Bird Community Traits in Recently Burned and Unburned Parts of the Northeastern Pantanal, Brazil: A Preliminary Approach
Abstract
:1. Introduction
2. Materials and Methods
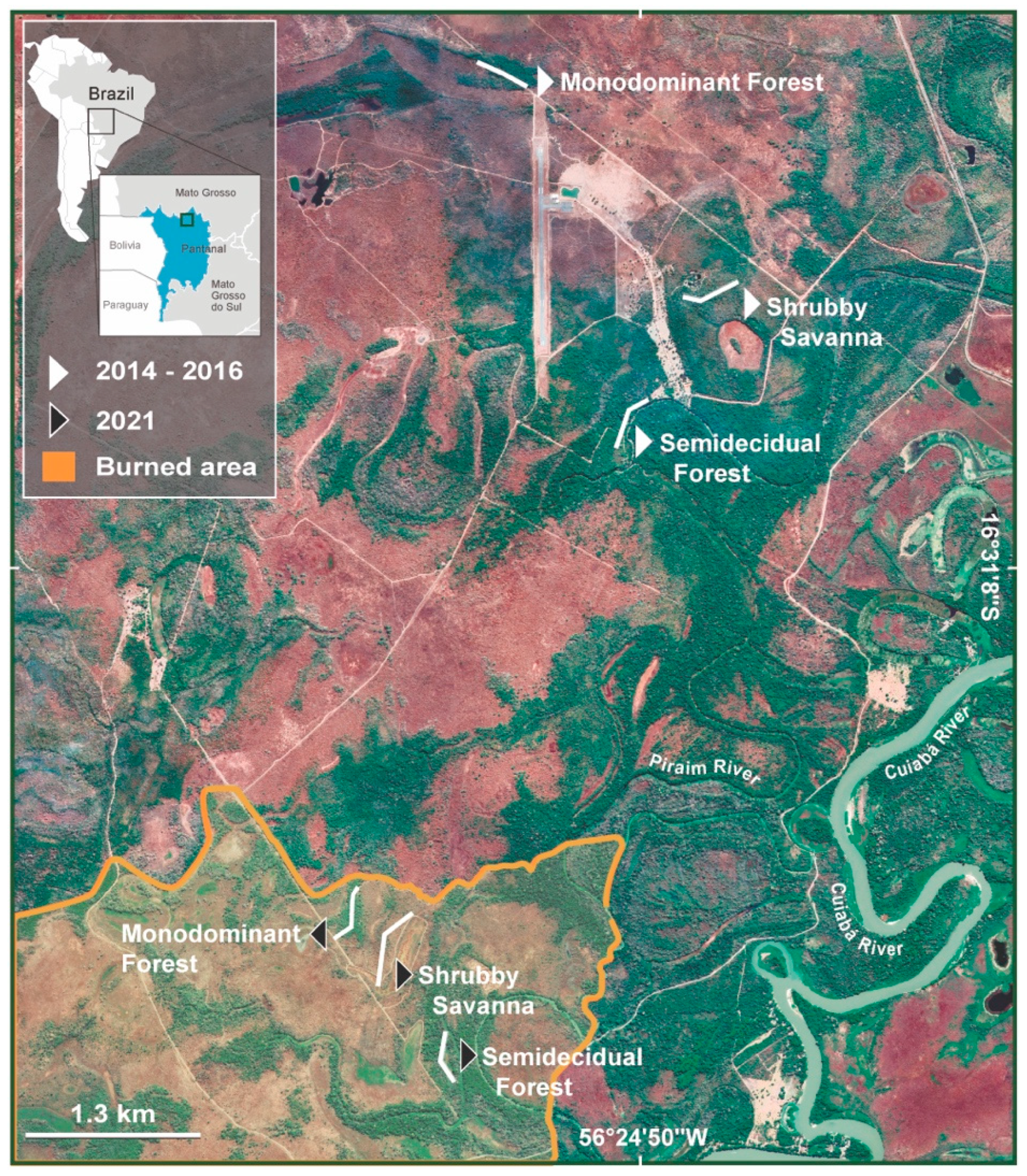
2.1. Study Area
2.2. Bird Survey
2.3. Mist Nets
2.4. Point Counts
2.5. Autonomous Acoustic Recordings
2.6. Bird Functional Traits
2.7. Statistical Analysis
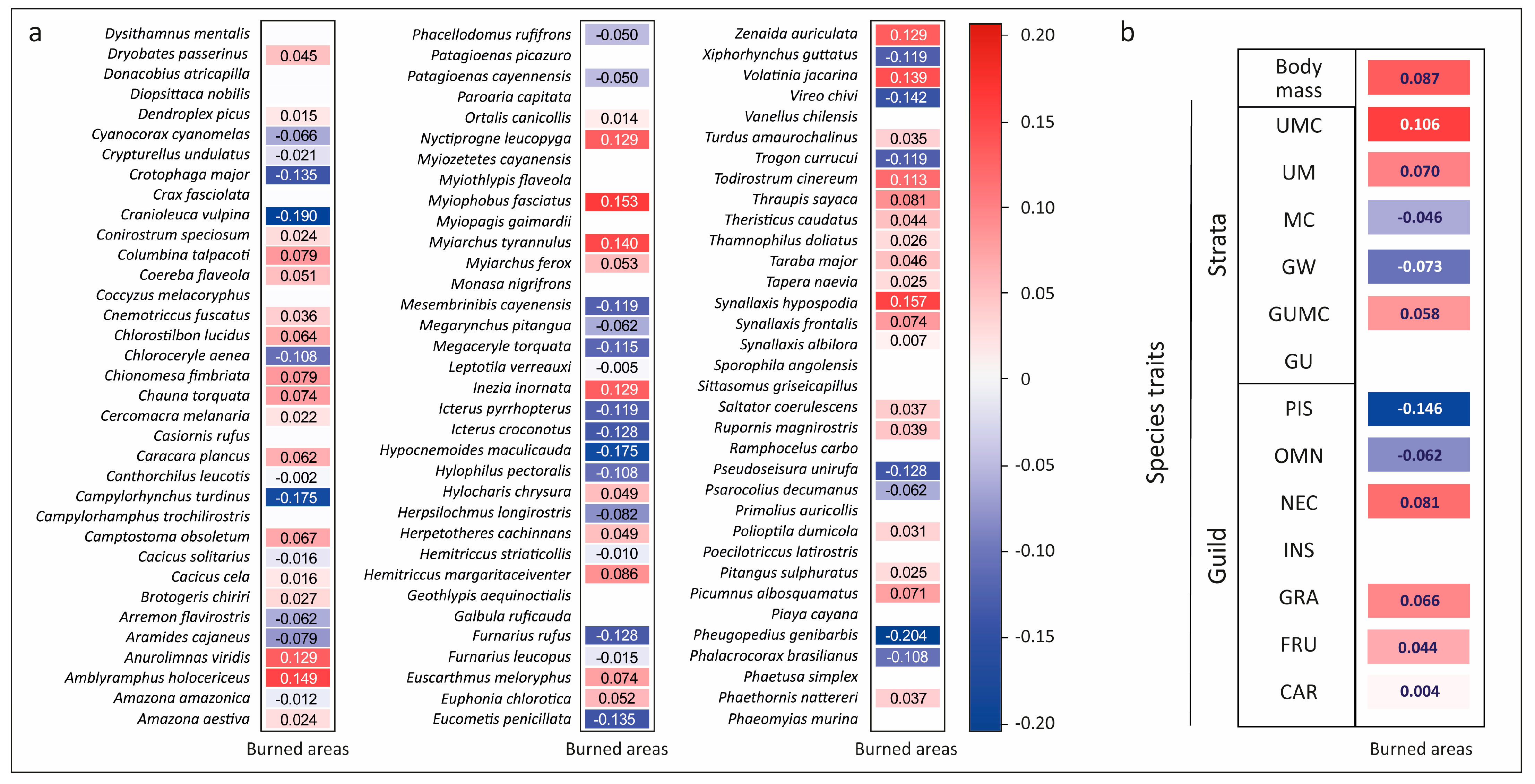
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Order/Family/Species | Name | Guild | Strata | Body Mass (g) | Unburned Areas | Burned Areas | Total |
|---|---|---|---|---|---|---|---|
| Tinamiformes | |||||||
| Tinamidae | |||||||
| Crypturellus undulatus (Temminck, 1815) | Undulated Tinamou | OMN | GU (G) | 564.4 | 38 | 19 | 57 |
| Anseriformes | |||||||
| Anhimidae | |||||||
| Chauna torquata (Oken, 1816) | Southern Screamer | OMN | GW | 4400.0 | 3 | 11 | 14 |
| Anatidae | |||||||
| Dendrocygna viduata (Linnaeus, 1766) | White-faced Whistling Duck | OMN | GW | 690.0 | 1 | 0 | 1 |
| Dendrocygna autumnalis (Linnaeus, 1758) | Black-bellied Whistling Duck | OMN | GW | 755.3 | 0 | 2 | 2 |
| Galliformes | |||||||
| Cracidae | |||||||
| Penelope ochrogaster (Pelzeln, 1870) | Chestnut-bellied Guan | OMN | MC | 1179.7 | 1 | 0 | 1 |
| Pipile cujubi (Pelzeln, 1858) | Red-throated Piping-Guan | FRU | MC | 1195.8 | 0 | 1 | 1 |
| Ortalis canicollis (Wagler, 1830) | Chaco Chachalaca | OMN | UMC | 539.0 | 36 | 38 | 74 |
| Crax fasciolata (Spix, 1825) | Bare-faced Curassow | OMN | GU (G) | 2600.0 | 4 | 2 | 6 |
| Columbiformes | |||||||
| Columbidae | |||||||
| Patagioenas picazuro (Temminck, 1813) | Picazuro Pigeon | FRU | GUMC | 279.0 | 5 | 3 | 8 |
| Patagioenas cayennensis (Bonnaterre, 1792) | Pale-vented Pigeon | FRU | MC | 229.0 | 7 | 2 | 9 |
| Leptotila verreauxi (Bonaparte, 1855) | White-tipped Dove | GRA | GU | 146.9 | 36 | 25 | 61 |
| Zenaida auriculata (Des Murs, 1847) | Eared Dove | GRA | GUMC (GUM) | 110.2 | 0 | 4 | 4 |
| Claravis pretiosa (Ferrari-Perez, 1886) | Blue Ground Dove | FRU | GUMC (GUM) | 68.2 | 1 | 2 | 3 |
| Columbina talpacoti (Temminck, 1811) | Ruddy Ground Dove | GRA | GU (G) | 46.0 | 4 | 16 | 20 |
| Columbina picui (Temminck, 1813) | Picui Ground Dove | GRA | GU (G) | 47.0 | 0 | 1 | 1 |
| Cuculiformes | |||||||
| Cuculidae | |||||||
| Guira guira (Gmelin, 1788) | Guira Cuckoo | OMN | GU | 141.0 | 1 | 0 | 1 |
| Crotophaga major (Gmelin, 1788) | Greater Ani | OMN | GUMC (GUM) | 148.3 | 7 | 0 | 7 |
| Crotophaga ani (Linnaeus, 1758) | Smooth-billed Ani | OMN | GUMC (GUM) | 110.1 | 2 | 1 | 3 |
| Tapera naevia (Linnaeus, 1766) | Striped Cuckoo | INS | GUMC (GUM) | 48.4 | 12 | 16 | 28 |
| Dromococcyx pavoninus (Pelzeln, 1870) | Pavonine Cuckoo | INS | GU | 46.4 | 1 | 0 | 1 |
| Piaya cayana (Linnaeus, 1766) | Squirrel Cuckoo | OMN | MC | 102.0 | 4 | 2 | 6 |
| Coccyzus melacoryphus (Vieillot, 1817) | Dark-billed Cuckoo | INS | MC | 49.7 | 3 | 1 | 4 |
| Caprimulgiformes | |||||||
| Caprimulgidae | |||||||
| Nyctiprogne leucopyga (Spix, 1825) | Band-tailed Nighthawk | INS | GUMC (GUM) | 27.3 | 0 | 4 | 4 |
| Apodiformes | |||||||
| Trochilidae | |||||||
| Phaethornis nattereri (Berlepsch, 1887) | Cinnamon-throated Hermit | NEC | GU (U) | 3.1 | 7 | 12 | 19 |
| Phaethornis pretrei (Lesson and Delattre, 1839) | Planalto Hermit | NEC | GU (U) | 5.6 | 0 | 1 | 1 |
| Heliomaster furcifer (Shaw, 1812) | Blue-tufted Starthroat | NEC | MC | 5.4 | 1 | 2 | 3 |
| Chlorostilbon lucidus (Shaw, 1812) | Glittering-bellied Emerald | NEC | UMC | 3.5 | 4 | 12 | 16 |
| Chionomesa fimbriata (Gmelin, 1788) | Glittering-throated Emerald | NEC | UMC | 4.9 | 7 | 28 | 35 |
| Hylocharis chrysura (Shaw, 1812) | Gilded Hummingbird | NEC | UMC | 4.5 | 2 | 5 | 7 |
| Gruiformes | |||||||
| Aramidae | |||||||
| Aramus guarauna (Linnaeus, 1766) | Limpkin | CAR | GW | 1080.0 | 2 | 0 | 2 |
| Rallidae | |||||||
| Anurolimnas viridis (Statius Muller, 1776) | Russet-crowned Crake | OMN | GU (G) | 64.3 | 0 | 4 | 4 |
| Aramides cajaneus (Statius Muller, 1776) | Gray-cowled Wood-Rail | OMN | GU (G) | 397.0 | 13 | 2 | 15 |
| Charadriiformes | |||||||
| Charadriidae | |||||||
| Vanellus chilensis (Molina, 1782) | Southern Lapwing | INS | GU (G) | 327.0 | 2 | 2 | 4 |
| Rynchopidae | |||||||
| Rynchops niger (Linnaeus, 1758) | Black Skimmer | PIS | GW (W) | 297.7 | 1 | 0 | 1 |
| Laridae | |||||||
| Phaetusa simplex (Gmelin, 1789) | Large-billed Tern | PIS | GW (W) | 235.0 | 3 | 1 | 4 |
| Eurypygiformes | |||||||
| Eurypygidae | |||||||
| Eurypyga helias (Pallas, 1781) | Sunbittern | CAR | GU (U) | 210.0 | 1 | 0 | 1 |
| Suliformes | |||||||
| Phalacrocoracidae | |||||||
| Phalacrocorax brasilianus (Gmelin, 1789) | Neotropic Cormorant | CAR | GW (W) | 1239.3 | 4 | 0 | 4 |
| Pelecaniformes | |||||||
| Ardeidae | |||||||
| Tigrisoma lineatum (Boddaert, 1783) | Rufescent Tiger-Heron | CAR | GW | 813.0 | 2 | 0 | 2 |
| Butorides striata (Linnaeus, 1758) | Striated Heron | CAR | GW | 201.5 | 3 | 0 | 3 |
| Ardea alba (Linnaeus, 1758) | Great Egret | CAR | GW | 871.3 | 0 | 1 | 1 |
| Pilherodius pileatus (Boddaert, 1783) | Capped Heron | CAR | GW | 568.6 | 1 | 0 | 1 |
| Egretta thula (Molina, 1782) | Snowy Egret | CAR | GW | 371.0 | 0 | 1 | 1 |
| Threskiornithidae | |||||||
| Mesembrinibis cayennensis (Gmelin, 1789) | Green Ibis | OMN | GW | 756.0 | 5 | 0 | 5 |
| Theristicus caerulescens (Vieillot, 1817) | Plumbeous Ibis | OMN | GW | 1500.0 | 1 | 2 | 3 |
| Theristicus caudatus (Boddaert, 1783) | Buff-necked Ibis | OMN | GU (G) | 1726.0 | 4 | 8 | 12 |
| Cathartiformes | |||||||
| Cathartidae | |||||||
| Coragyps atratus (Bechstein, 1793) | Black Vulture | CAR | GU (G) | 1881.7 | 1 | 0 | 1 |
| Cathartes burrovianus (Cassin, 1845) | Lesser Yellow-headed Vulture | CAR | GU (G) | 935.0 | 0 | 1 | 1 |
| Accipitriformes | |||||||
| Accipitridae | |||||||
| Busarellus nigricollis (Latham, 1790) | Black-collared Hawk | CAR | GW | 766.1 | 1 | 0 | 1 |
| Rostrhamus sociabilis (Vieillot, 1817) | Snail Kite | CAR | GW | 366.9 | 1 | 0 | 1 |
| Buteogallus urubitinga (Gmelin, 1788) | Great Black Hawk | CAR | GUMC | 1152.9 | 0 | 2 | 2 |
| Rupornis magnirostris (Gmelin, 1788) | Roadside Hawk | CAR | GUMC (GUM) | 269.0 | 9 | 16 | 25 |
| Strigiformes | |||||||
| Strigidae | |||||||
| Glaucidium brasilianum (Gmelin, 1788) | Ferruginous Pygmy- Owl | CAR | MC | 75.1 | 0 | 3 | 3 |
| Trogoniformes | |||||||
| Trogonidae | |||||||
| Trogon curucui (Linnaeus, 1766) | Blue-crowned Trogon | OMN | MC | 54.0 | 5 | 0 | 5 |
| Coraciiformes | |||||||
| Momotidae | |||||||
| Momotus momota (Linnaeus, 1766) | Amazonian Motmot | OMN | UM | 115.0 | 1 | 0 | 1 |
| Alcedinidae | |||||||
| Megaceryle torquata (Linnaeus, 1766) | Ringed Kingfisher | PIS | GW (W) | 317.0 | 12 | 0 | 12 |
| Chloroceryle amazona (Latham, 1790) | Amazon Kingfisher | PIS | GW (W) | 126.4 | 1 | 0 | 1 |
| Chloroceryle aenea (Pallas, 1764) | American Pygmy Kingfisher | PIS | GW (W) | 13.8 | 4 | 0 | 4 |
| Galbuliformes | |||||||
| Galbulidae | |||||||
| Galbula ruficauda (Cuvier, 1816) | Rufous-tailed Jacamar | INS | UM | 26.5 | 19 | 15 | 34 |
| Bucconidae | |||||||
| Monasa nigrifrons (Spix, 1824) | Black-fronted Nunbird | INS | GUMC (GUM) | 80.7 | 10 | 7 | 17 |
| Piciformes | |||||||
| Ramphastidae | |||||||
| Ramphastos toco (Statius Muller, 1776) | Toco Toucan | OMN | MC | 618.0 | 1 | 1 | 2 |
| Picidae | |||||||
| Picumnus albosquamatus (d’Orbigny, 1840) | White-wedged Piculet | INS | UMC | 11.9 | 5 | 17 | 22 |
| Dryobates passerinus (Linnaeus, 1766) | Little Woodpecker | INS | MC | 32.1 | 6 | 12 | 18 |
| Campephilus melanoleucos (Gmelin, 1788) | Crimson-crested Woodpecker | OMN | MC | 256.0 | 0 | 2 | 2 |
| Dryocopus lineatus (Linnaeus, 1766) | Lineated Woodpecker | OMN | MC | 183.2 | 1 | 1 | 2 |
| Celeus lugubris (Malherbe, 1851) | Pale-crested Woodpecker | INS | MC | 137.0 | 2 | 1 | 3 |
| Piculus chrysochloros (Vieillot, 1818) | Golden-green Woodpecker | INS | MC | 88.0 | 1 | 0 | 1 |
| Falconiformes | |||||||
| Falconidae | |||||||
| Herpetotheres cachinnans (Linnaeus, 1758) | Laughing Falcon | CAR | UMC | 623.6 | 2 | 5 | 7 |
| Micrastur semitorquatus (Vieillot, 1817) | Collared Forest-Falcon | CAR | UMC | 621.7 | 1 | 0 | 1 |
| Caracara plancus (Miller, 1777) | Crested Caracara | OMN | GU (G) | 1078.6 | 1 | 5 | 6 |
| Psittaciformes | |||||||
| Psittacidae | |||||||
| Myiopsitta monachus (Boddaert, 1783) | Monk Parakeet | FRU | MC | 120.0 | 1 | 0 | 1 |
| Brotogeris chiriri (Vieillot, 1818) | Yellow-chevroned Parakeet | FRU | UMC | 61.6 | 21 | 29 | 50 |
| Amazona aestiva (Linnaeus, 1758) | Turquoise-fronted Parrot | FRU | MC | 451.0 | 6 | 8 | 14 |
| Amazona amazonica (Linnaeus, 1766) | Orange-winged Parrot | FRU | MC | 370.0 | 20 | 12 | 32 |
| Anodorhynchus hyacinthinus (Latham, 1790) | Hyacinth Macaw | FRU | MC | 1331.0 | 0 | 2 | 2 |
| Eupsittula aurea (Gmelin, 1788) | Peach-fronted Parakeet | FRU | UM | 84.6 | 2 | 0 | 2 |
| Primolius auricollis (Cassin, 1853) | Yellow-collared Macaw | FRU | UMC | 245.0 | 4 | 1 | 5 |
| Ara ararauna (Linnaeus, 1758) | Blue-and-yellow Macaw | FRU | MC | 1125.0 | 0 | 1 | 1 |
| Diopsittaca nobilis (Linnaeus, 1758) | Red-shouldered Macaw | FRU | MC | 150.9 | 5 | 3 | 8 |
| Psittacara leucophthalmus (Statius Muller, 1776) | White-eyed Parakeet | FRU | MC | 158.0 | 1 | 2 | 3 |
| Passeriformes | |||||||
| Thamnophilidae | |||||||
| Taraba major (Vieillot, 1816) | Great Antshrike | INS | GU | 59.2 | 14 | 28 | 42 |
| Thamnophilus doliatus (Linnaeus, 1764) | Barred Antshrike | INS | UM | 27.0 | 18 | 24 | 42 |
| Thamnophilus pelzelni (Hellmayr, 1924) | Planalto Slaty-Antshrike | INS | UM | 20.9 | 0 | 1 | 1 |
| Thamnophilus amazonicus (Sclater, 1858) | Amazonian Antshrike | INS | GU (U) | 18.7 | 1 | 0 | 1 |
| Dysithamnus mentalis (Temminck, 1823) | Plain Antvireo | INS | UM | 14.9 | 2 | 2 | 4 |
| Herpsilochmus longirostris (Pelzeln, 1868) | Large-billed Antwren | INS | MC | 12.8 | 9 | 1 | 10 |
| Formicivora rufa (Wied, 1831) | Rusty-backed Antwren | INS | GU (U) | 10.8 | 0 | 3 | 3 |
| Cercomacra melanaria (Ménétries, 1835) | Mato Grosso Antbird | INS | GU | 19.0 | 39 | 48 | 87 |
| Hypocnemoides maculicauda (Pelzeln, 1868) | Band-tailed Antbird | INS | GU (U) | 11.8 | 23 | 0 | 23 |
| Furnariidae | |||||||
| Sittasomus griseicapillus (Vieillot, 1818) | Olivaceous Woodcreeper | INS | MC | 13.1 | 4 | 5 | 9 |
| Dendrocolaptes platyrostris (Spix, 1825) | Planalto Woodcreeper | INS | UM | 61.7 | 0 | 2 | 2 |
| Xiphorhynchus guttatus (Lafresnaye, 1850) | Buff-throated Woodcreeper | INS | UMC | 59.7 | 5 | 0 | 5 |
| Dendroplex picus (Gmelin, 1788) | Straight-billed Woodcreeper | INS | UM | 41.3 | 12 | 13 | 25 |
| Campylorhamphus trochilirostris (Lichtenstein, 1820) | Red-billed Scythebill | INS | MC | 32.6 | 3 | 3 | 6 |
| Lepidocolaptes angustirostris (Vieillot, 1818) | Narrow-billed Woodcreeper | INS | UM | 29.6 | 1 | 0 | 1 |
| Furnarius leucopus (Swainson, 1838) | Pale-legged Hornero | INS | GU (G) | 54.8 | 42 | 24 | 66 |
| Furnarius rufus (Gmelin, 1788) | Rufous Hornero | OMN | GU (G) | 46.4 | 6 | 0 | 6 |
| Phacellodomus rufifrons (Wied, 1821) | Rufous-fronted Thornbird | INS | UMC | 24.6 | 6 | 1 | 7 |
| Phacellodomus ruber (Vieillot, 1817) | Greater Thornbird | INS | GU | 41.0 | 1 | 1 | 2 |
| Cranioleuca vulpina (Pelzeln, 1856) | Rusty-backed Spinetail | INS | MC | 15.7 | 28 | 1 | 29 |
| Pseudoseisura unirufa (d’Orbigny and Lafresnaye, 1838) | Rufous Cacholote | INS | GUMC (GUM) | 44.9 | 6 | 0 | 6 |
| Certhiaxis cinnamomeus (Gmelin, 1788) | Yellow-chinned Spinetail | INS | GU | 15.2 | 2 | 1 | 3 |
| Synallaxis albilora (Pelzeln, 1856) | White-lored Spinetail | INS | GU | 14.9 | 47 | 43 | 90 |
| Synallaxis hypospodia (Sclater, 1874) | Cinereous-breasted Spinetail | INS | GU (U) | 16.9 | 0 | 7 | 7 |
| Synallaxis frontalis (Pelzeln, 1859) | Sooty-fronted Spinetail | INS | GU | 14.0 | 2 | 7 | 9 |
| Pipridae | |||||||
| Neopelma pallescens (Lafresnaye, 1853) | Pale-bellied Tyrant- Manakin | OMN | UM | 18.2 | 1 | 0 | 1 |
| Antilophia galeata (Lichtenstein, 1823) | Helmeted Manakin | FRU | MC | 21.5 | 2 | 0 | 2 |
| Pipra fasciicauda (Hellmayr, 1906) | Band-tailed Manakin | FRU | UM | 15.9 | 1 | 0 | 1 |
| Tityridae | |||||||
| Pachyramphus viridis (Vieillot, 1816) | Green-backed Becard | INS | MC | 21.0 | 1 | 0 | 1 |
| Pachyramphus polychopterus (Vieillot, 1818) | White-winged Becard | INS | MC | 20.8 | 3 | 0 | 3 |
| Tyrannidae | |||||||
| Leptopogon amaurocephalus (Tschudi, 1846) | Sepia-capped Flycatcher | INS | UM | 11.7 | 3 | 0 | 3 |
| Tolmomyias sulphurescens (Spix, 1825) | Yellow-olive Flycatcher | INS | MC | 14.3 | 0 | 1 | 1 |
| Hemitriccus striaticollis (Lafresnaye, 1853) | Stripe-necked Tody-Tyrant | INS | MC (M) | 8.6 | 13 | 8 | 21 |
| Hemitriccus margaritaceiventer (d’Orbigny and Lafresnaye, 1837) | Pearly-vented Tody-Tyrant | INS | UM | 8.4 | 2 | 8 | 10 |
| Poecilotriccus latirostris (Pelzeln, 1868) | Rusty-fronted Tody-Flycatcher | INS | GU (U) | 8.1 | 18 | 14 | 32 |
| Todirostrum cinereum (Linnaeus, 1766) | Common Tody-Flycatcher | INS | UMC | 6.3 | 2 | 16 | 18 |
| Inezia inornata (Salvadori, 1897) | Plain Tyrannulet | INS | MC | 12.0 | 0 | 4 | 4 |
| Euscarthmus meloryphus (Wied, 1831) | Fulvous-crowned Scrub-Tyrant | INS | GU (U) | 6.8 | 1 | 6 | 7 |
| Camptostoma obsoletum (Temminck, 1824) | Southern Beardless-Tyrannulet | OMN | MC | 8.1 | 7 | 22 | 29 |
| Elaenia flavogaster (Thunberg, 1822) | Yellow-bellied Elaenia | OMN | UMC | 24.8 | 1 | 1 | 2 |
| Elaenia parvirostris (Pelzeln, 1868) | Small-billed Elaenia | OMN | UMC | 13.8 | 1 | 0 | 1 |
| Elaenia chiriquensis (Lawrence, 1865) | Lesser Elaenia | OMN | UMC | 15.4 | 0 | 1 | 1 |
| Myiopagis gaimardii (d’Orbigny, 1839) | Forest Elaenia | OMN | MC (C) | 12.0 | 4 | 3 | 7 |
| Myiopagis viridicata (Vieillot, 1817) | Greenish Elaenia | OMN | MC | 11.5 | 0 | 1 | 1 |
| Phaeomyias murina (Spix, 1825) | Mouse-colored Tyrannulet | OMN | UMC | 10.0 | 2 | 2 | 4 |
| Attila bolivianus (Lafresnaye, 1848) | Dull-capped Attila | INS | MC | 39.5 | 1 | 0 | 1 |
| Legatus leucophaius (Vieillot, 1818) | Piratic Flycatcher | FRU | MC | 22.2 | 1 | 0 | 1 |
| Pitangus sulphuratus (Linnaeus, 1766) | Great Kiskadee | OMN | GUMC | 62.9 | 15 | 20 | 35 |
| Philohydor lictor (Lichtenstein, 1823) | Lesser Kiskadee | INS | UMC | 25.5 | 1 | 0 | 1 |
| Megarynchus pitangua (Linnaeus, 1766) | Boat-billed Flycatcher | OMN | MC | 69.9 | 8 | 2 | 10 |
| Myiodynastes maculatus (Statius Muller, 1776) | Streaked Flycatcher | INS | MC | 43.2 | 2 | 0 | 2 |
| Myiozetetes cayanensis (Linnaeus, 1766) | Rusty-margined Flycatcher | INS | UMC | 25.9 | 9 | 6 | 15 |
| Empidonomus varius (Vieillot, 1818) | Variegated Flycatcher | INS | MC | 27.1 | 1 | 0 | 1 |
| Tyrannus savana (Daudin, 1802) | Fork-tailed Flycatcher | OMN | UMC | 31.9 | 1 | 0 | 1 |
| Casiornis rufus (Vieillot, 1816) | Rufous Casiornis | INS | UMC | 24.8 | 3 | 3 | 6 |
| Myiarchus ferox (Gmelin, 1789) | Short-crested Flycatcher | INS | UM | 27.5 | 12 | 28 | 40 |
| Myiarchus tyrannulus (Statius Muller, 1776) | Brown-crested Flycatcher | INS | UM | 35.5 | 0 | 5 | 5 |
| Myiophobus fasciatus (Statius Muller, 1776) | Bran-colored Flycatcher | INS | GU (U) | 9.9 | 0 | 14 | 14 |
| Pyrocephalus rubinus (Boddaert, 1783) | Vermilion Flycatcher | INS | UM | 14.4 | 0 | 2 | 2 |
| Cnemotriccus fuscatus (Wied, 1831) | Fuscous Flycatcher | INS | UM | 13.6 | 19 | 31 | 50 |
| Lathrotriccus euleri (Cabanis, 1868) | Euler’s Flycatcher | INS | GU (U) | 11.3 | 0 | 1 | 1 |
| Vireonidae | |||||||
| Cyclarhis gujanensis (Gmelin, 1789) | Rufous-browed Peppershrike | OMN | UMC | 28.8 | 0 | 2 | 2 |
| Hylophilus pectoralis (Sclater, 1866) | Ashy-headed Greenlet | INS | MC | 11.6 | 11 | 0 | 11 |
| Vireo chivi (Vieillot, 1817) | Chivi Vireo | OMN | MC | 16.1 | 8 | 0 | 8 |
| Corvidae | |||||||
| Cyanocorax cyanomelas (Vieillot, 1818) | Purplish Jay | OMN | MC | 207.0 | 20 | 4 | 24 |
| Hirundinidae | |||||||
| Stelgidopteryx ruficollis(Vieillot, 1817) | Southern Rough-winged Swallow | INS | UM | 16.1 | 2 | 0 | 2 |
| Progne tapera (Linnaeus, 1766) | Brown-chested Martin | INS | UMC | 32.0 | 0 | 1 | 1 |
| Tachycineta albiventer (Boddaert, 1783) | White-winged Swallow | INS | UM | 17.7 | 1 | 0 | 1 |
| Troglodytidae | |||||||
| Troglodytes aedon (Naumann, 1823) | House Wren | INS | GU (U) | 10.9 | 0 | 1 | 1 |
| Campylorhynchus Turdinus (Wied, 1831) | Thrush-like Wren | INS | MC | 32.6 | 23 | 0 | 23 |
| Pheugopedius genibarbis (Swainson, 1838) | Moustached Wren | INS | GU (U) | 19.2 | 31 | 0 | 31 |
| Cantorchilus leucotis (Lafresnaye, 1845) | Buff-breasted Wren | INS | GU (U) | 19.4 | 34 | 25 | 59 |
| Polioptilidae | |||||||
| Polioptila dumicola (Vieillot, 1817) | Masked Gnatcatcher | INS | MC | 7.0 | 16 | 24 | 40 |
| Donacobiidae | |||||||
| Donacobius atricapilla (Linnaeus, 1766) | Black-capped Donacobius | INS | GU (U) | 36.8 | 5 | 3 | 8 |
| Turdidae | |||||||
| Turdus rufiventris (Vieillot, 1818) | Rufous-bellied Thrush | OMN | GUMC (GUM) | 69.4 | 1 | 1 | 2 |
| Turdus amaurochalinus (Cabanis, 1850) | Creamy-bellied Thrush | OMN | GUMC | 57.9 | 8 | 13 | 21 |
| Fringillidae | |||||||
| Euphonia chlorotica (Linnaeus, 1766) | Purple-throated Euphonia | FRU | MC | 11.0 | 7 | 16 | 23 |
| Passerellidae | |||||||
| Arremon flavirostris (Bonaparte, 1850) | Saffron-billed Sparrow | OMN | GU | 26.1 | 8 | 2 | 10 |
| Icteridae | |||||||
| Psarocolius decumanus (Pallas, 1769) | Crested Oropendola | OMN | MC | 206.3 | 7 | 1 | 8 |
| Cacicus solitarius (Vieillot, 1816) | Solitary Black Cacique | OMN | UM | 79.8 | 20 | 11 | 31 |
| Cacicus cela (Linnaeus, 1758) | Yellow-rumped Cacique | OMN | MC | 85.5 | 8 | 9 | 17 |
| Icterus croconotus (Wagler, 1829) | Orange-backed Troupial | OMN | MC | 40.0 | 6 | 0 | 6 |
| Icterus pyrrhopterus (Vieillot, 1819) | Variable Oriole | OMN | MC | 35.4 | 5 | 0 | 5 |
| Amblyramphus holosericeus (Scopoli, 1786) | Scarlet-headed Blackbird | OMN | GU (U) | 70.4 | 0 | 6 | 6 |
| Agelaioides badius (Vieillot, 1819) | Grayish Baywing | OMN | GU (G) | 45.3 | 0 | 1 | 1 |
| Parulidae | |||||||
| Geothlypis aequinoctialis (Gmelin, 1789) | Masked Yellowthroat | INS | GU (U) | 13.1 | 8 | 5 | 13 |
| Setophaga pitiayumi (Vieillot, 1817) | Tropical Parula | OMN | MC (C) | 6.8 | 0 | 3 | 3 |
| Myiothlypis flaveola (Baird, 1865) | Flavescent Warbler | INS | GU | 13.2 | 22 | 18 | 40 |
| Thraupidae (Cabanis, 1847) | |||||||
| Nemosia pileata (Boddaert, 1783) | Hooded Tanager | OMN | MC | 16.0 | 0 | 2 | 2 |
| Hemithraupis guira (Linnaeus, 1766) | Guira Tanager | FRU | MC | 12.0 | 0 | 1 | 1 |
| Conirostrum speciosum (Temminck, 1824) | Chestnut-vented Conebill | INS | UMC | 8.8 | 6 | 8 | 14 |
| Volatinia jacarina (Linnaeus, 1766) | Blue-black Grassquit | GRA | GU | 9.9 | 1 | 13 | 14 |
| Tachyphonus rufus (Boddaert, 1783) | White-lined Tanager | FRU | UMC | 34.4 | 1 | 2 | 3 |
| Eucometis penicillata (Spix, 1825) | Gray-headed Tanager | OMN | UM | 27.0 | 7 | 0 | 7 |
| Ramphocelus carbo (Pallas, 1764) | Silver-beaked Tanager | OMN | UMC | 25.9 | 30 | 25 | 55 |
| Sporophila angolensis (Linnaeus, 1766) | Chestnut-bellied Seed-Finch | GRA | GU (U) | 13.0 | 2 | 2 | 4 |
| Sporophila caerulescens (Vieillot, 1823) | Double-collared Seedeater | GRA | GU | 9.7 | 0 | 2 | 2 |
| Sporophila collaris (Boddaert, 1783) | Rusty-collared Seedeater | GRA | GU | 13.5 | 1 | 0 | 1 |
| Saltator coerulescens (Vieillot, 1817) | Bluish-gray Saltator | OMN | UMC | 54.9 | 22 | 37 | 59 |
| Thlypopsis sordida (d’Orbigny and Lafresnaye, 1837) | Orange-headed Tanager | OMN | UMC | 17.0 | 0 | 1 | 1 |
| Coereba flaveola (Linnaeus, 1758) | Bananaquit | OMN | UMC | 10.0 | 13 | 29 | 42 |
| Paroaria capitata (d’Orbigny and Lafresnaye, 1837) | Yellow-billed Cardinal | OMN | GUMC (GUM) | 37.8 | 2 | 3 | 5 |
| Thraupis sayaca (Linnaeus, 1766) | Sayaca Tanager | FRU | MC | 32.5 | 6 | 25 | 31 |
| Thraupis palmarum (Wied, 1821) | Palm Tanager | OMN | MC | 39.0 | 0 | 1 | 1 |
References
- Goldammer, J.G. Fire in the Tropical Biota—Ecosystem Processes and Global Challenges, Ecological Studies; Springer: Berlin, Germany, 1990; Volume 84, 497p. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global “herbivore”: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, J.; Myers, R.; Fulks, W. Fire, ecosystems, and people: A preliminary assessment of fire as a global conservation issue. Georg. Wright Forum 2005, 22, 78–87. [Google Scholar]
- Pivello, V.R.; Vieira, I.; Christianini, A.V.; Ribeiro, D.B.; Menezese, L.S.; Berlinck, C.N.; Melog, F.P.L.; Marengoh, J.A.; Tornquisti, C.G.; Tomasj, W.M.; et al. Understanding Brazil’s catastrophic fires: Causes, consequences and policy needed to prevent future tragedies. Perspect. Ecol. Conserv. 2021, 19, 233–255. [Google Scholar] [CrossRef]
- Coutinho, L.M. Fire in the ecology of Brazilian Cerrado. In Fire in the Tropical Biota: Ecological Processes and Global Challenges; Goldammer, J.G., Ed.; Springer: Berlin, Germany, 1990; pp. 82–105. [Google Scholar]
- Ledru, M.P. Late Quaternary history and evolution of the cerrados as revealed by palynological records. In The Cerrados of Brazil—Ecology and Natural History of a Neotropical Savanna; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 33–50. [Google Scholar]
- Simon, M.F.; Grether, R.; Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a Neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.F.; Pennington, T. Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Gloria, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef]
- Fidelis, A.; Rosalem, P.; Zanzarini, V.; Camargos, L.S.; Martins, A.R. From ashes to flowers: A savanna sedge initiates flowers 24 h after fire. Ecology 2019, 100, e02648. [Google Scholar] [CrossRef]
- Mistry, J. Fire in the Cerrado (savannas) of Brazil: An ecological review. Prog. Phys. Geogr. 1998, 224, 425–448. [Google Scholar] [CrossRef]
- Ramos-Neto, M.B.; Pivello, V.R. Lightning fires in a Brazilian savanna national park: Rethinking management strategies. J. Environ. Manag. 2000, 26, 675–684. [Google Scholar] [CrossRef]
- Adeney, J.M.; Ginsberg, J.R.; Russell, G.J.; Kinnaird, M.F. Effects of an ENSO-related fire on birds of a lowland tropical forest in Sumatra. Anim. Conserv. 2006, 9, 292–301. [Google Scholar] [CrossRef]
- O’Reilly, L.; Ogada, D.L.; Palmer, T.M.; Keesing, F. Effects of fire on bird diversity and abundance in an East African savanna. Afr. J. Ecol. 2006, 44, 165–170. [Google Scholar] [CrossRef]
- Sitters, H.; Di Stefano, J.; Christie, F.J.; Sunnucks, P.; York, A. Bird diversity increases after patchy prescribed fire: Implications from a before–after control–impact study. Int. J. Wildland Fire 2015, 24, 690–701. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z. Effects of fire on the bird communities of tropical woodlands and open forests in northern Australia. Aust. J. Ecol. 1990, 15, 1–22. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Brock, C.; Fisher, A.; Milne, B.D. Response of birds and reptiles to fire regimes on pastoral land in the Victoria River District, northern territory. Rangel. J. 1999, 21, 24–38. [Google Scholar] [CrossRef]
- Smucker, K.M.; Hutto, R.L.; Steele, B.M. Changes in bird abundance after wildfire: Importance of fire severity and time since fire. Ecol. Appl. 2005, 15, 1535–1549. [Google Scholar] [CrossRef]
- Reis, M.G.; Fieker, C.Z.; Dias, M.M. The influence of fire on the assemblage structure of foraging birds in grasslands of the Serra da Canastra National Park, Brazil. An. Acad. Bras. Cienc. 2016, 88, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Pons, P.; Wendenburg, C. The impact of fire and forest conversion into savanna on the bird communities of West Madagascan dry forests. Anim. Conserv. 2005, 8, 183–193. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Van Balen, S. Bird community changes in response to single and repeated fires in a lowland tropical rainforest of eastern Borneo. Biodivers. Conserv. 2006, 15, 4425–4451. [Google Scholar] [CrossRef]
- Albanesi, S.; Sebastián, S.D.; Bellis, L.M. Effects of fire disturbance on bird communities and species of mountain Serrano forest in central Argentina. J. For. Res. 2013, 19, 105–114. [Google Scholar] [CrossRef]
- Lee, J.S.; Cornwell, W.K.; Kingsford, R.T. Rainforest bird communities threatened by extreme fire. Glob. Ecol. Conserv. 2022, 33, e01985. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Avifaunal responses to single and recurrent wildfires in Amazonian forests. Ecol. Appl. 2004, 14, 1358–1373. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Ecological responses to el Niño-induced surface fires in central Brazilian Amazonia: Management implications for flammable tropical forests. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Beal-Neves, M.; Chiarani, E.; Ferreira, P.M.A.; Suertegaray Fontana, C. The role of fire disturbance on habitat structure and bird communities in South Brazilian Highland Grasslands. Sci. Rep. 2020, 10, 19708. [Google Scholar] [CrossRef] [PubMed]
- Chalmandrier, L.; Midgley, G.F.; Barnard, P.; Sirami, C. Effects of time since fire on birds in a plant diversity hotspot. Acta Oecol. 2013, 49, 99–106. [Google Scholar] [CrossRef]
- Barlow, J.; Haugaasen, T.; Peres, C.A. Effects of ground fires on understory bird assemblages in Amazonian forests. Biol. Conserv. 2002, 105, 157–169. [Google Scholar] [CrossRef]
- Ubaid, F.K. Efeitos do Fogo Sobre Comunidades de Aves no Pantanal Mato-Grossense. Doctoral Thesis, Universidade Estadual Paulista-UNESP, Botucatu, Brazil, 2014. [Google Scholar]
- Fontaine, J.B.; Donato, D.C.; Robinson, D.W.; Law, B.E.; Boone, J.K. Bird communities following high-severity fire: Response to single and repeat fires in a mixed-evergreen forest, Oregon, USA. For. Ecol. Manag. 2009, 257, 1496–1504. [Google Scholar] [CrossRef]
- Franklin, M.J.M.; Major, R.E.; Bedward, M.; Bradstock, R.A. Relative avian mobility linked to use of fire-affected resources in forested landscapes. For. Ecol. Manag. 2021, 97, 119484. [Google Scholar] [CrossRef]
- Lee, J.S.; Callaghan, C.T.; Cornwell, W.K. Using citizen science to measure recolonisation of birds after the Australian 2019–2020 mega-fires. Austral Ecol. 2021, 48, 31–40. [Google Scholar] [CrossRef]
- Lee, A.T.K.; Herrmann, E.; Retief, E.F.; van der Westhuizen-Coetzer, E.; Seymour, C.L. The impact of a massive wildfire event on avian species richness and abundance in an arid African savanna ecosystem. J. Arid. Environ. 2023, 217, 105039. [Google Scholar] [CrossRef]
- Watson, S.J.; Taylor, R.S.; Nimmo, D.G.; Kelly, L.T.; Clarke, M.F.; Bennett, A.F. Post-fire bird communities and spatial patterns of fire. Anim. Conserv. 2012, 15, 499–507. [Google Scholar] [CrossRef]
- Thielen, D.; Schuchmann, K.-L.; Ramoni-Perazzi, P.; Marquez, M.; Quintero, J.I.; Marques, M.I. Quo vadis Pantanal? Expected precipitation extremes and drought dynamics from changing sea surface temperature. PLoS ONE 2020, 15, e0227437. [Google Scholar] [CrossRef] [PubMed]
- Furness, R.W.; Greenwood, J.J.D. Birds as Monitors of Environmental Change; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Siddig, A.A.H.; Ellison, A.M.; Ochsc, A.; Villar-Leemand, C.; Laub, K.M. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef]
- Leal Filho, W.; Azeiteiro, U.M.; Salvia, A.L.; Fritzen, B.; Libonati, R. Fire in paradise: Why the Pantanal is burning. Environ. Sci. Policy 2021, 123, 31–34. [Google Scholar] [CrossRef]
- Manrique-Pineda, D.A.; Souza, E.B.; Paranhos Filho, A.C.; Encina, C.C.C.; Damasceno-Junior, G.A. Fire, flood and monodominance of Tabebuia aurea in Pantanal. For. Ecol. Manag. 2021, 479, 118599. [Google Scholar] [CrossRef]
- Pott, A.; Oliveira, A.K.; Damasceno-Junior, G.A.; Silva, J.S. Plant diversity of the Pantanal wetland. Braz. J. Biol. 2011, 71, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Damasceno-Junior, G.A.; Pott, A.; Paranhos Filho, A.C.; Suarez, Y.R.; Parolin, P. Regeneration of riparian forests of the Brazilian Pantanal under flood and fire influence. For. Ecol. Manag. 2014, 331, 256–263. [Google Scholar] [CrossRef]
- Damasceno-Junior, G.A.; Roque, F.O.; Garcia, L.C.; Ribeiro, D.B.; Tomas, W.M.; Scremin-Dias, E.; Dias, F.A.; Libonati, R.; Rodrigues, J.A.; Lemos, F.; et al. Lessons to be learned from the wildfire catastrophe of 2020 in the Pantanal. Wetl. Sci. Pract. 2021, 38, 107–115. [Google Scholar] [CrossRef]
- Marengo, J.A.; Cunha, A.P.; Cuartas, L.A.; Deusdara Leal, K.R.; Broedel, E.; Seluchi, M.E.; Michelin, C.M.; Baião, C.F.P.; Ângulo, E.C.; Almeida, E.K.; et al. Extreme drought in the Brazilian Pantanal in 2019–2020: Characterization, causes, and impacts. Front. Water 2021, 3, 639204. [Google Scholar] [CrossRef]
- Garcia, L.C.; Szabo, J.K.; Roque, F.O.; Pereira, A.M.M.; Cunha, C.N.; Damasceno-Júnior, G.A.; Morato, R.G.; Tomas, W.M.; Libonati, R.; Ribeiro, D.B. Record breaking wildfires in the world’s largest continuous tropical wetland: Integrative fire management is urgently needed for both biodiversity and humans. J. Environ. Manag. 2021, 293, 112870. [Google Scholar] [CrossRef]
- Libonati, R.; Belém, L.B.C.; Rodrigues, J.A.; Santos, F.L.M.; Sena, C.A.P.; Pinto, M.M.; Carvalho, I.A. Sistema ALARMES-Alerta da Área Queimada Pantanal, Situação Final de 2020; Laboratório de Aplicações de Satélites Ambientais—UFRJ: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Martins, P.I.; Belém, L.B.C.; Szabo, J.K.; Libonati, R.; Couto Garcia, L. Prioritizing areas for wildfire prevention and post-fire restoration in the Brazilian Pantanal. Ecol. Eng. 2022, 176, 106517. [Google Scholar] [CrossRef]
- Jankauskaite, G.; Delegido, J. Assessing fire impacts on the Pantanal wetland using Sentinel-2 imagery. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Frizzo, T.L.; Bonizario, C.; Borges, M.P.; Vasconcelos, H. Revisão dos efeitos do fogo sobre a fauna de formações savânicas do Brasil. Oecol. Aust. 2011, 15, 365–379. [Google Scholar] [CrossRef]
- Arruda, F.V.; Sousa, D.G.; Teresa, F.B.; Prado, V.H.M.; Cunha, H.F.; Izzo, T.J. Trends and gaps of the scientific literature about the effects of fire on Brazilian Cerrado. Biota Neotrop. 2018, 18, e20170426. [Google Scholar] [CrossRef]
- Berlinck, C.N.; Lima, L.H.A.; Carvalho Junior, A.R. Historical survey of research related to fire management and fauna conservation in the world and in Brazil. Biota Neotrop. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Cintra, R.; Sanaiotti, T.M. Fire effects on the composition of a bird community in an Amazonian Savanna (Brazil). Braz. J. Biol. 2005, 65, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Mestre, L.A.M.; Cochrane, M.A.; Barlow, J. Long-term changes in bird communities after wildfires in the central brazilian amazon. Biotropica 2013, 45, 480–488. [Google Scholar] [CrossRef]
- Chiarani, E.; Bettio, M.; Fontana, C.S. Temporal changes in bird communities in areas with different histories of fire disturbance in highland grasslands of Brazil. PLoS ONE 2020, 5, e0243070. [Google Scholar] [CrossRef]
- Nunes, A.P.; Posso, S.R.; Frota, A.V.B.; Vitorino, B.D.; Laps, R.R.; Donatelli, R.J.; Straube, F.C.; Pivatto, M.A.C.; Oliveira, D.M.M.; Carlos, B.; et al. Birds of the Pantanal floodplains, Brazil: Historical data, diversity, and conservation. Pap. Avulsos Zool. 2021, 61, e20216182. [Google Scholar] [CrossRef]
- Tomas, W.M.; Berlinck, C.N.; Chiaravalloti, R.M.; Faggioni, G.P.; Strussmann, C.; Libonati, R.; Abrahao, C.R.; Alvarenga, G.V.; Bacellar, A.E.F.; Batista, F.R.Q.; et al. Counting the dead: 17 million vertebrates directly killed by the 2020 wildfires in the Pantanal wetland, Brazil. Res. Sq. 2021, 1–16. [Google Scholar] [CrossRef]
- Dwyer, J.K.; Block, W.M. Effects of wildfire on densities of secondary cavity-nesting birds in ponderosa pine forests of northern Arizona. In Fire and Forest Ecology: Innovative Silviculture and Vegetation Management, No. 21; Moser, W.K., Moser, C.E., Eds.; Tall Timbers Research Station: Tallahassee, FL, USA, 2000; pp. 151–156. [Google Scholar]
- Milesi, F.A.; Marone, L.; Lopez De Casenave, J.; Cueto, V.R.; Mezquida, E.T. Management guilds as indicators of environmental conditions: A case study with birds and habitat disturbances in the central Monte desert, Argentina. Ecol. Austral 2002, 12, 149–161. [Google Scholar]
- Whittingham, M.J.; Evans, K.L. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 2004, 146, 210–220. [Google Scholar] [CrossRef]
- Schieck, J.; Song, S.J. Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: Literature review and meta-analyses. Can. J. For. Res. 2006, 36, 1299–1318. [Google Scholar] [CrossRef]
- Stojanovic, D.; Webb nee Voogdt, J.; Webb, M.; Cook, H.; Heinsohn, R. Loss of habitat for a secondary cavity nesting bird after wildfire. For. Ecol. Manag. 2016, 360, 235–241. [Google Scholar] [CrossRef]
- Doherty, T.S.; Geary, W.L.; Jolly, C.J.; Macdonald, K.J.; Miritis, V.; Watchorn, D.J.; Cherry, M.J.; Conner, L.M.; González, T.M.; Legge, S.M.; et al. Fire as a driver and mediator of predator–prey interactions. Biol. Rev. 2022, 97, 1539–1558. [Google Scholar] [CrossRef]
- dos Santos Ferreira, B.H.; Oliveira, M.R.; Rodrigues, J.A.; Fontoura, F.M.; Guedes, N.M.; Szabo, J.K.; Libonati, R.; Garcia, L.C. Wildfires jeopardise habitats of Hyacinth Macaw (Anodorhynchus hyacinthinus), a flagship species for the conservation of the brazilian Pantanal. Wetlands 2023, 43, 47. [Google Scholar] [CrossRef]
- Cintra, R.; Yamashita, C. Habitats, abundância e ocorrência das espécies de aves do Pantanal de Poconé, Mato Grosso, Brasil. Pap. Avulsos Zool. 1990, 37, 1–21. [Google Scholar]
- Figueira, J.E.C.; Cintra, R.; Viana, L.R.; Yamashita, C. Spatial and temporal patterns of bird species diversity in the Pantanal of Mato Grosso, Brazil: Implications for conservation. Braz. J. Biol. 2006, 66, 393–404. [Google Scholar] [CrossRef]
- Figueira, J.E.C.; Mourão, F.A.; Coelho, A.S. Habitat heterogeneity and climatic seasonality structure the avifauna trophic guilds in the Brazilian Pantanal wetland. Can. J. Zool. 2011, 89, 1206–1213. [Google Scholar] [CrossRef]
- Donatelli, R.J.; Eaton, D.P.; Sementili-Cardoso, G.; Vianna, R.M.; Gerotti, R.W.; Rodrigues, F.G.; Martins, R.M. Temporal and spatial variation of richness and abundance of the community of birds in the Pantanal wetlands of Nhecolândia (Mato Grosso do Sul, Brazil). Rev. Biol. Trop. 2017, 65, 1358–1380. [Google Scholar] [CrossRef]
- De Deus, F.F.; Arieira, J.; Schuchmann, K.-L.; Tissiani, A.S.O.; Marques, M.I. Avian beta-diversity in a Neotropical wetland: Effects of flooding and vegetation. Wetlands 2020, 40, 1513–1527. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalvez, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Heckman, C.W. The Pantanal of Poconé; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Catian, G.; Scremin-Dias, K.; Pott, A. Reproductive phenology of macrophyte community in response to wetland flooding cycle. Oecologia Aust. 2019, 23, 856–873. [Google Scholar] [CrossRef]
- ICV. Balanço dos Incêndios em Mato Grosso em 2020; Nota Técnica Instituto Centro de Vida: Cuiabá, Brazil, 2000; pp. 1–9. [Google Scholar]
- Silgueiro, V.; Souza, C.O.; Muller, E.O.; Silva, C.J. Dimensions of the 2020 wildfire catastrophe in the Pantanal wetland: The case of the municipality of Poconé, Mato Grosso, Brazil. Res. Soc. Dev. 2021, 10, e08101522619. [Google Scholar] [CrossRef]
- De Deus, F.F.; Burs, K.; Fieker, C.Z.; Tissiani, A.S.O.; Marques, M.I.; Schuchmann, K.-L. Mammal prevalence after the fire catastrophe in northeastern Pantanal, Brazil. Pap. Avulsos Zool. 2023, 63, e202363022. [Google Scholar] [CrossRef]
- Remsen, J.V.; Areta, J.I.; Bonaccorso, E.; Claramunt, S.; Del-Rio, G.; Jaramillo, A.; Lane, D.F.; Robbins, M.B.; Stiles, F.G.; Zimmer, K.J. A Classification of the Bird Species of South America. Museum of Natural Science, Louisiana State University, USA. Available online: http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm (accessed on 31 May 2023).
- Bibby, C.J.; Buckland, S.T. Bias of bird census results due to detectability varying with habitat. Acta Oecol. 1987, 8, 103–112. [Google Scholar]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques; Academic Press: London, UK, 2000. [Google Scholar]
- Robbins, C.S. Effect of time of day on bird activity. Stud. Avian Biol. 1981, 6, 275–286. [Google Scholar]
- Stotz, D.F.; Fitzpatrick, J.W.; Parker III, T.A.; Moskovits, D.K. Neotropical Birds: Ecology and Conservation; University of Chicago Press: Chicago, MI, USA, 1996. [Google Scholar]
- Wilman, H.; Belmaker, J.; Simpson, J.; De La Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Dunning, J.B., Jr. CRC Handbook of Avian Body Masses; CRC Press: Boca Raton, USA, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.-H.; Li, C.-F.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.-L.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblage. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Brown, A.M.; Warton, D.I.; Andrew, N.R.; Binns, M.; Cassis, G.; Gibb, H. The fourth–corner solution–using predictive models to understand how species traits interact with the environment. Methods Ecol. Evol. 2014, 5, 344–352. [Google Scholar] [CrossRef]
- Warton, D.I.; Shipley, B.; Hastie, T. CATS regression—A model–based approach to studying trait-based community assembly. Methods Ecol. Evol. 2015, 6, 389–398. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund-an R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Eddelbuettel, D.; Wilshire, J.; Warton, D. mvabund: Statistical Methods for Analysing Multivariate Abundance Data. R Package Version 4.2.1. Available online: https://CRAN.R-project.org/package=mvabund (accessed on 18 May 2022).
- Warton, D.I. Eco-Stats: Data Analysis in Ecology: From t-Tests to Multivariate Abundances; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- De Deus, F.F.; Schuchmann, K.-L.; Tissiani, A.S.O.; Nogueira, W.; Marques, M.I. Avian biodiversity assessment studies in a Neotropical wetland—The combination of sampling methods makes the difference. Pap. Avulsos Zool. 2023, 63, e202363015. [Google Scholar] [CrossRef]
- Sakar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6–4. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 December 2022).
- Kinnaird, M.F.; O’Brien, T.G. Ecological effects of wildfire on lowland rainforest in Sumatra. Conserv. Biol. 1998, 12, 954–956. Available online: http://www.jstor.org/stable/2387568 (accessed on 12 September 2023). [CrossRef]
- Libonati, R.; DaCamara, C.C.; Peres, L.F.; Sander de Carvalho, L.A.; Garcia, L.C. Rescue Brazil’s burning Pantanal wetlands. Nature 2020, 588, 217–219. [Google Scholar] [CrossRef]
- Davis, M.A.; Peterson, D.W.; Reich, P.B.; Crozier, M.; Query, T.; Mitchell, E.; Huntington, J.; Bazakas, P. Restoring savanna using fire: Impact on the breeding bird community. Restor. Ecol. 2000, 8, 30–40. [Google Scholar] [CrossRef]
- Rush, S.; Klaus, N.; Keyes, T.; Petrick, J.; Cooper, R. Fire severity has mixed benefits to breeding bird species in the southern Appalachians. For. Ecol. Manag. 2012, 263, 94–100. [Google Scholar] [CrossRef]
- Mardiastuti, A. Response and impact of fire on bird community in the tropical rainforest: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 504, 012001. [Google Scholar] [CrossRef]
- De Deus, F.F.; Schuchmann, K.-L. Temporal Dynamics in Pantanal hummingbird assemblages is triggered by flood pulse. Ornitol. Neotrop. 2023, 34, 71–77. [Google Scholar] [CrossRef]
- Tunes, P.; Alves, V.N.; Valentin-Silva, A.; Batalha, M.A.; Guimarães, E. Does fire affect the temporal pattern of trophic resource supply to pollinators and seed-dispersing frugivores in a Brazilian savanna community? Plant Ecol. 2016, 218, 345–357. [Google Scholar] [CrossRef]
- Recher, H.; Allen, D.; Gowing, G. The impact of wildfire on birds in an intensively logged forest. In Birds of Eucalypt Forests and Woodlands: Ecology, Conservation, Management; Keast, A., Recher, H., Ford, H., Saunders, D., Eds.; Surrey Beatty in association with the Royal Australasian Ornithologists Union: Chipping Norton, Australia, 1985; pp. 283–290. [Google Scholar]
- Valentine, L.E.; Schwarzkopf, L.; Johnson, C.N.; Grice, A.C. Burning season influences the response of bird assemblages to fire in tropical savannas. Biol. Conserv. 2007, 137, 90–101. [Google Scholar] [CrossRef]
- Prada, M.; Marini-Filho, O.J.; Price, P.W. Insects in flower heads of Aspilia foliacea (Asteraceae) after a fire in a central Brazilian savanna: Evidence for the plant vigor hypothesis. Biotropica 1995, 27, 513–518. [Google Scholar] [CrossRef]
- Lepesqueur, C.; Morais, H.C.; Diniz, I.R. Accidental fire in the Cerrado: Its impact on communities of caterpillars on two species of Erythroxylum. Psyche 2012, 2012, 101767. [Google Scholar] [CrossRef]
- Canedo-Júnior, E.O.; Gonçalves Cuissi, R.; de Almeida Curi, N.H.; Ramos Demetrio, G.; Lasmar, C.J.; Malves, K.; Rodrigues Ribas, C. Can anthropic fires affect epigaeic and hypogaeic Cerrado ant (Hymenoptera: Formicidae) communities in the same way? Rev. Biol. Trop. 2016, 64, 95–104. [Google Scholar] [CrossRef]
- Cunha, H.F.; Ramalho, W.P.; Dias, A.M.; Peixoto, B.R.; Jesus, G.S.; Oliveira, J.P.; Silva, T.M.P. Post-fire recovery of arthropod assemblage in an area of Brazilian savanna. EntomoBrasilis 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Durigan, G.; Pilon, N.A.L.; Abreu, R.C.R.; Hoffmann, W.A.; Martins, M.; Fiorillo, B.F.; Antunes, A.Z.; Carmignotto, A.P.; Maravalhas, J.B.; Vieira, J.; et al. No net loss of species diversity after prescribed fires in the Brazilian savanna. Front. For. Glob. Change 2020, 3, 13. [Google Scholar] [CrossRef]
- Gonçalves, T.F.; Correa, C.M.; Audino, L.D.; Vaz-de-Mello, F.Z.; Fontoura, F.M.; Guedes, N.M. Quantifying the post-fire recovery of taxonomic and functional diversity of dung beetles in the Brazilian Pantanal. Ecol. Entomol. 2022, 47, 601–612. [Google Scholar] [CrossRef]
- Vieira, E.M.; Andrade, I.; Price, P.W. Fire effects on a Palicourea rigida (Rubiaceae) gall midge: A test of the plant vigor hypothesis. Biotropica 1996, 28, 210–217. [Google Scholar] [CrossRef]
- Alves-Silva, E. Post fire resprouting of Banisteriopsis malifolia (Malpighiaceae) and the role of extrafloral nectaries on the associated ant fauna in a Brazilian savanna. Sociobiology 2011, 58, 327–339. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr. Cinereous-breasted Spinetail (Synallaxis hypospodia). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Kirwan, G.M.; Farnsworth, A.; del Hoyo, J.; Lebbin, D.J.; Collar, N.; Boesman, P.F.D. Bran-colored Flycatcher (Myiophobus fasciatus). In Birds of the World; Billerman, S.M., Sly, N.D., Eds.; Version 2.1; Cornell Lab of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar] [CrossRef]
- Fitzpatrick, J.W. Plain Tyrannulet (Inezia inornata). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Fraga, R. Scarlet-headed Blackbird (Amblyramphus holosericeus). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Taylor, B. Russet-crowned Crake (Rufirallus viridis). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.1; Cornell Lab of Ornithology: Ithaca, NY, USA, 2023. [Google Scholar] [CrossRef]
- Kroodsma, D.E.; Brewer, D. Moustached Wren (Pheugopedius genibarbis). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Schulenberg, T.S.; Kirwan, G.M. Band-tailed Antbird (Hypocnemoides maculicauda). In Birds of the World; Schulenberg, T.S., Ed.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr. Rufous Cacholote (Pseudoseisura unirufa). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Marantz, C.A.; del Hoyo, J.; Collar, N.; Aleixo, A.; Bevier, L.R.; Kirwan, G.M.; Patten, M.A. Buff-throated Woodcreeper (Xiphorhynchus guttatus). In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Version 1.0; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species, Version 2022-2. Available online: https://www.iucnredlist.org. (accessed on 13 September 2023).
- Şekericoğlu, C.H.; Daily, G.C.; Ehrlich, P.R. Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. USA 2004, 101, 18042–18047. [Google Scholar] [CrossRef] [PubMed]
- Şekericoğlu, C.H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Junk, W.J.; da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Damasceno-Junior, G.A.; Pereira, A.M.M.; Oldeland, J.; Parolin, P.; Pott, A. Fire, flood and Pantanal vegetation. In Flora and Vegetation of the Pantanal Wetland, Plant and Vegetation; Damasceno-Junior, G.A., Pott, A., Eds.; Springer: Berlin, Germany, 2021; pp. 661–688. [Google Scholar]

| Sample Completeness Profiles (Figure 2a) | |||
| Completeness | q = 0 | q = 1 | q = 2 |
| Unburned areas | 76.6% (+/−0.12) | 96.3% (+/−0.01) | 99.8% (+/−0.001) |
| Burned areas | 85.1% (+/−0.11) | 97.0% (+/−0.01) | 99.8% (+/−0.001) |
| Asymptotic analysis (Figure 2b,c) | |||
| Diversity | q = 0 | q = 1 | q = 2 |
| Unburned areas | |||
| Asymptotic | 189.2 (+/−33.8) | 87.9 (+/−5.5) | 59.5 (+/−4.7) |
| Empirical | 145.0 (+/−8.9) | 80.2 (+/−4.7) | 56.6 (+/−4.2) |
| Undetected | 44.2 | 7.7 | 2.9 |
| Burned areas | |||
| Asymptotic | 151.7 (+/−24.2) | 77.2 (+/−4.4) | 55.6 (+/−3.8) |
| Empirical | 129.0 (+/−7.7) | 71.5 (+/−4.1) | 52.9 (+/−3.7) |
| Undetected | 22.7 | 5.7 | 2.7 |
| Non-asymptotic coverage-based rarefaction and extrapolation (Figure 2d) | |||
| Maximum standardized coverage Cmax = 98.6% | |||
| Diversity | q = 0 | q = 1 | q = 2 |
| Unburned areas | 171.7 (+25.0/−24.0) | 84.8 (+5.4/−5.5) | 58.0 (+4.4/−4.5) |
| Burned areas | 140.9 (+/−18.0) | 73.7 (+/−4.3) | 53.8 (+/−3.6) |
| Evenness among species abundances (Figure 2e) | |||
| Diversity | Pielou J’ | q = 1 | q = 2 |
| Unburned areas | 0.86 | 0.49 (+/−0.05) | 0.33 (+/−0.04) |
| Burned areas | 0.87 | 0.52 (+/−0.06) | 0.38 (+/−0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuchmann, K.-L.; Burs, K.; de Deus, F.; Fieker, C.Z.; Tissiani, A.S.; Marques, M.I. Bird Community Traits in Recently Burned and Unburned Parts of the Northeastern Pantanal, Brazil: A Preliminary Approach. Sustainability 2024, 16, 2321. https://doi.org/10.3390/su16062321
Schuchmann K-L, Burs K, de Deus F, Fieker CZ, Tissiani AS, Marques MI. Bird Community Traits in Recently Burned and Unburned Parts of the Northeastern Pantanal, Brazil: A Preliminary Approach. Sustainability. 2024; 16(6):2321. https://doi.org/10.3390/su16062321
Chicago/Turabian StyleSchuchmann, Karl-L., Kathrin Burs, Filipe de Deus, Carolline Zatta Fieker, Ana Silvia Tissiani, and Marinêz I. Marques. 2024. "Bird Community Traits in Recently Burned and Unburned Parts of the Northeastern Pantanal, Brazil: A Preliminary Approach" Sustainability 16, no. 6: 2321. https://doi.org/10.3390/su16062321






