1. Introduction
Land use change is the driver that has the largest global impact on biodiversity worldwide [
1]. Since human settlement is a prevailing source of land use change all over the world [
2], rapid urbanization has become a great threat to natural resources in recent years. Half of the world’s population dwells in urbanized areas, which cover approximately 2% of the Earth’s terrestrial surface [
3,
4]. Moreover, more buildings and infrastructure are supposed to be constructed in the next 50 years than have been built throughout human history [
5,
6], which will possibly make the problem much worse in urban areas within a few decades, while they are already characterized by low biodiversity, the introduction of non-native species, and simplified species composition and community structures with extensive degradation of natural habitats and attendant biota [
4,
7].
Many ecological studies however treat urban areas as homogeneous entities and combine all anthropogenic factors into one aggregated variable, while urbanization is actually multidimensional and highly variable across time and space [
8]. Similarly, most of the studies examining the composition of local species consider urban areas as one land use type, without categorizing it in subtypes according to the density, structure or function of built-up areas. This although [
9,
10] have showed that plants in urban areas can find some specific isolated places which show significant differences to the general character of the area, mentioning some plants that do not always have to be in the same conditions even within homogenous-looking small urban areas, owing to such artificial structural elements as harbors, revetments, walls, etc.
As for walls, composed of brick, stone, mortar or concrete, they are widely distributed in the urban ecosystem [
3]. However, still very limited studies have focused on the differences in vegetation composition and their proportions on the surfaces of walls depending on the ecological characteristics of their locations. Vegetation can however be easily affected by the near environment. Furthermore, some plant species are favored by urban environments and this situation has often been mentioned by some studies on the comparison of species traits of flora between urban and open rural landscapes [
9,
11,
12,
13]. In general, urbanization is a process that changes flora through a series of filters, which influence habitat availability, the spatial arrangement of habitats, the pool of plant species and the evolutionary selection pressures on populations persisting in urbanized areas [
14]. Despite the massive and pervasive human perturbation, urban ecosystems can provide a variety of substrata for colonization by vegetation and wildlife [
3]. There is no doubt that walls are a characteristic habitat found in human settlements and their surroundings [
15]. As soon as these artificial vertical habitats were formed, nature’s cliff-hangers would begin to colonize the vacant niches [
16]. While vertical surfaces—especially walls—carve lands from hill slopes in urban areas have become much beset, owing to new instruction technologies, this logically made these vertical surfaces very essential platforms to make important contributions to the biodiversity of urban areas.
As urban areas have been under real commercial and residential pressure for decades, lack of green areas in many urban parts of the world has been a common issue among politicians, scientists, NGOs and finally residents. In spite of the fact that urban areas have to possess more green areas so that the people living in them can be physically and psychologically healthier, it is not that easy to find “suitable” places to create these areas since many horizontal surfaces are too costly for this kind of “economically valuable” part of the city. Therefore, vertical greening in urban areas became a popular way to have some nature in cities while these vertical surfaces—at least so far—have no active commercial or residential economic value compared with horizontal surfaces in the urban areas of the world and they are good abodes for spontaneous or synanthropic plant species. Especially masonry is ecologically quite similar to nature’s rocky cliffs [
17]. Supporting this idea, many researchers claim they have some common features such as bare stone surfaces and cracks, soil, humus, moisture and available space so that wall vegetation can attach and survive [
7,
18,
19].
This study focuses on the wall vegetation compositions shaped by ecological parameters both in urban and in sub-urban areas. Since the creation of more vertical green surfaces in urban areas strongly depends on their ecological and economic suitability, this study aims to assess the differences between the wall vegetation that can spontaneously survive in urban and in sub-urban areas; and the basic parameters affecting them and their compositions so that this mechanism could be adapted to create more suitable green surfaces in order to increase ecological quality in urban areas in the near future. To make this possible, analysis of the detailed information gleaned in this study helps (1) to enumerate the wall vegetation depending on the ecological characteristics; (2) to evaluate if there are different wall vegetation typologies in urban and sub-urban areas; (3) to evaluate growth conditions and basic challenges for the wall vegetation; and (4) to discuss how to use this information to make cities ecologically better while artificial green surfaces in urban areas are still expensive, especially because of the high requirements of artificially used green wall plants.
3. Research Findings
3.1. The Walls
Trabzon is hilly. As the center of the region, this extremely compact city often needs to build walls to accommodate the roads, buildings and several other main functions by shaping the hill slopes. Retaining walls are often classified in terms of their relative mass, flexibility, and anchorage condition [
27], and because of its geomorphological characteristics, Trabzon is a city in which one can see different types of walls in urban and sub-urban parts of the city. While stone walls are intuitively known to be hostile to vegetation establishment due to verticality, lack of substrate, inadequate nutrient and moisture supply and dominance by sterile stone surfaces, masonry retaining walls often offers opportunities for plant life [
3]. In spite of the fact that it is quite easy to observe that concrete walls, one of the most common wall types in the research area, have big challenges for plant life; nature often finds a way to send its representatives even to the most difficult wall surfaces, using narrow gaps between blocks, joints and cracks that surprisingly allow many ready-for-fight plants to send their roots into the wall, which are filled with a mixture of different substrates such as soil, sand and dust (
Figure 3 and
Figure 4).
Walls in the study area were mainly constructed from pre-fabricated concrete elements (67.1%) while the rest (32.9%) are made of stone or brick. However, concrete walls were obviously more common in urban parts (82.8%) compared with the sub-urban (51.4%). The walls are mostly vertical (93.3%) and older than 10 years (56.7%). The most common land use functions were the circulation of vehicles and pedestrians at the foot (91.7%) and residential (35%) at the crest. As with the joints that are the main spaces for plants to hold, most of the walls had mortared joints (65%) while only 28.3% of the walls had no joint and the rest (6.7%) had open joints. Most of the walls (71.7%) had no drainage holes, which is one of the main ways that water leaks through wall surfaces and bring life for plants. Retaining walls were the most common walls (83.3%), while the rest (16.7%) were just borders in the study. Walls in the study area mainly face north (45%), followed by east (31.7%), west (18.3%) and south (5%).
Despite there being no official or unofficial record that we have found so far reporting any wall damage by vegetation in the city, it is not hard to say that people generally believe plants on a wall can damage its solidity. As a result of this generally well-known belief and some conflicts between vegetation and buildings, pavements and some other artificial structures—like those reported by [
28,
29,
30,
31]—it is seldom possible to see wall vegetation being removed from the wall surfaces in the city. However, the number of wall trees is known to be tiny in comparison with the hundreds to thousands of tons of wall materials, and this vegetation could be ecologically very useful, so that maintenance or repair work should be phased over several years to allow some regeneration of wall plants after consolidation from as-yet untouched sections, wherever possible [
3,
32].
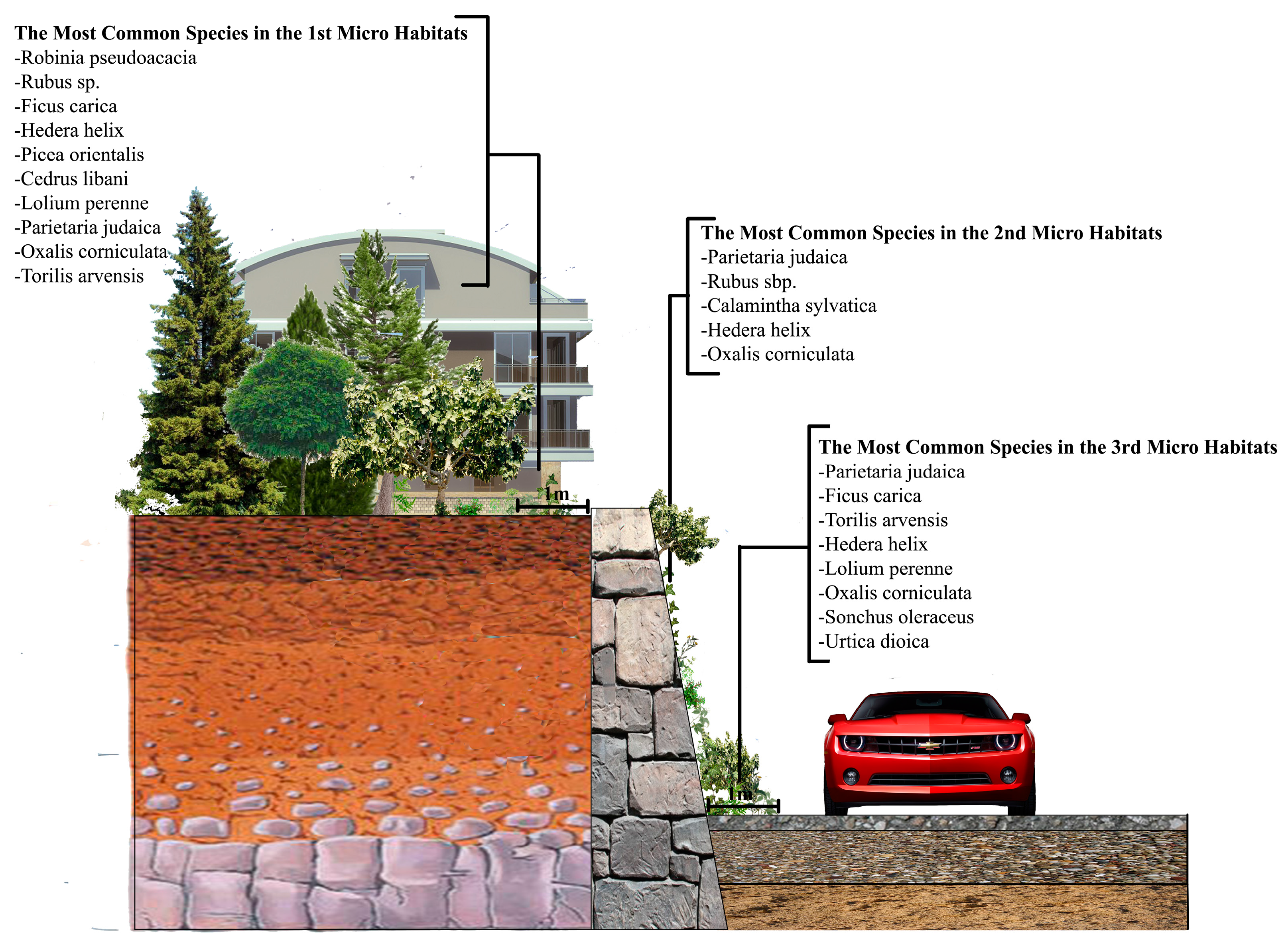
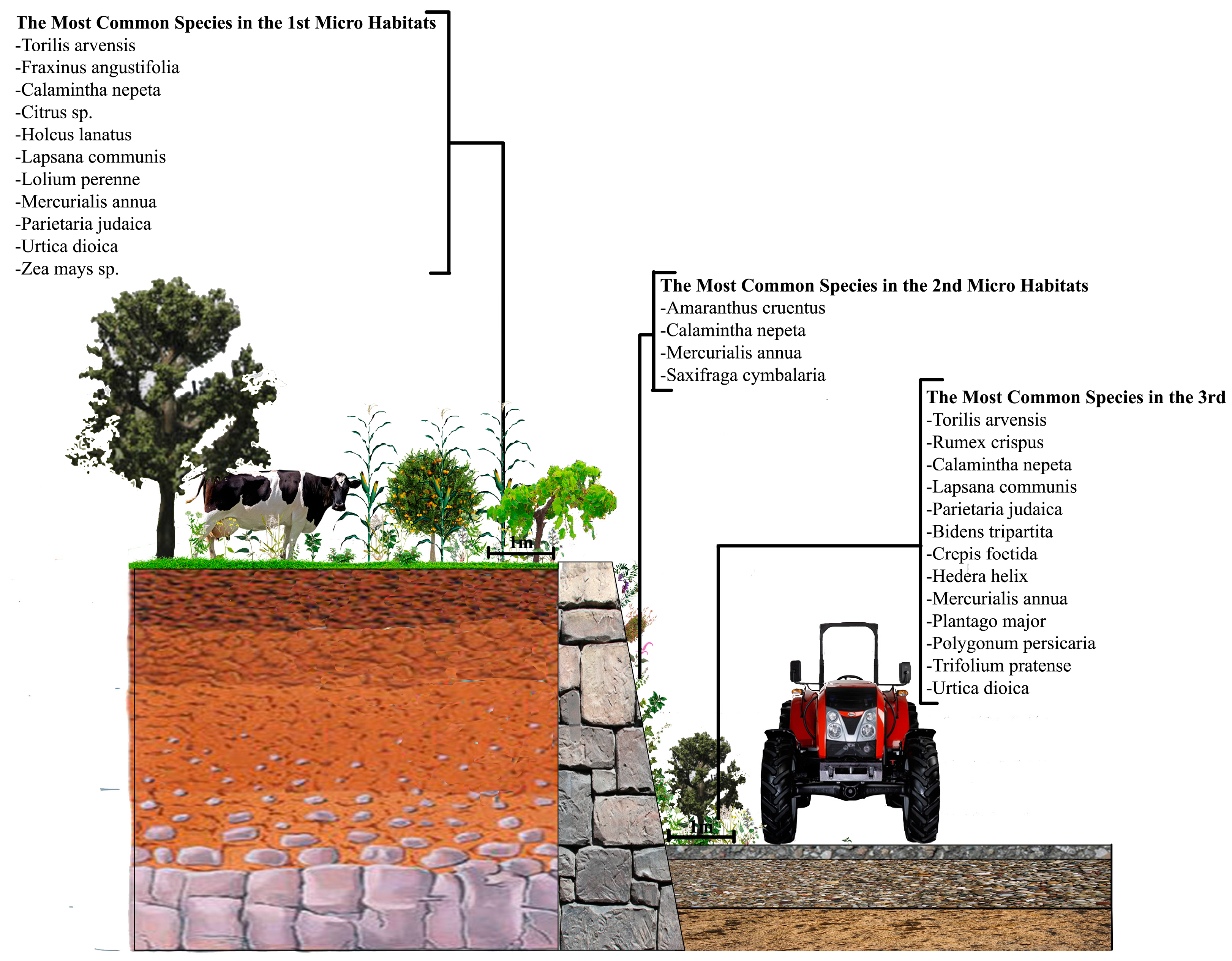
3.2. The Wall Vegetation
All the wall vegetation, including microhabitat I, II and III in the research area, has 196 species in 69 families, 40 of which (57.97%) is represented by only one species. The most dominant family is Asteraceae and it has 30 species. As for the urban and sub-urban areas, we found 119 species in 52 families in the urban areas, while this was 131 species in 49 families in the sub-urban parts. While Asteraceae is still the most dominant family in the both urban and sub-urban areas, it has 16 species in the urban areas and 23 in the sub-urban areas.
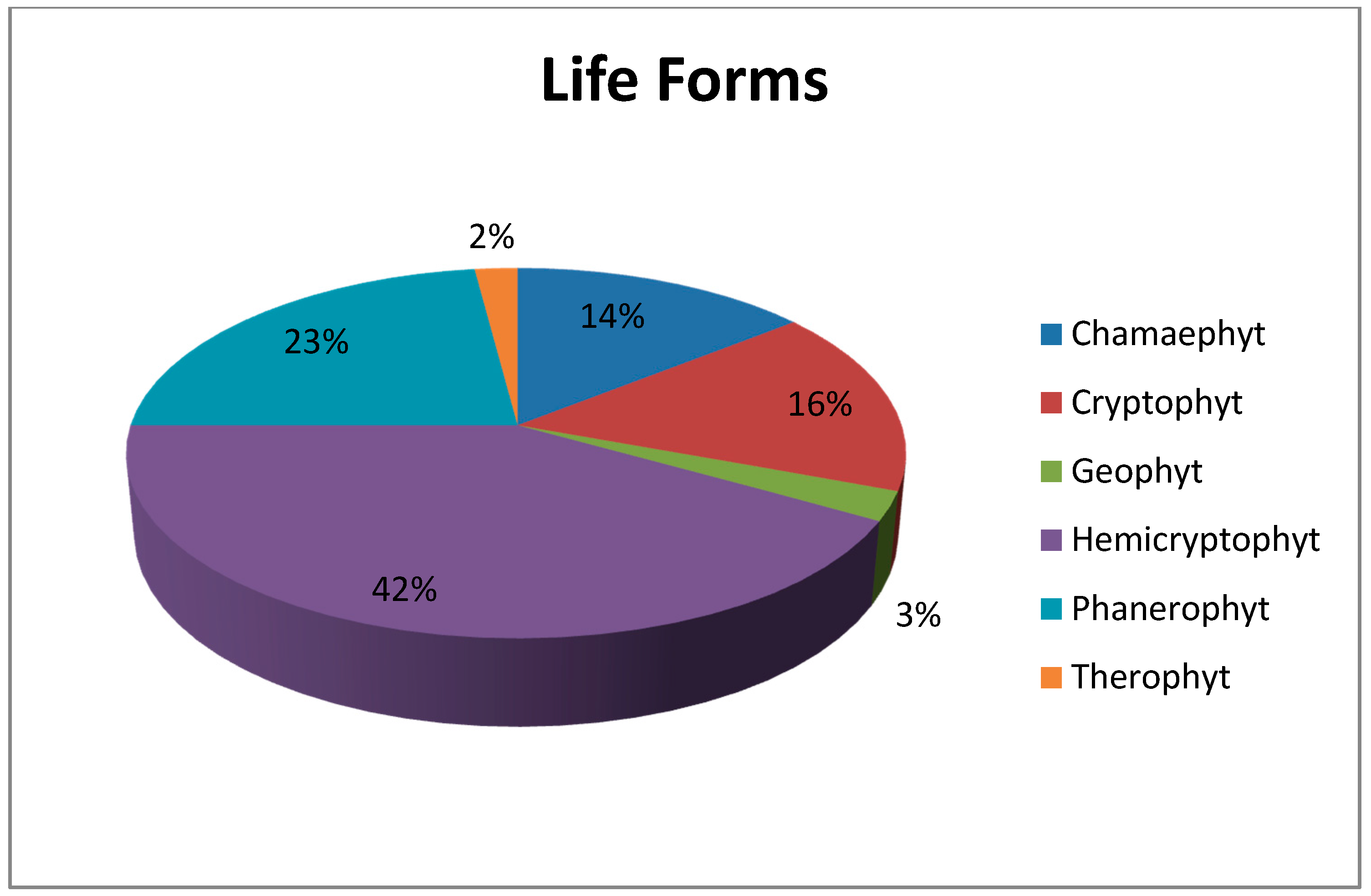
Another important vegetation definition parameter, life forms, also varies in the research area. The research area totally possesses six different life forms. While 81 species out of 196 are Hemicryptophyts (42%), other life forms and the number of the plant species within them are Phanerophyt (45 species; 23%), Crytophyt (33 species; 16%), Chamaephyt (28 species; 14%), Geophyt (five species; 3%) and Therophyt (four species; 2%) (
Figure 5).
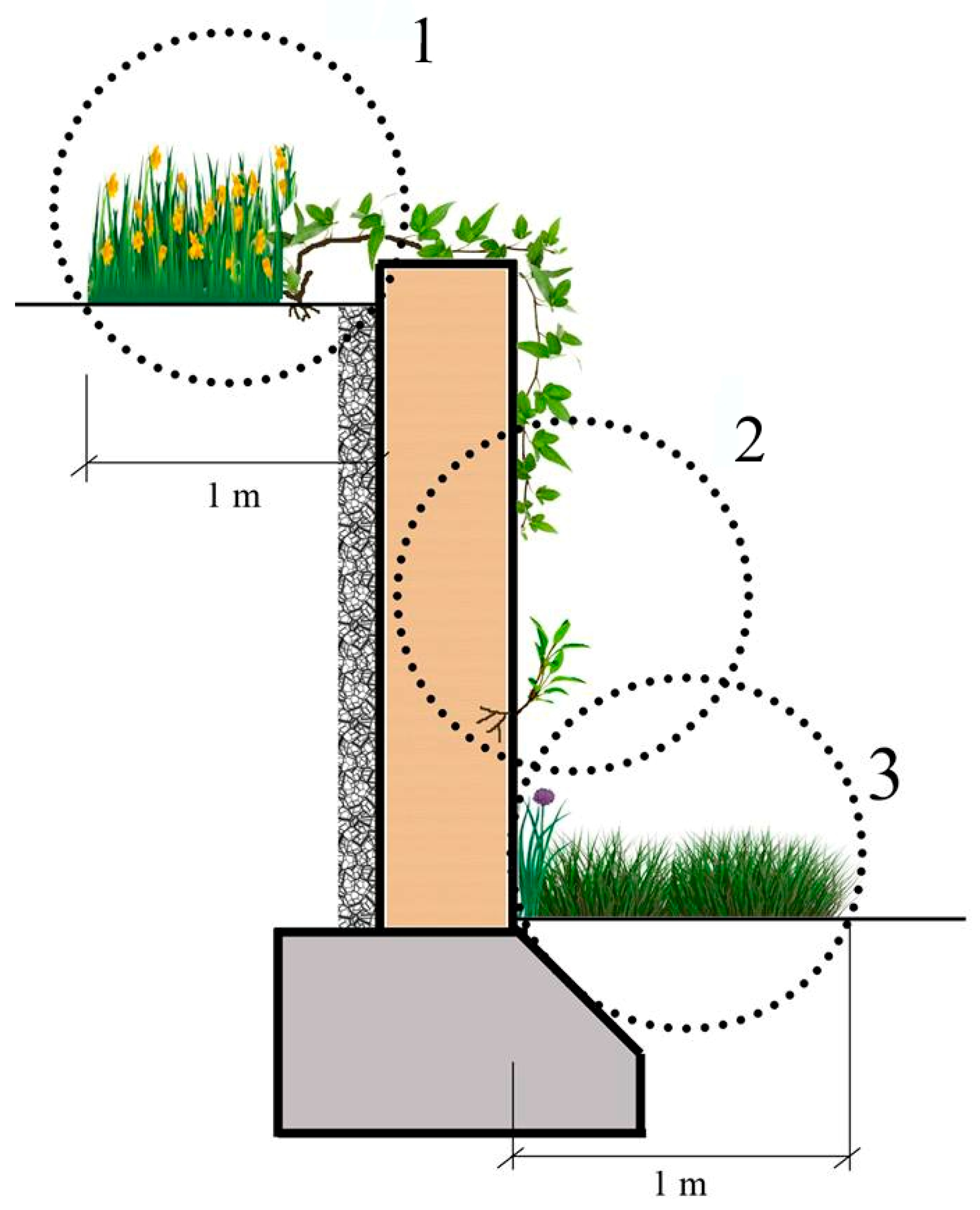
In the urban and sub-urban areas, some specifically artificially conditioned areas, just like walls, were colonized only by plant species with specific adaptations for development and reproduction [
33]. Furthermore, the diversity of the flora found on the walls is known to vary depending on many different criteria such as geography [
3], plant characteristics [
34] and even the history of the cities [
35]. Herbaceous plants are generally considered the most common among wall vegetation all over the world. For example, while a study reported 77.6% of the most common higher plants were herbaceous on the walls of several towns in the Mediterranean [
34], the same rate was around 93% on the walls of Sao Paulo, Brazil [
35]. For comparison, this study similarly founded 77% herbaceous plant on the walls in both urban and sub-urban areas. However, as one of the most logical arguments to prove that studying urban and sub-urban areas separately was a good idea, the herbaceous plant rate was only 1.8% in the sub-urban areas, which means that locations even within a city might have quite different wall vegetation components. While Ficus carica was the only woody plant species we found on the walls of the sub-urban areas, the other woody plant species Corylus avellana, Robinia pseudoacacia, Prunus sp. and finally Ficus carica again were seen on the walls of the urban areas.
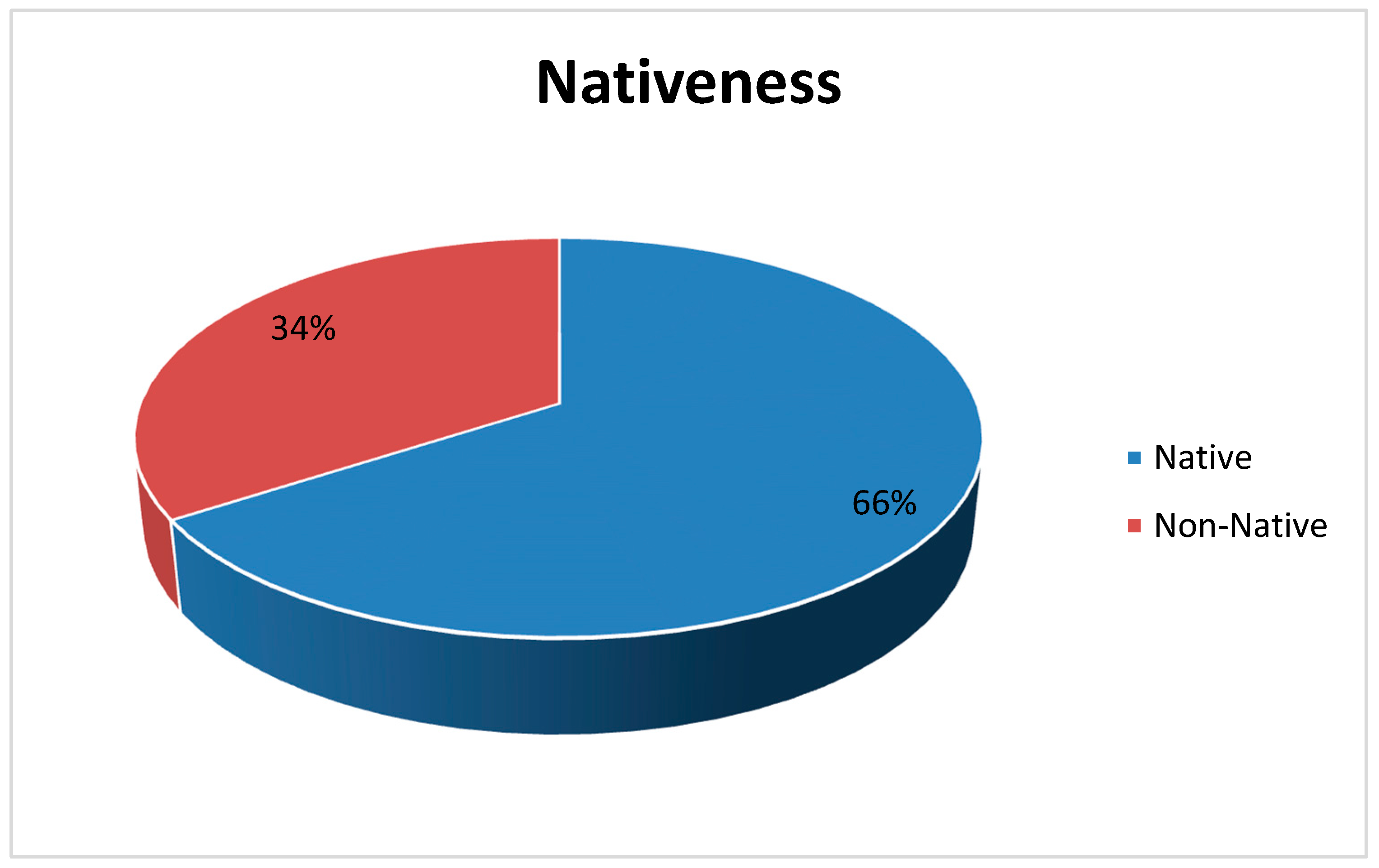
As for native plants, 129 species (66%) out of 196 are known to be native to Trabzon, while the rest (67 species out of 196, which means 34%) seem to be introduced to the city (
Figure 6).
When focusing on the plants growing in the microhabitats II, which have literally been called “wall vegetation” by many previous researchers, as the plants directly attach themselves to the wall by sending their roots into it, it is still possible to see some interesting results. While Parietaria judaica is the most common (40%) species on the walls in the urban areas—confirming its common name “pellitory of the wall”—the same plant species, surprisingly, is hardly seen on the walls in the sub-urban areas, with a rate of 10% (
Figure 7 and
Figure 8). The signature wall plant, however, is still very popular in the scientific world not only owing to its expansive ability to grow on the walls in urban environments, but also the allergenic potential of its pollens.
Only one found species on the wall surfaces in the urban areas (3.2%) is non-native to Trabzon city. This would have been seen as “ordinary”, if we had not found that 26.4% of the wall vegetation in the semi-urban areas was non-native, mainly because of the introduced plant species especially found in drainage holes on the surface of the walls. These “alien” species were probably brought by people attempting to create attractive walls in semi-urban parts, or they were just escapees from the surrounding ornamental gardens, as we often saw that people who lived very close to the randomly selected walls were keeping the same species in their private gardens. Campsis radicans, Pelargonium hybrida, Philadelphus coronaries, Ligustrum japonica and Beta vulgaris are the most common species within this group. Although the semi-urban areas generally represented more naturalistic ecological characteristics compared with the urban parts, the people living in semi-urban areas were likely to have more time to deal with the environment in which they were living, and the walls in the urban parts were clearly more prone to artificial vegetation clearing by the municipality, especially on the fatal parts, such as drainage holes.
4. Discussion and Results
When examining the relations between vegetation characteristics and habitat variables, six significant factors were identified (
Table 2). Factor one involves the location of the walls, which means whether the wall is in the urban or sub-urban parts of the research area. Factor two is the coverage on the walls by plants growing in the first, second and third microhabitats, which means that these plants do not have to grow on the vertical surface of the walls, they can climb from the foot or drop from the crest. Factor three involves wall vegetation coverage, which means the coverage by the plants only growing on the vertical surfaces of the walls. Factor four is species richness, meaning the number of plant species collected from the walls. Factor five is land use type at the crest (or behind the wall, in other words) and, finally, factor six is the sealing of the joints, which determine whether the wall surfaces have any cracks, joints or any other small niches so that plants can attach, or whether these were already artificially filled. In spite of the fact that some important factors were directly or indirectly mentioned in previous studies, such as surface moisture, aspect, wind, etc. [
3,
15,
16,
34,
36,
37], we could not find any statistically strong argument between them and wall vegetation occurrence.
However, it is possible to say that the location of the walls has important effects on the coverage by plants, wall vegetation coverage and the species richness on the wall surfaces. According to this, the walls in the sub-urban areas have a higher coverage rate and more plant species on their surfaces than the ones in the urban areas. As with the sealing of joints (mortared joints and no joints), which clearly affect plant coverage on walls, it can be defined as an important factor keeping plants away from wall surfaces.
Although we could not find any correlation between surface moisture by sight, which is a well mentioned factor in previous studies, and any other factors regarding wall vegetation, another vital factor for wall vegetation, land use behind walls, shows a strong correlation with wall vegetation coverage. Land use behind walls or at the crest, as we mentioned in this study, is known to be one of the most important reasons for moisture availability, if it is not the most important. Especially naturally-vegetated fields behind walls provide a great source of moisture for the wall surfaces as they are the best areas for infiltration. When a wall has a well-vegetated field at the crest, it can gradually get water from the field even when there is no rainfall during long periods. Infiltrated rainwater soaking the soil behind walls supplies seepage out through weep holes and joints [
16]. Cracks or joints are the other main ways for water to get to the surface. Apart from water, a well-vegetated field can fertilize walls because it naturally has a great deal of organic material, which is also an essential eutrophic outflow source for wall vegetation, which has great difficulty in creating strong root systems in such difficult and challenging habitats.
One of the most desirable results of the study was to see which habitat characteristics would affect the plant coverage and species richness on walls in the early stages of the study. To determine that, multiple regression analysis was performed (
Table 3,
Table 4 and
Table 5).
It is clear that the most effective parameters that affect wall vegetation cover on the surface of walls are isolation on vertical surfaces, wall material, average direct sunshine duration on the wall and the main material at the crest (
Table 5). First of all, plants need to find holding in vacant niches on the wall and to take nutrients and water, and a wall surface is not the easiest place to do this. The more vacant niches on the wall, the more nutrients and water are available for wall vegetation (
Figure 9). In the study area, the most common joint material is mixed sand–lime–cement and no data is available to identify their rate. However, this material often loses its solidity due to climatic and other physical effects and lets wall vegetation take nutrients and water so that they can survive and grow. On the contrary, it is nearly impossible to see wall vegetation on concrete walls that have perfect and smooth surfaces if they do not have any drainage holes or an unexpected crack (
Figure 10).
Apart from precipitation mostly carried by wind to the surface of walls, the most valuable water resource for wall vegetation is the water stored by the mass behind the wall. As a result of this, land use and the material at the crest are vital for the wall vegetation. If water and nutrients can easily be stored and carried to the surface of the wall, these challenging mostly vertical areas might turn into appropriate habitats for the vegetation. While there are already limited water opportunities, high water loss risks due to wind and sunlight, and low nutrient potential on wall surfaces, the water stored at the crest and the nutrients in it are really important for the vegetation. We clearly found that the walls which have some land use types requiring impermeable surfaces such as asphalt, concrete etc., at the crest have relatively less vegetation on their vertical surfaces (
Figure 11) while those which have more natural materials such as soil, meadows, pasture etc., at the crest have more, as they are capable of possessing more water and nutrients that can leak into walls so that the wall vegetation can make use of it (
Figure 12).
The sealing of joints, another major parameter that affects wall vegetation, is often made to protect walls against water creating mortared joints, believing that this would make walls more durable. When there is no isolation on a wall, which means that wall has open joints, plants can easily attach themselves to the wall and water can easily leak through the wall surface. Since wind and sunlight generally make wall surfaces extremely dry and therefore poor in nutrients, these mortared joints can easily be defined as one of the most challenging characteristics for wall vegetation. Nevertheless, many plant species can still be seen on walls all in the study area (
Table 6).
There is no doubt that we need more ecologically sustainable cities and therefore more green areas, and vertical gardens are really ideal for urban areas. Focusing on spontaneous wall vegetation in urban areas should be a good start to create economically and ecologically sustainable green walls for cities as they already exist in similar conditions. At this point, we believe that understanding wall vegetation dynamics will definitely help humanity to create healthier cities.
The remaining vegetated habitats in urban areas often contain low plant diversity as a result of erosion, trampling, pollution, invasion or the cultivation of a few non-native species and replacing the native species that are lost with the wide-spread presence of “weedy” non-native species. This can be defined as a great conservation challenge, while managing the large amount of residential vegetation in ways that promote native plants and animals could make a significant contribution to conservation [
6]. Therefore, it can be said that green walls are supposed to support urban areas ecologically, and using some invasive or at least popular exotic plant species only because they can survive in the difficult conditions of wall ecology might not be logical or sustainable, even if they seem to create an attractive green wall surface. The use of native species is ideal to support biodiversity and they are generally the best ecosystem services providers, it would be great to use them on green walls as much as possible. Plus, even the non-native plant species that we found in this study make a contribution to urban ecosystems as they were already able to survive on the wall surfaces spontaneously. While the main purpose of humanity should be to create more sustainable environments by paying less ecologically and economically, there is no doubt that work on sustainable green wall systems should pay more attention to wall vegetation characteristics to reach the ideal, artificial green walls is the best way to follow both in urban and sub-urban areas.