Hydrolases Control Soil Carbon Sequestration in Alpine Grasslands in the Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
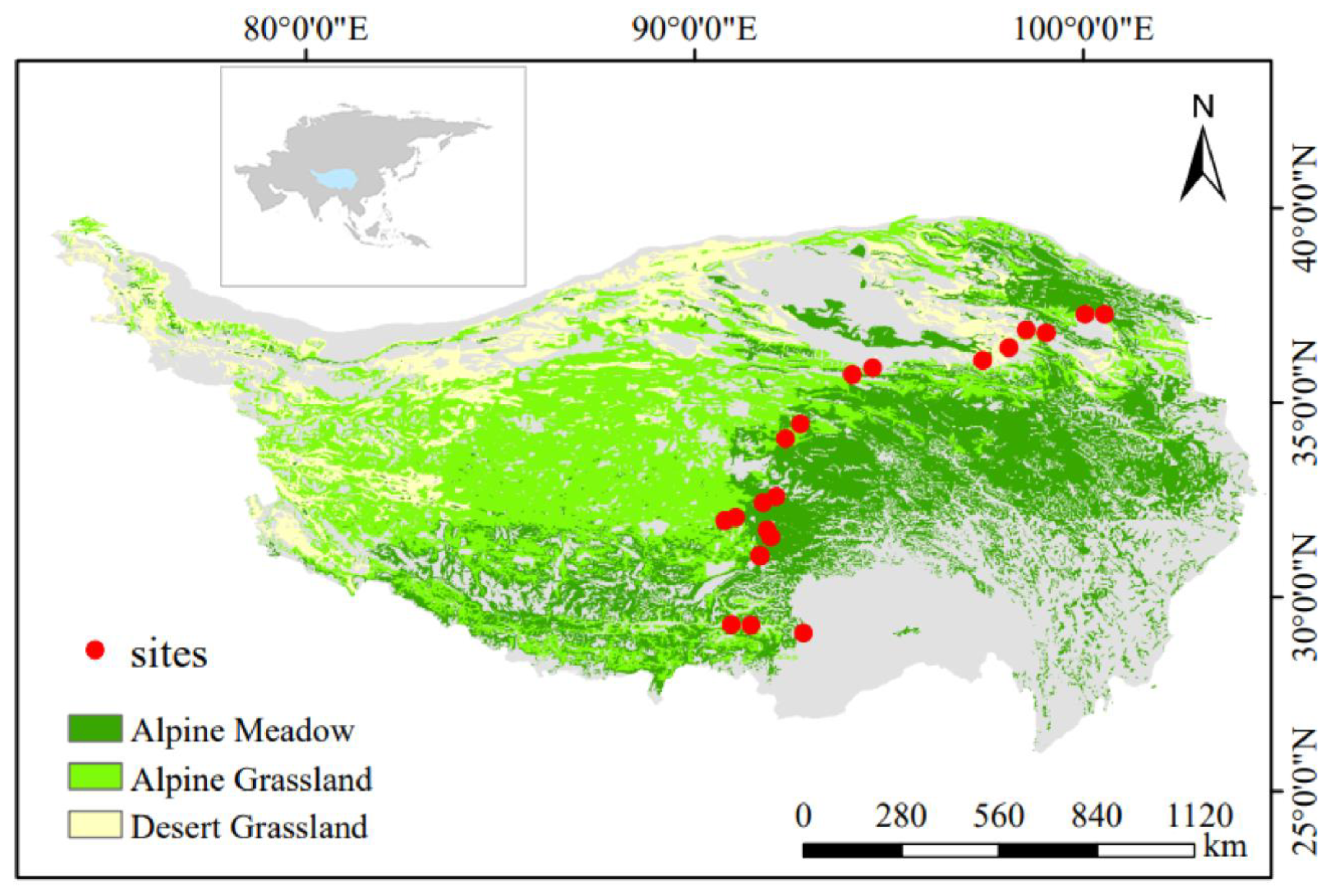
2.1. Study Area
2.2. Plant Surveys and Soil Sampling
2.3. Soil Properties
2.4. Determination of Soil Extracellular Enzymes
2.5. Amino Sugar Analysis
2.6. Parameters Related to Soil Microbial Source Carbon
2.7. Statistical Analysis
3. Results
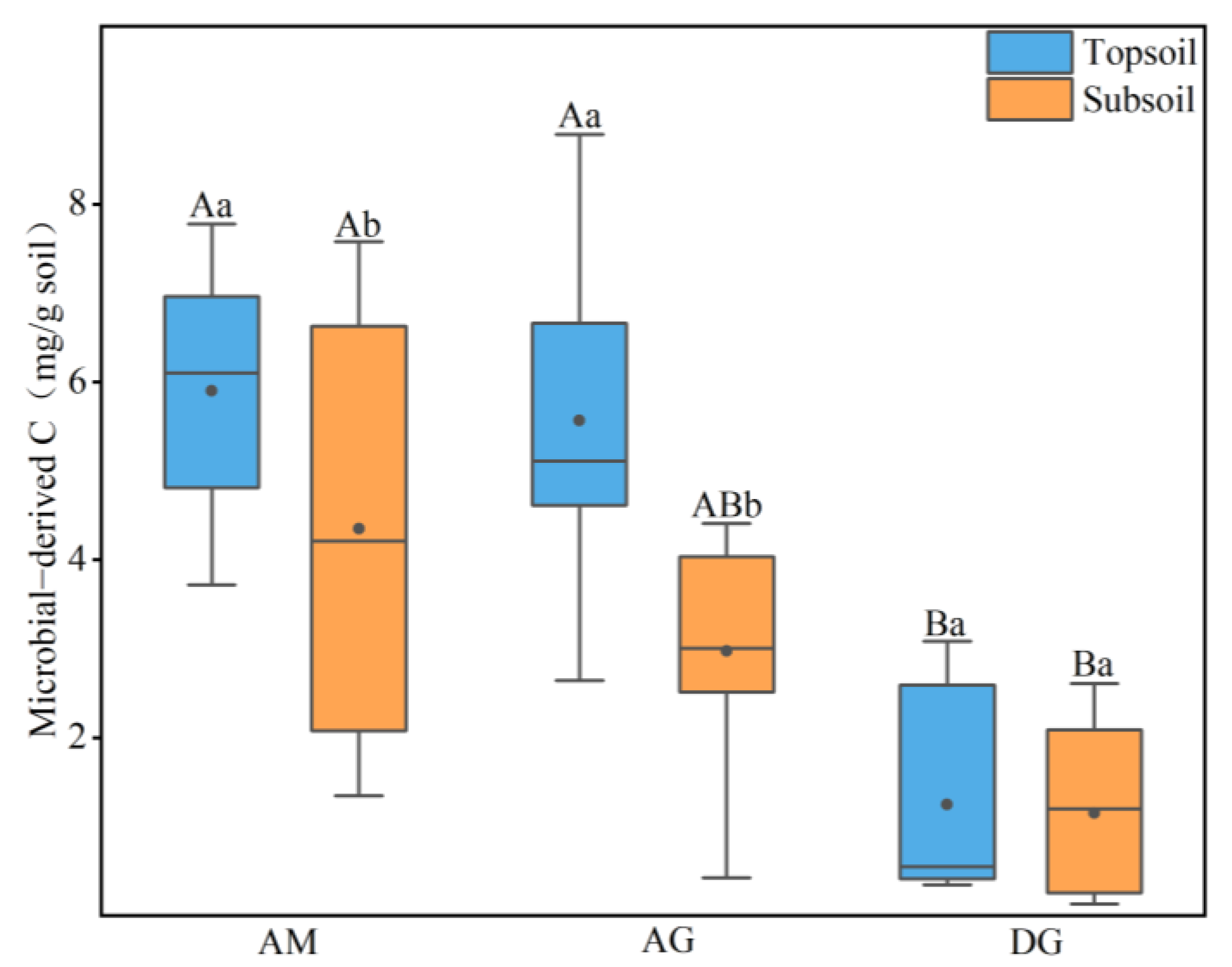
3.1. Dynamics of Amino Sugar in Soil
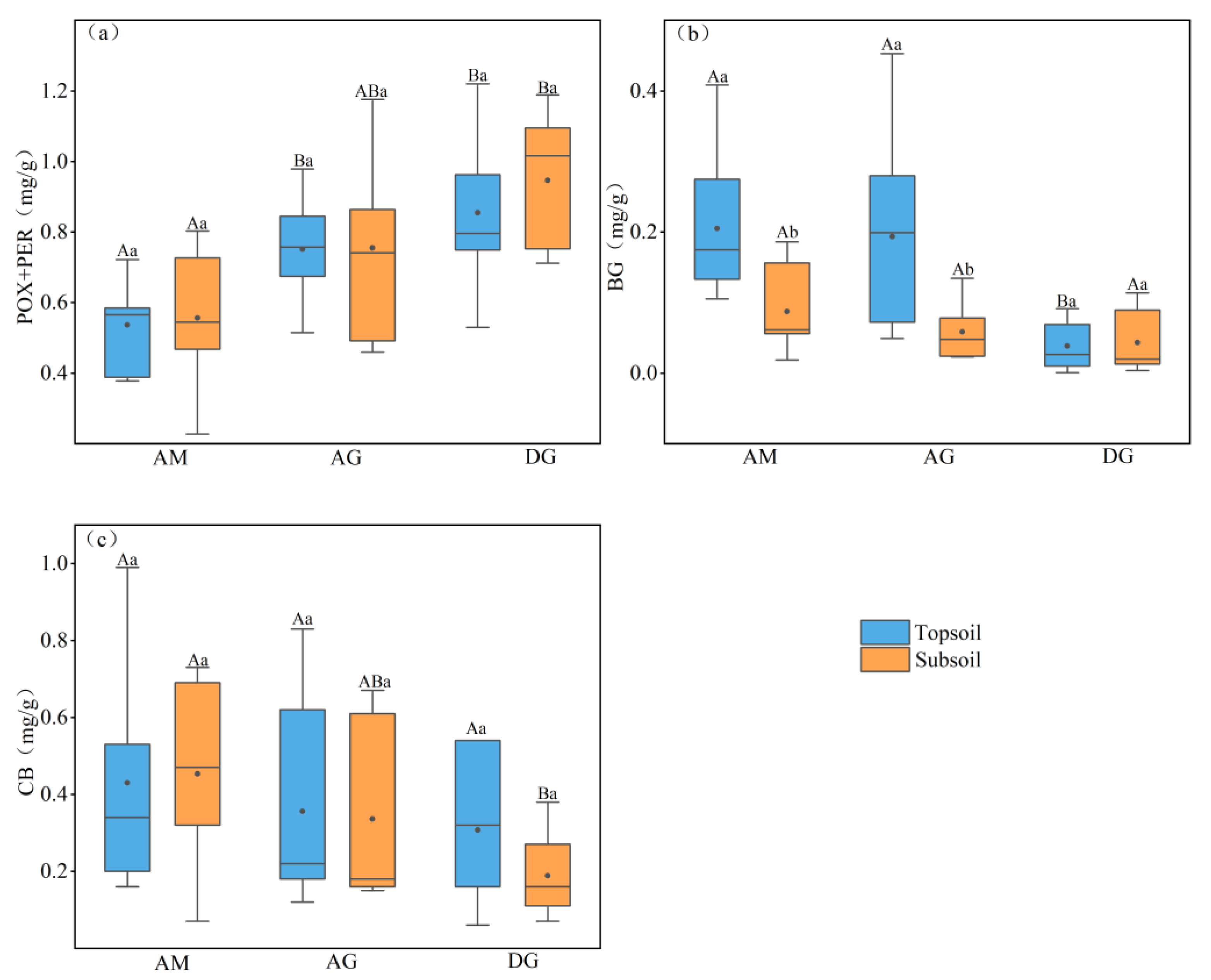
3.2. Soil Extracellular Enzyme
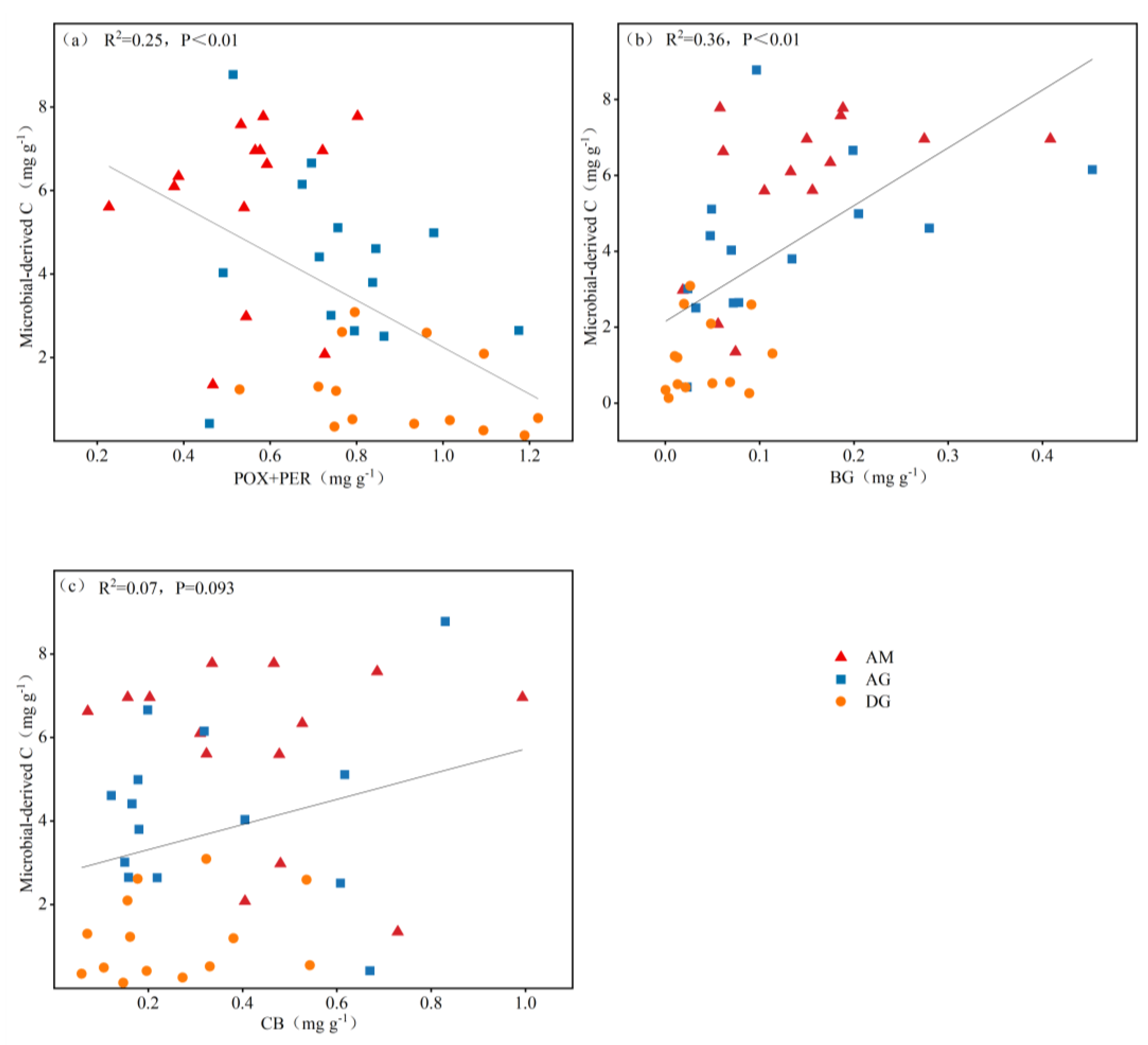
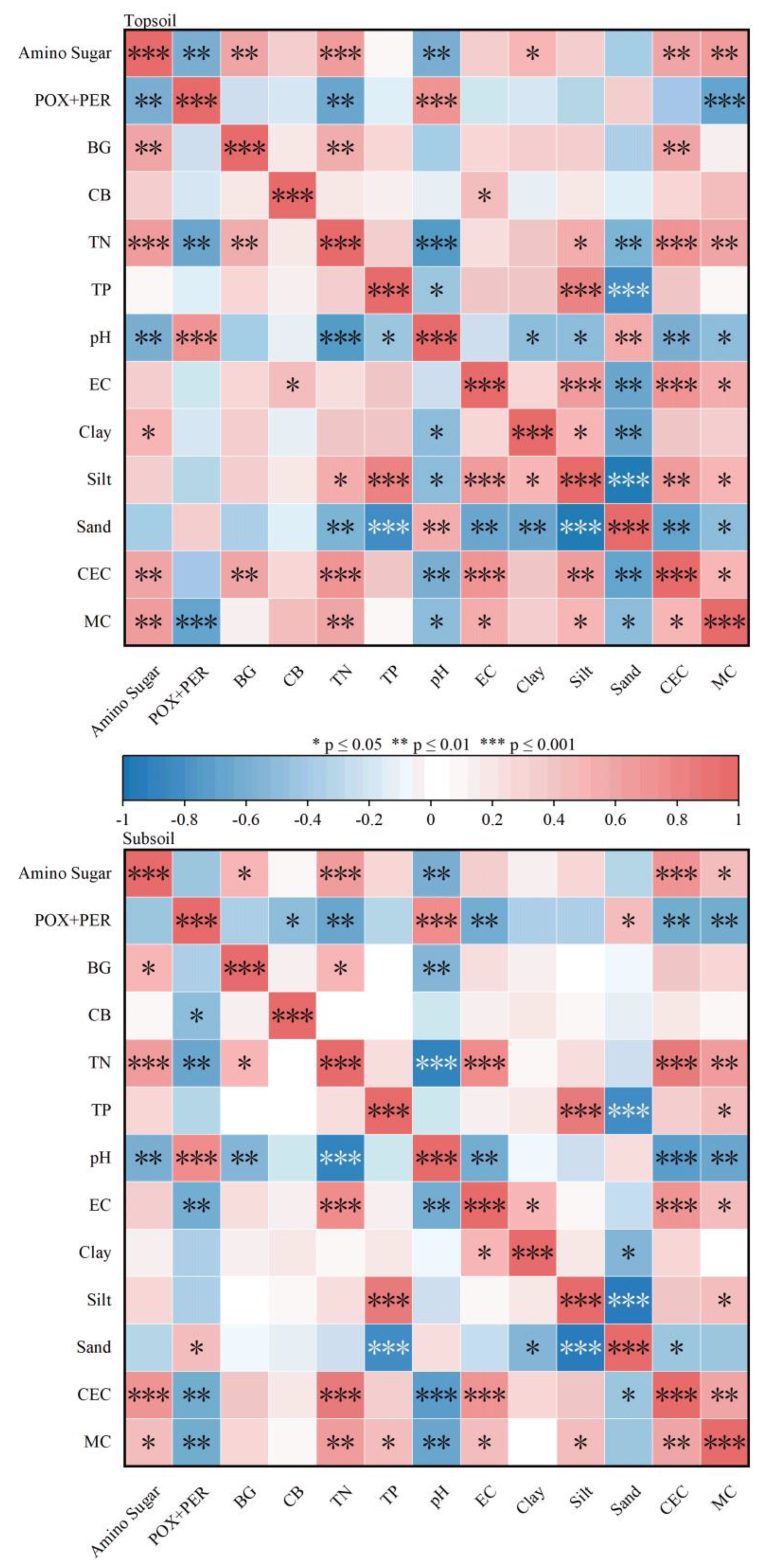
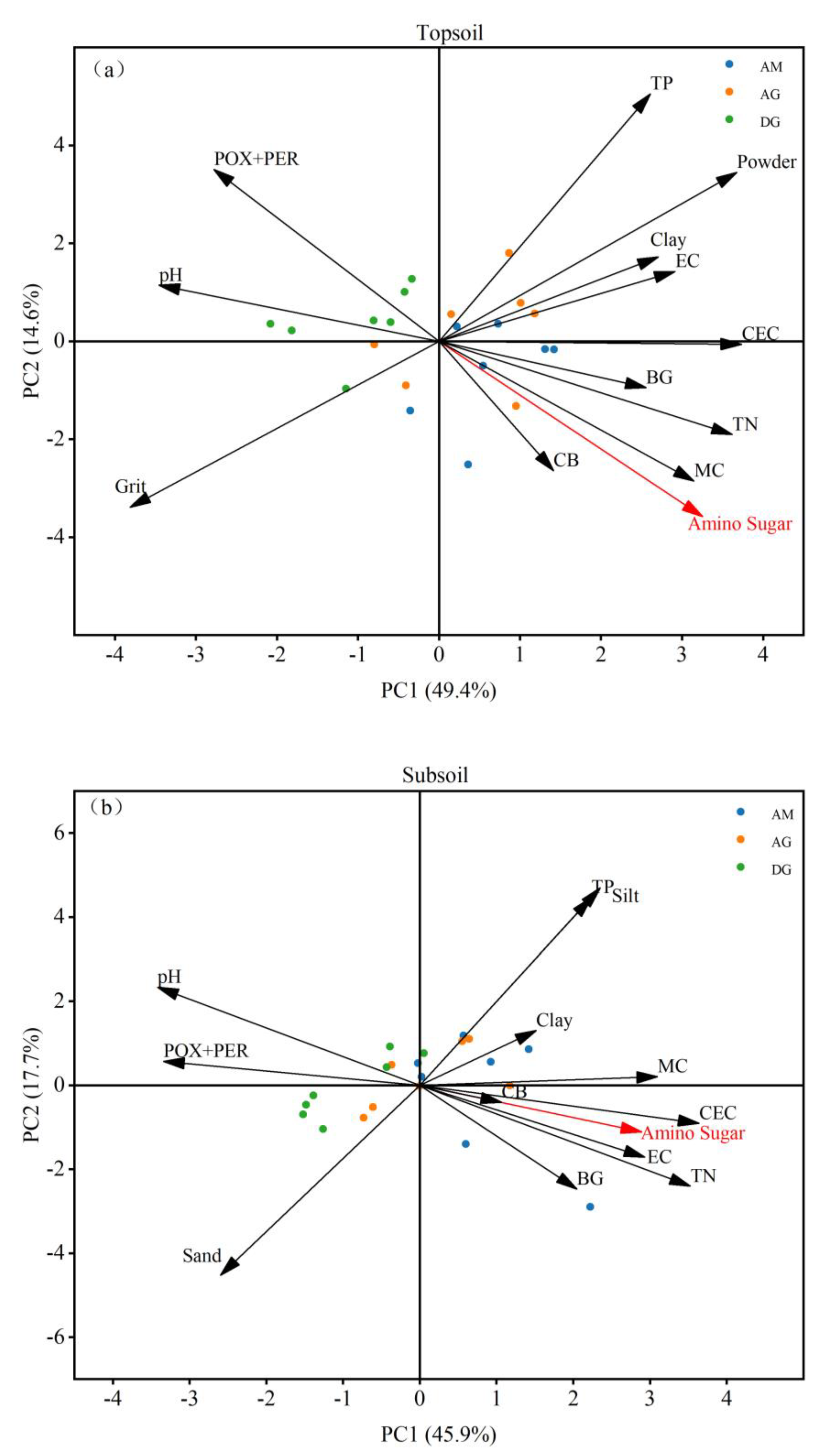
3.3. Influence of Soil Physical and Chemical Properties on Extracellular Enzyme Activities of Amino Sugars and Soil Enzymes
4. Discussion
4.1. Influence of Different Grassland Types and Soil Depth on Soil Extracellular Enzymes and Amino Sugar Content
4.2. Effect of Different Soil Extracellular Enzymes on Amino Sugars
4.3. Influence of Basic Physical and Chemical Properties of Soil on the Accumulation of Extracellular Enzymes and Amino Sugar Content in Soil
4.4. Analysis of Soil Carbon Sequestration Mechanism in Alpine Grassland
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgiou, K.; Jackson, R.B.; Vinduskova, O.; Abramoff, R.Z.; Ahlstrom, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Gerke, J. The Central Role of Soil Organic Matter in Soil Fertility and Carbon Storage. Soil Syst. 2022, 6, 33. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Wang, Y.; Liang, C.; An, S.; Soromotin, A.; Kuzyakov, Y. Increasing contribution of microbial residues to soil organic carbon in grassland restoration chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Liang, C. Soil microbial carbon pump: Mechanism and appraisal. Soil Ecol. Lett. 2020, 2, 241–254. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R.-w. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Xue, Y.; Chen, D.; Chen, F.; Thompson, L.; Cui, P.; Koike, T.; Lau, W.K.M.; Lettenmaier, D.; Mosbrugger, V.; et al. Recent Third Pole’s Rapid Warming Accompanies Cryospheric Melt and Water Cycle Intensification and Interactions between Monsoon and Environment: Multidisciplinary Approach with Observations, Modeling, and Analysis. Bull. Amer. Meteorol. Soc. 2019, 100, 423–444. [Google Scholar] [CrossRef]
- Chen, H.; Ju, P.; Zhu, Q.; Xu, X.; Wu, N.; Gao, Y.; Feng, X.; Tian, J.; Niu, S.; Zhang, Y.; et al. Carbon and nitrogen cycling on the Qinghai-Tibetan Plateau. Nat. Rev. Earth Environ. 2022, 3, 701–716. [Google Scholar] [CrossRef]
- Liu, S.; Zamanian, K.; Schleuss, P.-M.; Zarebanadkouki, M.; Kuzyakov, Y. Degradation of Tibetan grasslands: Consequences for carbon and nutrient cycles. Agric. Ecosyst. Environ. 2018, 252, 93–104. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, W.; Xue, K.; Wang, S.; Zhang, L.; Hu, R.; Zeng, H.; Xu, X.; Li, Y.; Jiang, L.; et al. Grassland changes and adaptive management on the Qinghai-Tibetan Plateau. Nat. Rev. Earth Environ. 2022, 3, 668–683. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhang, J.; Zhao, M.; He, N. Changes in Soil Particulate Organic Carbon and Their Response to Changing Environments on the Tibetan Plateau, Mongolian Plateau, and Loess Plateau, China. J. Soil Sci. Plant Nutr. 2023, 23, 420–430. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Ma, T.; Huang, L.; Pu, Q.; Hao, M.; Zhang, X. Distribution and mineralization of organic carbon and nitrogen in forest soils of the southern Tibetan Plateau. CATENA 2017, 156, 298–304. [Google Scholar] [CrossRef]
- Yang, R.-M.; Zhang, G.-L.; Yang, F.; Zhi, J.-J.; Yang, F.; Liu, F.; Zhao, Y.-G.; Li, D.-C. Precise estimation of soil organic carbon stocks in the northeast Tibetan Plateau. Sci. Rep. 2016, 6, 21842. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, S.; Jiang, N.; Sun, J.; Cao, R.; Ling, X.; Fang, B.; Zhang, L.; Zhang, L.; Xu, X.; et al. Plant phenology changes and drivers on the Qinghai-Tibetan Plateau. Nat. Rev. Earth Environ. 2022, 3, 633–651. [Google Scholar] [CrossRef]
- Xia, M.; Jia, K.; Zhao, W.; Liu, S.; Wei, X.; Wang, B. Spatio-temporal changes of ecological vulnerability across the Qinghai-Tibetan Plateau. Ecol. Indic. 2021, 123, 107274. [Google Scholar] [CrossRef]
- Tang, L.; Dong, S.; Sherman, R.; Liu, S.; Liu, Q.; Wang, X.; Su, X.; Zhang, Y.; Li, Y.; Wu, Y.; et al. Changes in vegetation composition and plant diversity with rangeland degradation in the alpine region of Qinghai-Tibet Plateau. Rangel. J. 2015, 37, 107–115. [Google Scholar] [CrossRef]
- Tian, P.; Lu, H.; Feng, W.; Guan, Y.; Xue, Y. Large decrease in streamflow and sediment load of Qinghai-Tibetan Plateau driven by future climate change: A case study in Lhasa River Basin. CATENA 2020, 187, 104340. [Google Scholar] [CrossRef]
- Ding, J.; Chen, L.; Zhang, B.; Liu, L.; Yang, G.; Fang, K.; Chen, Y.; Li, F.; Kou, D.; Ji, C.; et al. Linking temperature sensitivity of soil CO2 release to substrate, environmental, and microbial properties across alpine ecosystems. Glob. Biogeochem. Cycles 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Shimizu, M.; Wariishi, H. Development of a sample preparation method for fungal proteomics. FEMS Microbiol. Lett. 2005, 247, 17–22. [Google Scholar] [CrossRef]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. C. R. Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.; Winsley, T.; Ferrari, B.C.; Siciliano, S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Chaudhari, S.K.; Singh, R.; Kundu, D.K. Rapid textural analysis for saline and alkaline soils with different physical and chemical properties. Soil Sci. Soc. Am. J. 2008, 72, 431–441. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013, 81, e50961. [Google Scholar] [CrossRef]
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Ma, T.; Dai, G.; Zhu, S.; Chen, D.; Chen, L.; Lu, X.; Wang, X.; Zhu, J.; Zhang, Y.; Ma, W.; et al. Distribution and Preservation of Root- and Shoot-Derived Carbon Components in Soils Across the Chinese-Mongolian Grasslands. J. Geophys. Res.-Biogeosci. 2019, 124, 420–431. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Chang. Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Hu, J.; Huang, C.; Zhou, S.; Liu, X.; Dijkstra, F.A. Nitrogen addition increases microbial necromass in croplands and bacterial necromass in forests: A global meta-analysis. Soil Biol. Biochem. 2022, 165, 108500. [Google Scholar] [CrossRef]
- Mellado-Vazquez, P.G.; Lange, M.; Bachmann, D.; Gockele, A.; Karlowsky, S.; Milcu, A.; Piel, C.; Roscher, C.; Roy, J.; Gleixner, G. Plant diversity generates enhanced soil microbial access to recently photosynthesized carbon in the rhizosphere. Soil Biol. Biochem. 2016, 94, 122–132. [Google Scholar] [CrossRef]
- Jia, Y.; Zhai, G.; Zhu, S.; Liu, X.; Schmid, B.; Wang, Z.; Ma, K.; Feng, X. Plant and microbial pathways driving plant diversity effects on soil carbon accumulation in subtropical forest. Soil Biol. Biochem. 2021, 161, 108375. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, Y.; Hu, S.; Chang, S.X. Plant mixture effects on carbon-degrading enzymes promote soil organic carbon accumulation. Soil Biol. Biochem. 2021, 163, 108457. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Erdel, E.; Simsek, U.; Kesimci, T.G. Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Turkiye. Sustainability 2023, 15, 1765. [Google Scholar] [CrossRef]
- Kim, J.I.; Yang, Y.; Kang, H. Fluorometric assay for phenol oxidase activity in soils and its controlling variables. Appl. Soil Ecol. 2024, 195, 105240. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Z.; Liu, Y.; Luo, Y.; Deng, Y.; Xu, X.; Liu, S.; Richter, A.; Shibistova, O.; Guggenberger, G.; et al. C:N:P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol. Fertil. Soils 2020, 56, 1093–1107. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Austin, A.T.; Cleland, E.E.; Frey, S.D.; Houlton, B.Z.; Wallenstein, M.D. Responses and feedbacks of coupled biogeochemical cycles to climate change: Examples from terrestrial ecosystems. Front. Ecol. Environ. 2011, 9, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, E.; Cao, Z.; Jia, J.; Liu, C.; Zhang, Z.; Wang, H.; Dai, G.; He, J.-S.; Feng, X. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Change Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef]
- Fang, K.; Kou, D.; Wang, G.; Chen, L.; Ding, J.; Li, F.; Yang, G.; Qin, S.; Liu, L.; Zhang, Q.; et al. Decreased Soil Cation Exchange Capacity Across Northern China’s Grasslands Over the Last Three Decades. J. Geophys. Res.-Biogeosci. 2017, 122, 3088–3097. [Google Scholar] [CrossRef]
- Hall, S.J.; Ye, C.; Weintraub, S.R.; Hockaday, W.C. Molecular trade-offs in soil organic carbon composition at continental scale. Nat. Geosci. 2020, 13, 687. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yuan, M.; Jian, S.; Gamage, L.; Parajuli, M.; Dzantor, K.E.; Hui, D.; Fay, P.A.; Li, J. Soil extracellular oxidases mediated nitrogen fertilization effects on soil organic carbon sequestration in bioenergy croplands. GCB Bioenergy 2021, 13, 1303–1318. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Van Horn, D.J.; Shah, J.J.F.; Findlay, S. Ecoenzymatic Stoichiometry in Relation to Productivity for Freshwater Biofilm and Plankton Communities. Microb. Ecol. 2010, 60, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.; Ma, Y.; Hu, B.; Yang, Y.; Tian, H.; Liu, X.; Meng, N.; Zhu, J.; Yan, D.; et al. The hidden risk: Changes in functional potentials of microbial keystone taxa under global climate change jeopardizing soil carbon storage in alpine grasslands. Environ. Int. 2024, 185, 108516. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Du, X.; Li, Y.; Wang, Z.; Jiang, S.; Li, Q. Effect of grassland degradation on soil quality and soil biotic community in a semi-arid temperate steppe. Ecol. Process. 2020, 9, 63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, X.; Sun, Y.; Wu, J.; Deng, T.; Yuan, M.; Duan, W.; Zhao, Y. Hydrolases Control Soil Carbon Sequestration in Alpine Grasslands in the Tibetan Plateau. Sustainability 2024, 16, 3508. https://doi.org/10.3390/su16093508
Zhang Y, Wang X, Sun Y, Wu J, Deng T, Yuan M, Duan W, Zhao Y. Hydrolases Control Soil Carbon Sequestration in Alpine Grasslands in the Tibetan Plateau. Sustainability. 2024; 16(9):3508. https://doi.org/10.3390/su16093508
Chicago/Turabian StyleZhang, Yuanye, Xia Wang, Yuxin Sun, Jinhong Wu, Tao Deng, Menghan Yuan, Wenhui Duan, and Yunfei Zhao. 2024. "Hydrolases Control Soil Carbon Sequestration in Alpine Grasslands in the Tibetan Plateau" Sustainability 16, no. 9: 3508. https://doi.org/10.3390/su16093508




