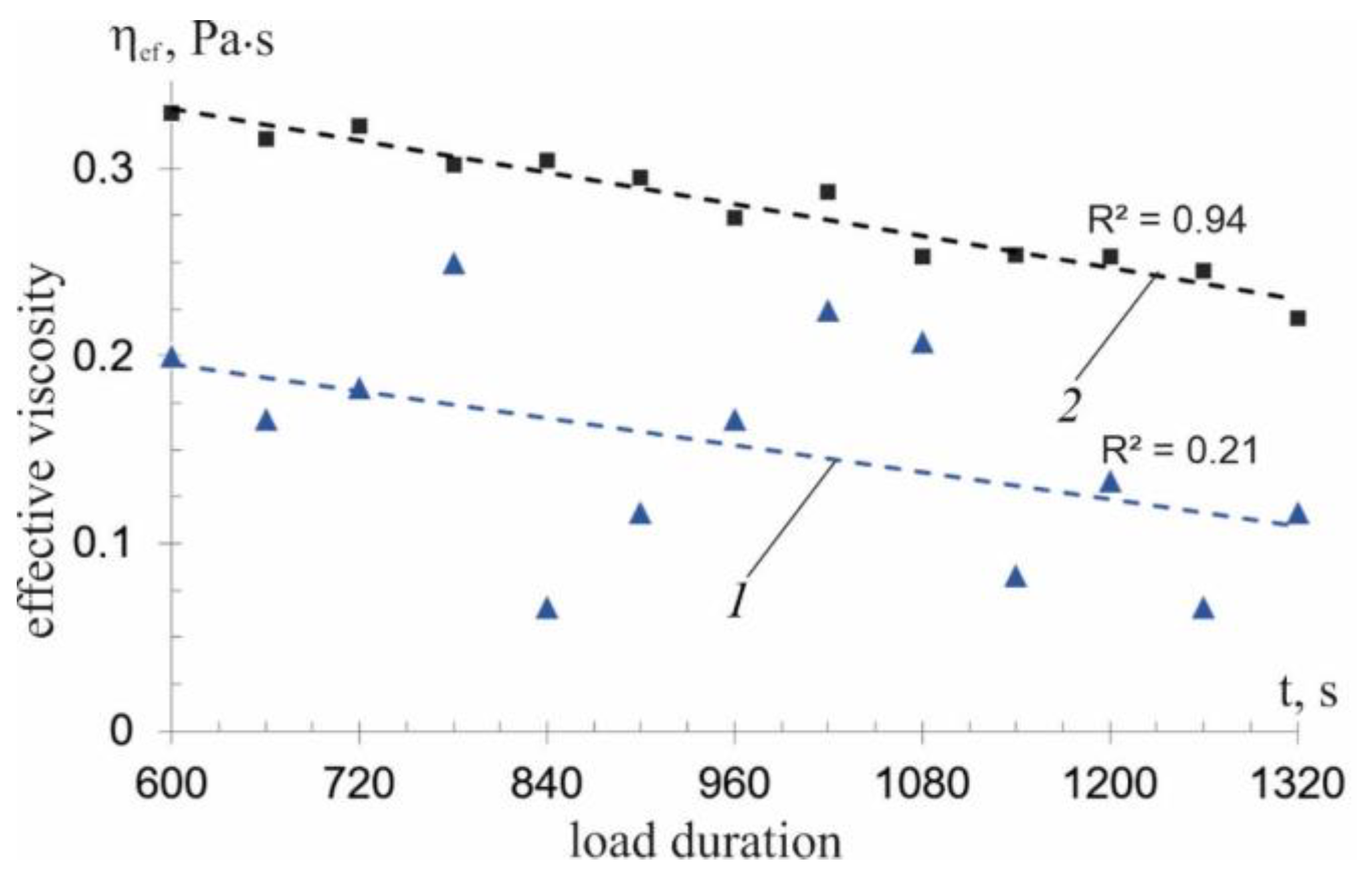
| Mass Concentration of HGMSA in H2O | Specific Processing Time | Average Speed of the Temperature Increase in the Suspension | Preferable Final Size of Monolithic and Aggregated Particles (Maximum) | Structural–Adsorption Indicators | |||
|---|---|---|---|---|---|---|---|
| Specific Surface Area | Sorption Pore Volume | Effective Pore Size Range | Effective Pore Radius | ||||
| Cm, % | , min/kg | , m2/g | , cm3/g | , nm | nm | ||
| 100 (original sample) | - | - | - | 310 | 0.890 | 1.0–13.8 | 4.56 |
| 70 | 0.08 | 0.06 | 65–250 (300) | 210 | 1.623 | 3.0–27.6 | 4.61 |
| 0.29 | 0.10 | 30–170 (250) | 81.3 | 1.084 | 3.2–40.0 | 7.13 | |
| 60 | 1.27 | 0.07 | 40–70 (120) | 60.6 | 1.064 | 1.2–20.0 | 2.77/5.39 |
| 1.39 | 0.18 | 30–60 (100) | 27 | 1.012 | 2.0–30.2 | 7.4 | |
| 40 | 1.8 | 0.04 | 20–70 (98) | 57.8 | 1.774 | 0.4–24.0 | 3.01/5.37 |
| 3.1 | 2.67 | 20–50 (85) | 9.8 | 1.062 | 1.0–28.8 | 6.6 | |
| Sample # | DPIE Processing Parameters | Adsorption and Textural Characteristics of Xerogels | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Cycles | Temperature | Final Size of Monoliths in Suspension | Humidity | Specific Surface Area | Boundary Sorption Pore Volume | Effective Pore Radius | Pore Size Range | Dimensions of Monoliths and Aggregates | |
| T, °C | δH, μm | W, % | Ssp, m2/g | Vs, cm3/g | ref max, nm | def, nm | δX, μm | ||
| 1 (initial HGMSA) | - | - | up to 2 × 104 | 86 | 310 | 0.89 | 4.60 | 3–8 | fragile loose-packed monoliths 1 μm ≥ 2 mm |
| 2 | 1 | 19–20 | 60–290 | 91 | 210 | 1.62 | 4.61 | 3–28 | monoliths 100–500 μm |
| 3 | 10 | 19–28 | 30–156 | 91 | 32 | 0.45 | 2.81/8.89 | 2–40 | densely packed monoliths >300 μm—85% 100–300 μm—7.6% |
| Sample # | DPIE Process Parameters | Dewatering Process Parameters | |||||
|---|---|---|---|---|---|---|---|
| Ratio of Suspension Components | Specific Time | Average Hydrogel Monoliths | Initial Humidity | Average Air Temperature in the Drying Chamber at the Inlet/Outlet | Final Humidity | Dispersion Characteristics of Xerogel Monoliths | |
| , min/kg | μm | Wp, % | , °C | Wf, % | μm | ||
| 4 | HGMSA:H2O 7:3 | 2 | 60–290 | 93 | 206/87 | 0.5 | <63—18% 63–100—56% |
| 5 | Cu2+/Zn-HGMSA:H2O 7:2 | 2 | 60–250 | 92 | 1.2 | <5–80—14%; 15–30—80% | |
| Suspension «HGMSA–H2O» | Hershel–Buckley Model | Ostwald–de Waele Model | Casson Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Equation Parameters | , % | Equation Parameters | , % | Equation Parameters | , % | |||||
| , Pa | k | n | k | n | Pa | K, Pa·s | ||||
| initial | 175.7 | 6.659 | 0.231 | 6 | 175.7 | 0.024 | 5 | 185.1 | 0.1 × 10−2 | 2 |
| after processing | 169.6 | 23.92 | 0.241 | 22 | 169.6 | 0.084 | 20 | 197.23 | 0.019 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshlak, H.; Basok, B.; Pavlenko, A.; Hrabova, T.; Opryshko, V. The Thermophysical Aspects of the Transformation of Porous Structures in Versatile Nanostructured Materials. Sustainability 2024, 16, 2673. https://doi.org/10.3390/su16072673
Koshlak H, Basok B, Pavlenko A, Hrabova T, Opryshko V. The Thermophysical Aspects of the Transformation of Porous Structures in Versatile Nanostructured Materials. Sustainability. 2024; 16(7):2673. https://doi.org/10.3390/su16072673
Chicago/Turabian StyleKoshlak, Hanna, Borys Basok, Anatoliy Pavlenko, Tatiana Hrabova, and Vitalii Opryshko. 2024. "The Thermophysical Aspects of the Transformation of Porous Structures in Versatile Nanostructured Materials" Sustainability 16, no. 7: 2673. https://doi.org/10.3390/su16072673








