Human Consumption of Non-Native Species in a Circular Economy: Determination of Persistent Organic Pollutants in the Invasive Signal Crayfish from a Baltic Coastal River and Its Assessment for Consumption
Abstract
:1. Introduction
- (1)
- to determine concentrations of persistent organic pollutants (PAHs, as above, and PCBs: 28, 52, 101, 118, 138, 156, 180) in the signal crayfish,
- (2)
- (3)
- to evaluate hazard quotients (HQ),
- (4)
- to calculate consumption limits for carcinogenic and noncarcinogenic health effects.
2. Materials and Methods
2.1. Sampling Strategy
2.2. Characteristics of the Study Area
2.3. Laboratory Analysis
2.3.1. Preparation of the Material for Analysis
2.3.2. Sample Processing and Analysis
2.4. Health Risk Assessment of Consumption of Crayfish
2.4.1. Hazard Quotient (HQ)
- RfD = oral reference dose (mg kg−1 day−1)
- ADD = average daily dose (mg kg−1 day−1)
- BM = consumer body mass (70 kg)
- Cm = measured concentration of chemical contaminant in fish (mg kg−1)
- CR = mean daily crayfish consumption rate (0.05 kg day−1)
2.4.2. Risk-Based Consumption Limits
- CRlim = maximum allowable crayfish consumption rate (kg d−1)
- ARL = maximum acceptable individual lifetime risk level (unit-less)
- BM = consumer body mass (70 kg)
- Cm = measured concentration of chemical contaminant in crayfish (mg kg−1)
- CSF = cancer slope factor (mg kg−1 day−1)
- RfD = oral reference dose (mg kg−1 day−1).
2.5. Statistical Analysis
3. Results
3.1. Persistent Organic Pollutants (PAHs and PCBs) in the Signal Crayfish
3.2. Human Health Risk Assessment Caused by Consumption of the Signal Crayfish from Different Sections of the River Wieprza and Its Tributary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. What is a Circular Economy? 2023. Available online: https://www.epa.gov/circulareconomy/what-circular-economy (accessed on 20 February 2024).
- Di Vaio, A.; Hasan, S.; Palladino, R.; Hassan, R. The transition towards circular economy and waste within accounting and accountability models: A systematic literature review and conceptual framework. Environ. Dev. Sustain. 2023, 25, 734–810. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species; European Parliament: Strasbourg, France, 2014. [Google Scholar]
- Lozan, J.L. On the threat to the European crayfish: A contribution with the study of the activity behaviour of four crayfish species (Decapoda: Astacidae). Limnologica 2000, 30, 156–161. [Google Scholar] [CrossRef]
- Holdich, D.M. A review of astaculture—Freshwater crayfish farming. Aquat. Living Resour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- Ackefors, H.E.G. Freshwater crayfish farming technology in the 1990s: European and global perspective. Fish Fish. 2000, 1, 337–359. [Google Scholar] [CrossRef]
- Krzywosz, T.; Chybowski, Ł.; Ulikowski, Ł. Rak sygnałowy w Polsce—Historia, stan obecny, perspektywy. Komun. Ryb. 1995, 24, 5–8. [Google Scholar]
- Holdich, D.M.; Harlioglu, M.M.; Firkins, I. Salinity adaptations of crayfish in British waters with particular references to Austropotamobius pallipes, Astacus leptodactylus and Pacifastacus leniusculus. Estuar. Coast. Shelf. Sci. 1997, 44, 147–154. [Google Scholar] [CrossRef]
- Rutledge, P.S.; Pritchard, A.W. Scope of activity in the crayfish Pacifastacus leniusculus. Am. J. Physiol. 1981, 240, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Holdich, D.M. The dangers of introducing alien animals with particular reference to crayfish. Freshw. Crayfish 1998, 7, 15–30. [Google Scholar]
- Śmietana, P.; Krzywosz, T. Determination of the rate of growth of Pacifastacus leniusculus, in Lake Pobłędzie using polymodal length frequency distribution analysis. Bull. Fr. Peche Piscic. 2006, 380–381, 1229–1244. [Google Scholar] [CrossRef]
- Heese, T. Nowe stanowisko raka sygnałowego w wodach otwartych—Dolna Wieprza. (New locality of the signal crayfish in open waters- downstream section of the Wieprza river). Przegląd Ryb. 2013, 1, 3–5. (In Polish) [Google Scholar]
- Dobrzycka-Krahel, A.; Skóra, M.; Raczyński, M.; Szaniawska, A. Signal crayfish Pacifastacus leniusculus—Distribution and invasion in the southern Baltic coastal river. Pol. J. Ecol. 2017, 65, 444–450. [Google Scholar] [CrossRef]
- CBD. United Nations Convention on Biological Diversity; Secretariat for the Convention on Biological Diversity: Montreal, QC, Canada, 1992. [Google Scholar]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Campbell, B.M.; Vermeulen, S.J.; Aggarwal, P.K.; Corner-Dolloff, C.; Girvetz, E.; Loboguerrero, A.M.; Ramirez-Villegas, J.; Rosenstock, T.; Sebastian, L.; Thornton, P.; et al. Reducing risks to food security from climate change. Glob. Food Sec. 2016, 11, 34–43. [Google Scholar] [CrossRef]
- Ilbironke, S.I.; Adepeju, A.B.; Otutu, O.; Oyedele, D.S.; Esan, Y.O. Nutritional evaluation and comparison study of seafood such as fish and crayfish suplement dietary. MOJ Food Process. Technol. 2018, 6, 73–76. [Google Scholar] [CrossRef]
- Peng, Q.; Nunes, L.M.; Greenfield, B.K.; Dang, F.; Zhong, H. Are Chinese consumers at risk due to exposure to metals in crayfish? A bioaccessibility—Adjusted probabilistic risk assessment. Environ. Int. 2016, 88, 261–268. [Google Scholar] [CrossRef]
- FAO. ASFIS List of Species for Fishery Statistics Purposes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Nędzarek, A.; Czerniejewski, P.; Tórz, A. Macroelements and trace elements in invasive signal crayfish (Pacifastacus leniusculus) from the Wieprza River (southern Baltic): Human health implications. Biol. Trace Elem. Res. 2019, 197, 304–315. [Google Scholar] [CrossRef]
- Buccini, J. The development of a global treaty on persistent organic pollutants (POPs). In The Handbook of Environmental Chemistry, per Persistent Organic Pollutants, 3rd ed.; Fiedler, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Ritter, L.; Solomon, K.R.; Forget, J.; Stemeroff, M.; O’Leary, C. Persistent Organic Pollutants; UNEP Chemicals: Geneva, Switzerland, 2007; p. 43. [Google Scholar]
- Vallack, H.W.; Bakker, D.J.; Brandt, I.; Broström-Lunden, E.; Brouwer, A.; Bull, K.R.; Gough, C.; Guardans, R.; Holoubek, I.; Jansson, B.; et al. Controlling persistent organic pollutants—What next? Environ. Toxicol. Pharmacol. 1998, 6, 143–175. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Parol, J.; Kubiak, M. Porównanie zawartości wielopierścieniowych węglowodorów aromatycznych (WWA) w wędzonych tradycyjnie rybach słodkowodnych. Content comparison of polycyclic aromatic hydrocarbons (PAHs) in traditionally smoked freshwater fish. Nauka Przyr. Technol. 2015, 9, 1–9. [Google Scholar] [CrossRef]
- Sapota, G.; Szaniawska, A.; Normant, M. Contamination by persistent organic pollutants of invasive species from the Baltic Sea region. Ocean. Hydrobiol. Stud. 2005, 34, 239–248. [Google Scholar]
- Windsor, F.M.; Pereira, M.G.; Tyler, C.R.; Ormerod, S.J. River organisms as indicators of the distribution and sources of persistent organic pollutants in contrasting catchments. Environ. Pollut. 2019, 255, 113144. [Google Scholar] [CrossRef]
- Scientific Committee on Food (SCF). Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. SCF/CS/CNTM/PAH/29 Final; Directorate C—Scientific Opinions; European Commission, Health and Consumer Protection Directorate-General: Brussels, Belgium, 2002. [Google Scholar]
- EU. Commission Recommendation (2005/108/EC) of 4 February 2005 on the further Investigation into the Levels of Polycyclic Aromatic Hydrocarbons in Certain Foods; European Parliament: Strasbourg, France, 2005. [Google Scholar]
- EFSA. Invitation to Submit Data: 10 October 2005–10 October 2006; European Food Safety Authority: Parma, Italy, 2006.
- Holmqvist, N.; Stenroth, P.; Berglund, O.; Nyström, P.; Graneli, W.; Larsson, P. Persistent organic pollutants (POP) in a benthic omnivore—A comparison between lake and stream crayfish populations. Chemosphere 2007, 66, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs; European Parliament: Strasbourg, France, 2011. [Google Scholar]
- EU. Commission Regulation (EU) No 1259/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Dioxins, Dioxin-like PCBs and Non Dioxin-like PCBs in Foodstuffs; European Parliament: Strasbourg, France, 2011. [Google Scholar]
- Vogt, G.; Keller, M.; Brandis, D. Occurrence of in the stone crayfish Austropotamobius torrentium from a population naturally mixed with the noble crayfish Astacus astacus. Dis. Aquat. Organ. 1996, 25, 233–238. [Google Scholar] [CrossRef]
- Reid, S.M.; Devlin, J. Effectiveness of stream sampling methods in capturing non-native rusty crayfish (Orconectes rusticus) in Ontario. Can. Field-Nat. 2014, 128, 111–118. [Google Scholar] [CrossRef]
- Szaniawska, A.; Normant, M.; Michałowska, M.; Kamińska, A. Morphometric characters of the freshwater American crayfish, Orconectes limosus Raf., from the Vistula Lagoon (Poland). Oceanol. Hydrobiol. Stud. 2005, 34 (Suppl. 1), 195–208. [Google Scholar]
- HELCOM. Technical Note on Determination of Polycyclic Aromatic Hydrocarbons in Biota; HELCOM: Helsinki, Finland, 2002. [Google Scholar]
- Bolałek, J. Fizyczne, Biologiczne i Chemiczne Badania Morskich Osadów Dennych; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2010. [Google Scholar]
- US EPA 1668. United States Environmental Protection Agency Method 1668. Toxic Polychlorinated Biphenyls by Isotope Dilution High Resolution Gas Chromatography/High Resolution Mass Spectrometry. Available online: https://f.hubspotusercontent00.net/hubfs/6549100/EPA%20Method%201668.pdf (accessed on 20 January 2020).
- Guo, W.; Archer, J.; Moore, M.; Bruce, J.; McLain, M.; Shojaee, S.; Zou, W.; Benjamin, L.A.; Adeuya, A.; Fairchild, R.; et al. QUICK: Quality and Usability Investigation and Control Kit for Mass Spectrometric Data from Detection of Persistent Organic Pollutants. Int. J. Environ. Res. Public Health 2019, 16, 4203. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, C.P.; Holtz, S.H.; Reinert, J.C.; Panyacosit, L.; Axelrad, D.A.; Woodruff, T.J. Dietary exposures to food contaminants across the United States. Environ. Res. 2000, 84, 170–185. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Guidance for Assessing Chemical Contaminant Data for Use in fish Advisories, Volume 2: Risk Assessment and Fish Consumption Limites, 3rd ed.; US EPA: Washington, DC, USA, 2000.
- US EPA. Fish Consumption and Environmental Justice. A Report Developed from the National Environmental Justice Advisory Council Meeting of December 3–6, 2001; US EPA: Washington, DC, USA, 2002; p. 169.
- Shakeri, A.; Shakeri, R.; Mehrabi, B. Potentially toxic elements and persistent organic pollutants in water and fish at Shahid Rajaei Dam, north of Iran. Int. J. Environ. Sci. Technol. 2015, 12, 2201–2212. [Google Scholar] [CrossRef]
- US EPA. Guidelines for Exposure Assessment; US Environmental Protection Agency, Federal Register: Washington, DC, USA, 1992; Volume 57, pp. 22888–22938.
- US EPA. Integrated Risk Information System for POPs Contaminants. US EPA, 1999. U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Aroclor 1254; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.
- ATSDR. Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors (Update); United States Agency for Toxic Substances and Disease Registry, Division of Toxicology SRC, Inc.: Atlanta, GA, USA, 2018; 154p.
- Tanabe, S.; Hidaka, H.; Kawano, M.; Tatsukawa, R. Global distribution and atmospheric transport of chlorinated hydrocarbons: HCH isomers and DDT compounds in the Western Pacific, Eastern Indian and Antarctic Ocean. J. Ocean. Soc. Japan 1982, 38, 137–148. [Google Scholar] [CrossRef]
- Tanabe, S.; Hidaka, H.; Tatsukawa, R. PCBs and chlorinated hydrocarbon pesticides in Anctarctic atmosphere and hydrosphere. Chemosphere 1983, 12, 277–288. [Google Scholar] [CrossRef]
- Kallenborn, R.; Hung, H.; Brorström-Lundén, E. Atmospheric long-range transport of persistent organic pollutants (POPs) into polar regions. Compr. Anal. Chem. 2015, 67, 411–432. [Google Scholar] [CrossRef]
- Domingo, J.L. Influence of cooking processes on the concentrations of toxic metals and various organic environmental pollutants in food: A review of the published literature. Crit. Rev. Food Sci. Nutr. 2010, 51, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Abramsson-Zetterberg, L.; Maurer, B.M. Fluoranthene and phenathrene, two predominant PAHs in heat prepared food, do not influence the frequency of micronucleated mouse erythrocytes induced by other PAHs. Toxicol. Rep. 2015, 2, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.M. Bioaccumulation in Marine Organisms. Effect of Contaminants from Oil Well Produced Water; Elsevier: Amsterdam, The Netherlands, 2002; 452p, ISBN 0-08-043716-8. [Google Scholar]
- Brette, F.; Shiels, H.; Galli, G.; Cros, C.; Incardona, J.P.; Scholz, N.L.; Block, B.A. A Novel Cardiotoxic Mechanism for a Pervasive Global Pollutant. Sci. Rep. 2017, 7, 41476. [Google Scholar] [CrossRef] [PubMed]
- Baars, A.; Theelen, R.; Janssen, P.; Hesse, J.; van Apeldoorn, M.; Meijerink, M.; Verdam, L.; Zeilmaker, M. Re-Evaluation of Human-Toxicity Maximum Permissible Risk Levels; Report No. 711701025; National Institute of Public Health and the Environment (RIVM): Bilthoveen, The Netherlands, 2002.
- Paulik, L.B.; Smith, B.W.; Bergmann, A.J.; Sower, G.J.; Forsberg, N.D.; Teeguarden, J.G.; Anderson, K.A. Passive samplers accurately predict PAH levels in resident crayfish. Sci. Total Environ. 2016, 544, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.C.; Amero, P.; Santoro, A.; Monnolo, A.; Simeoli, R.; Di Guida, F.; Mattace Raso, G.; Meli, R. Polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) alter leptin signaling and lipid metabolism in differentiated 3T3-L1 adipocytes. Toxicol. Appl. Pharmacol. 2014, 279, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Ohtsu, S.; Muroi, M.; Tanamoto, K. Effects of possible endocrine disrupting chemicals on bacterial component-induced activation of NF-kappaB. Biol. Pharm. Bull. 2006, 29, 2120–2122. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.L.; Widholm, J.J.; Rice, D.C. Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 2003, 111, 357–376. [Google Scholar] [CrossRef]
- Viluksela, M.; Heikkinen, P.; van der Ven, L.T.M.; Rendel, F.; Roos, R.; Esteban, J.; Korkalainen, M.; Lensu, S.; Miettinen, H.M.; Savolainen, K.; et al. Toxicological Profile of Ultrapure 2,29,3,4,49,5,59-Heptachlorbiphenyl (PCB 180) in Adult Rats. PLoS ONE 2014, 9, e104639. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ferrante, M.C.; Di Guida, F.; Pirozzi, C.; Lama, A.; Simeoli, R.; Clausi, M.T.; Monnolo, A.; Mollica, M.P.; Raso, G.M.; et al. Polychlorinated biphenyls (PCB 101, 153, and 180) impair murine macrophage responsiveness to lipopolysaccharide: Involvement of NF-κB pathway. Toxicol. Sci. 2015, 147, 255–269. [Google Scholar] [CrossRef]
- Hudina, A.; Lucič, A.; Žganec, K.; Jankowič, S. Characteristics and movement patterns of a recently established invasive Pacifastacus leniusculus population in the river Mura, Croatia. Knowl. Manag. Aquat. Ecosyst. 2011, 403, 07. [Google Scholar] [CrossRef]
- Montorya, M.; Habitb, E.; Fernandezc, P.; Grimaltc, J.O.; Kolokd, A.S.; Barrab, R.O.; Ferrer, J. Biotransport of persistent organic pollutants in the southern Hemisphere by invasive Chinook salmon (Oncorhynchus tshawytscha) in the rivers of northern Chilean Patagonia, a UNESCO biosphere reserve. Environ. Int. 2020, 142, 105803. [Google Scholar] [CrossRef] [PubMed]
- Gerig, B.S.; Janetski, D.J.; Chaloner, D.T.; Lamberti, G.A. Contaminant Biotransport by Pacific Salmon in the Great Lakes. Front. Ecol. Evol. 2020, 8, 199. [Google Scholar] [CrossRef]
- Binnington, M.J.; Wania, F. Clarifying relationships between persistent organic pollutant concentrations and age in wildlife biomonitoring: Individuals, cross-sections, and the roles of lifespan and sex. Environ. Toxicol. Chem. 2014, 33, 1415–1426. [Google Scholar] [CrossRef]
- US Fish and Wildlife Service. Signal Crayfish (Pacifastacus leniusculus). Ecological Risk Screening Summary; U.S. Fish and Wildlife Service: Washington, DC, USA, 2015; pp. 1–15.
- Ren, J.; Wang, X.; Wang, C.; Gong, P.; Wang, X.; Yao, T. Biomagnification of persistent organic pollutants along a high-altitude aquatic food chain in the Tibetan Plateau: Processes and mechanisms. Environ. Pollut. 2017, 220, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Morin, A.E.; Gobas, F.A.P.C. Food web-specific biomagnificaton of persistent organic pollutants. Science 2007, 317, 236–239. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, K. Non-carcinogenic Health Outcomes Associated with Polycyclic Aromatic Hydrocarbons (PAHs) Exposure in Humans: An Umbrella Review. Expo. Health 2023, 15, 95–111. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehunbert, R.T.; Scraborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a Join WHO/FAO Expert Consultation; WHO Technical Report Series 916; WHO: Geneva, Switzerland, 2003; 149p, ISBN 924120916X.
- Bohman, P.; Edsman, L. Status, management and conservation of crayfish in Sweden: Results and the way forward. Freshw. Crayfish 2011, 18, 19–26. [Google Scholar] [CrossRef]
- Vaeßen, S.; Hollert, H. Impacts of the North American signal crayfish (Pacifastacus leniusculus) on European ecosystems. Environ. Sci. Eur. 2015, 27, 33. [Google Scholar] [CrossRef]
- Skóra, M.E.; Dobrzycka-Krahel, A. The invasive signal crayfish Pacifastacus leniusculus resilience on its way to establishment in a European coastal river. Water 2024. under review. [Google Scholar]
- Dobrzycka-Krahel, A.; Skóra, M.E.; Lewicka, A.; Nowicka, A.; Szaniawska, A. If it is impossible to eradicate, let’s consume. Human food security in the sustainable management of the invasive signal crayfish settlement in a coastal Baltic river. Sustainability 2024. under review. [Google Scholar]
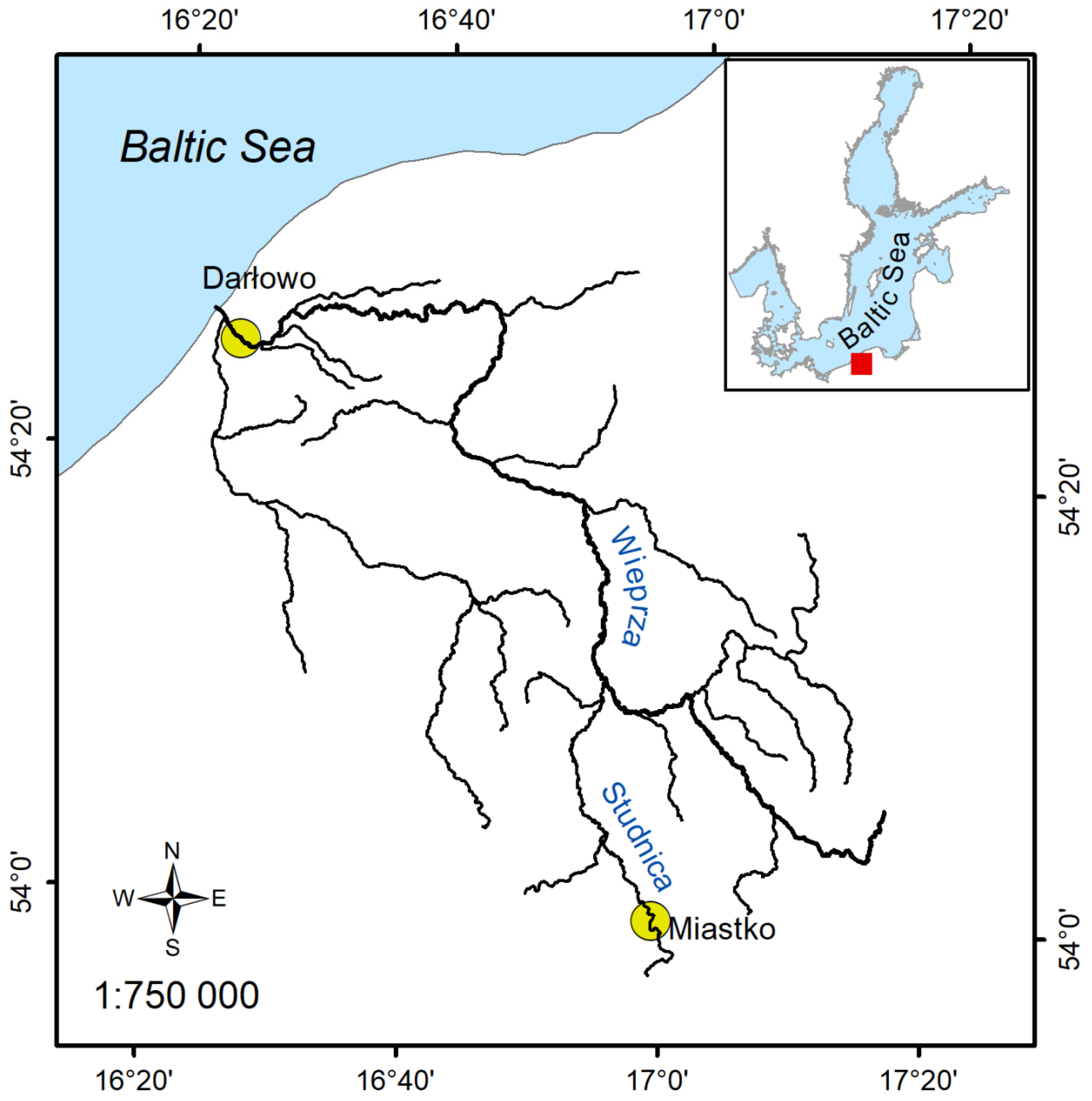
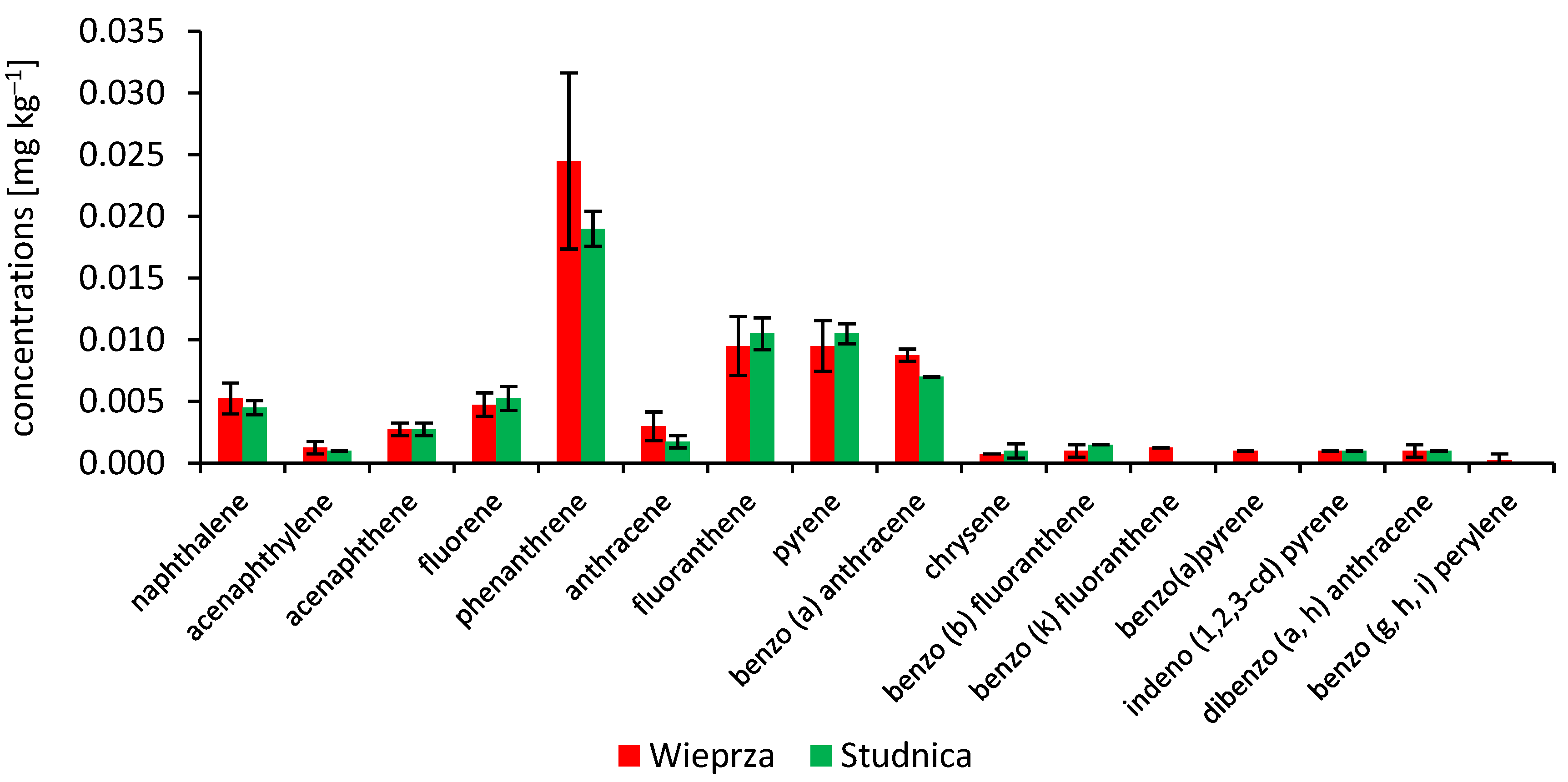
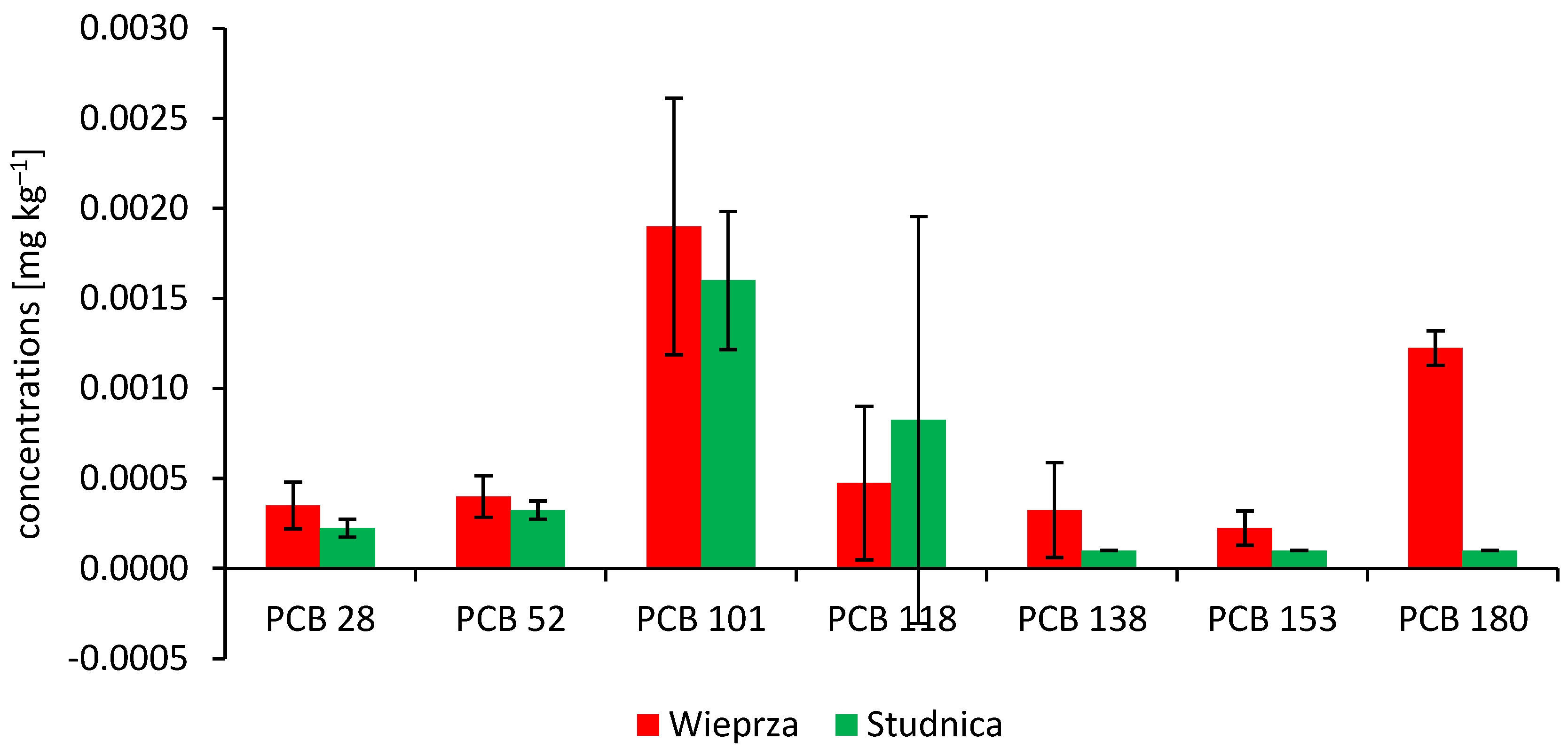

| Sampling Site | Total Length | Wet Mass | Concentrations of Persistent Organic Pollutants | |||||
|---|---|---|---|---|---|---|---|---|
| PAHs | PCBs | |||||||
| Mean [mm] | SD | Mean [g] | SD | Mean [mg kg−1] | SD | Mean [mg kg−1] | SD | |
| Wieprza | 97.55 | 8.95 | 30.100 | 10.00 | 0.067 | 0.0568 | 0.0049 | 0.00108 |
| Studnica | 106.20 | 12.79 | 39.322 | 21.01 | 0.056 | 0.035 | 0.0032 | 0.00138 |
| Persistent Organic Pollutants | Sampling Site | Hazard Quotient (HQ) | Carcinogenic Risk CRlim [g d−1] | Non-Carcinogenic Risk CRlim [g d−1] |
|---|---|---|---|---|
| PAHs | Wieprza | 0.160 * | 1.43 * | 313 * |
| Studnica | 0.133 * | 1.72 * | 375 * | |
| PCBs | Wieprza | 0.175 | 71.5 | 286 |
| Studnica | 0.114 | 109.38 | 437.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzycka-Krahel, A.; Skóra, M.E.; Malek, M. Human Consumption of Non-Native Species in a Circular Economy: Determination of Persistent Organic Pollutants in the Invasive Signal Crayfish from a Baltic Coastal River and Its Assessment for Consumption. Sustainability 2024, 16, 3532. https://doi.org/10.3390/su16093532
Dobrzycka-Krahel A, Skóra ME, Malek M. Human Consumption of Non-Native Species in a Circular Economy: Determination of Persistent Organic Pollutants in the Invasive Signal Crayfish from a Baltic Coastal River and Its Assessment for Consumption. Sustainability. 2024; 16(9):3532. https://doi.org/10.3390/su16093532
Chicago/Turabian StyleDobrzycka-Krahel, Aldona, Michał E. Skóra, and Marika Malek. 2024. "Human Consumption of Non-Native Species in a Circular Economy: Determination of Persistent Organic Pollutants in the Invasive Signal Crayfish from a Baltic Coastal River and Its Assessment for Consumption" Sustainability 16, no. 9: 3532. https://doi.org/10.3390/su16093532





