Exploring the Use of Unprocessed Waste Chicken Eggshells for UV-Protective Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples for the UV Aging Experiments
2.1.1. Polymers
2.1.2. Eggshells
2.1.3. Quartz Coverslip
2.2. UV Aging Chamber
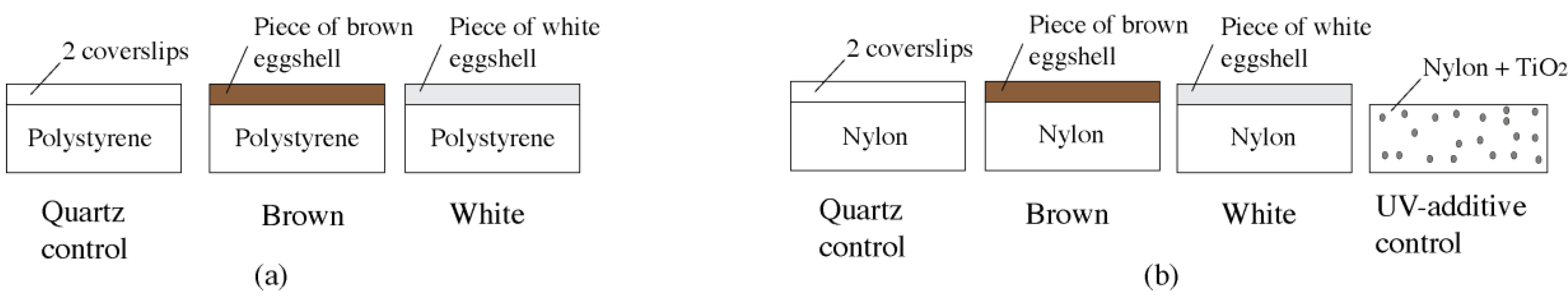
2.3. Specimen Installation
2.4. Experimental Setup
2.5. Temperature Measurement
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Statistical Analysis
2.8. UV Transmittance through Eggshell and TiO2 Particles Suspension Films
2.9. Scanning Electron Microscopy (SEM)
2.10. UV-Vis Spectrophotometry
3. Results and Discussion
3.1. UV Aging of Two Synthetic Polymers
3.1.1. Temperature Measurement
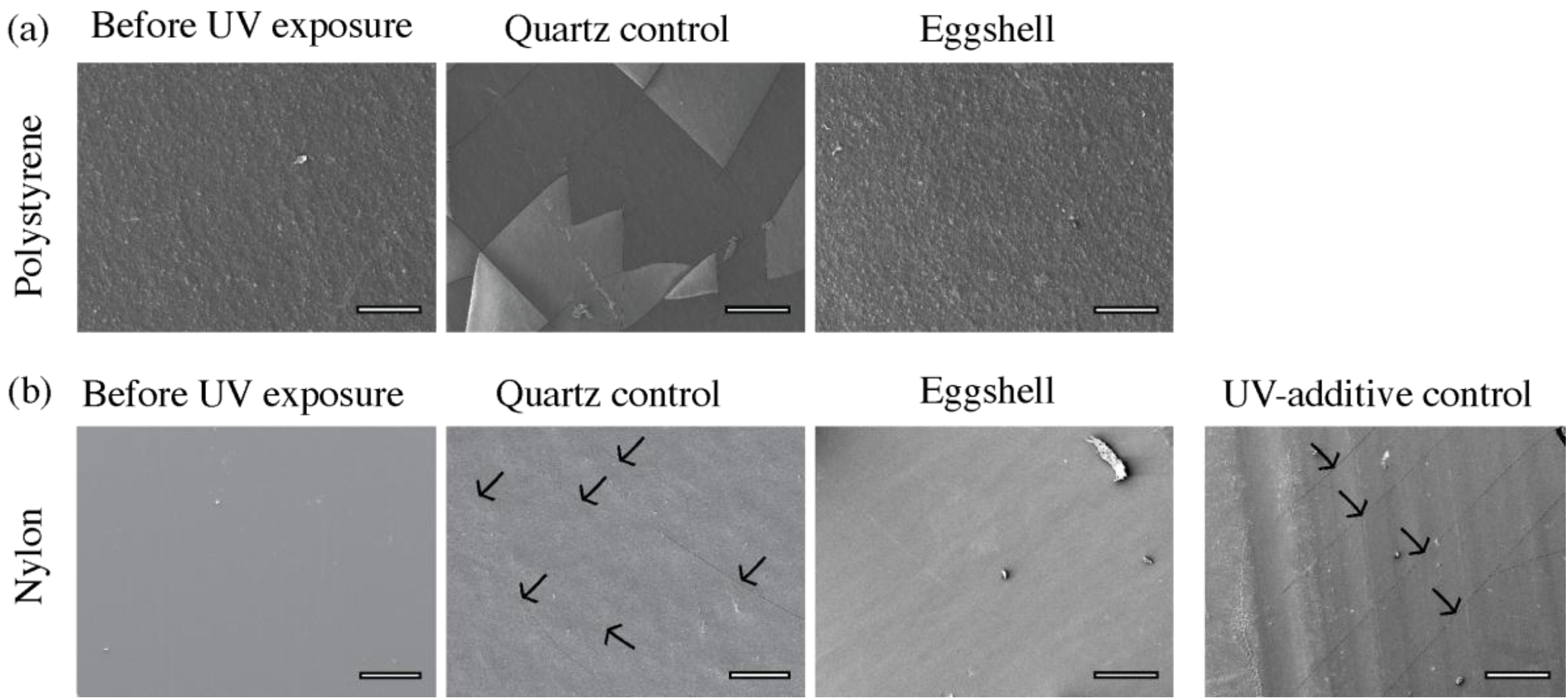
3.1.2. Scanning Electron Microscopy
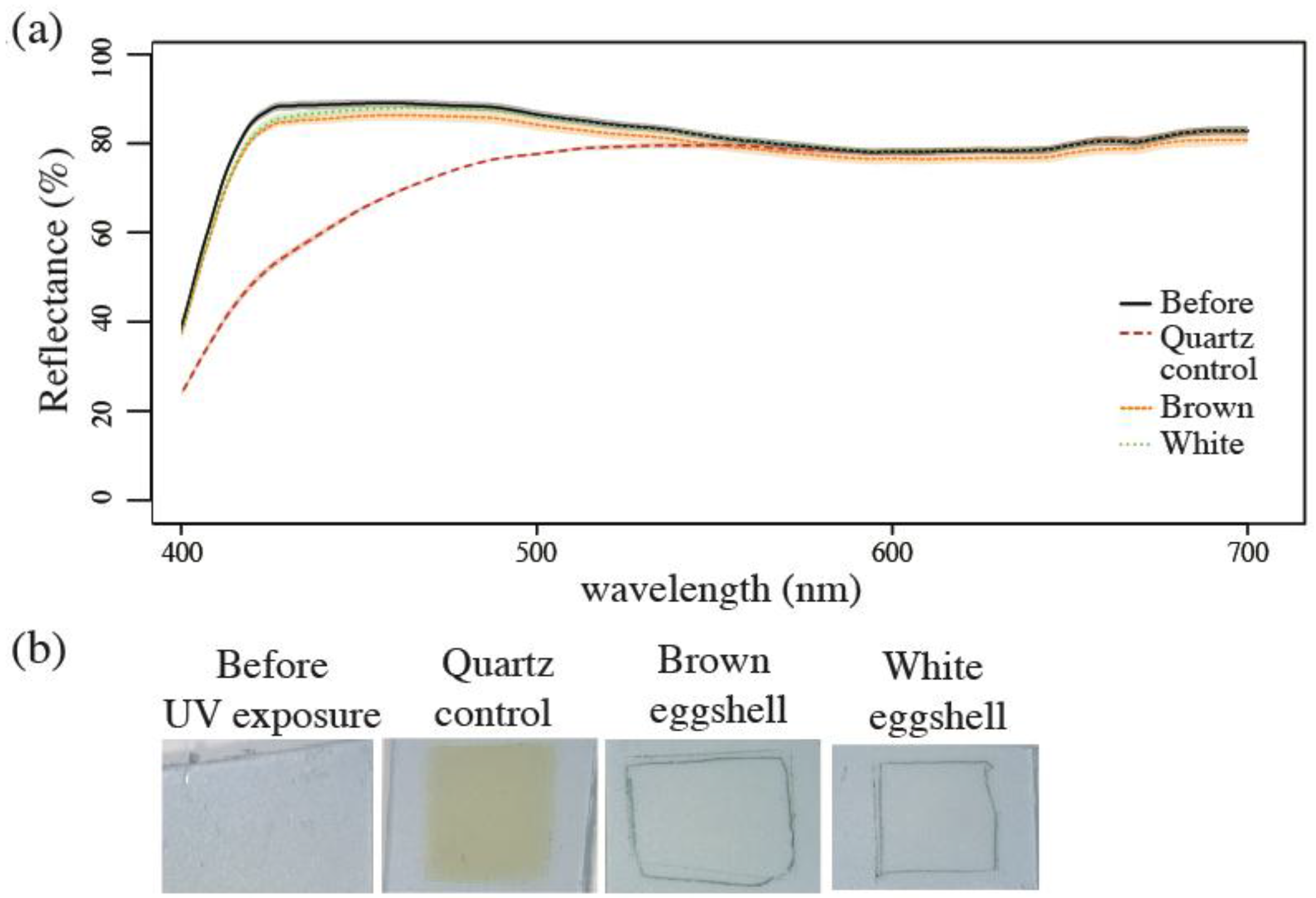
3.1.3. Spectrophotometry
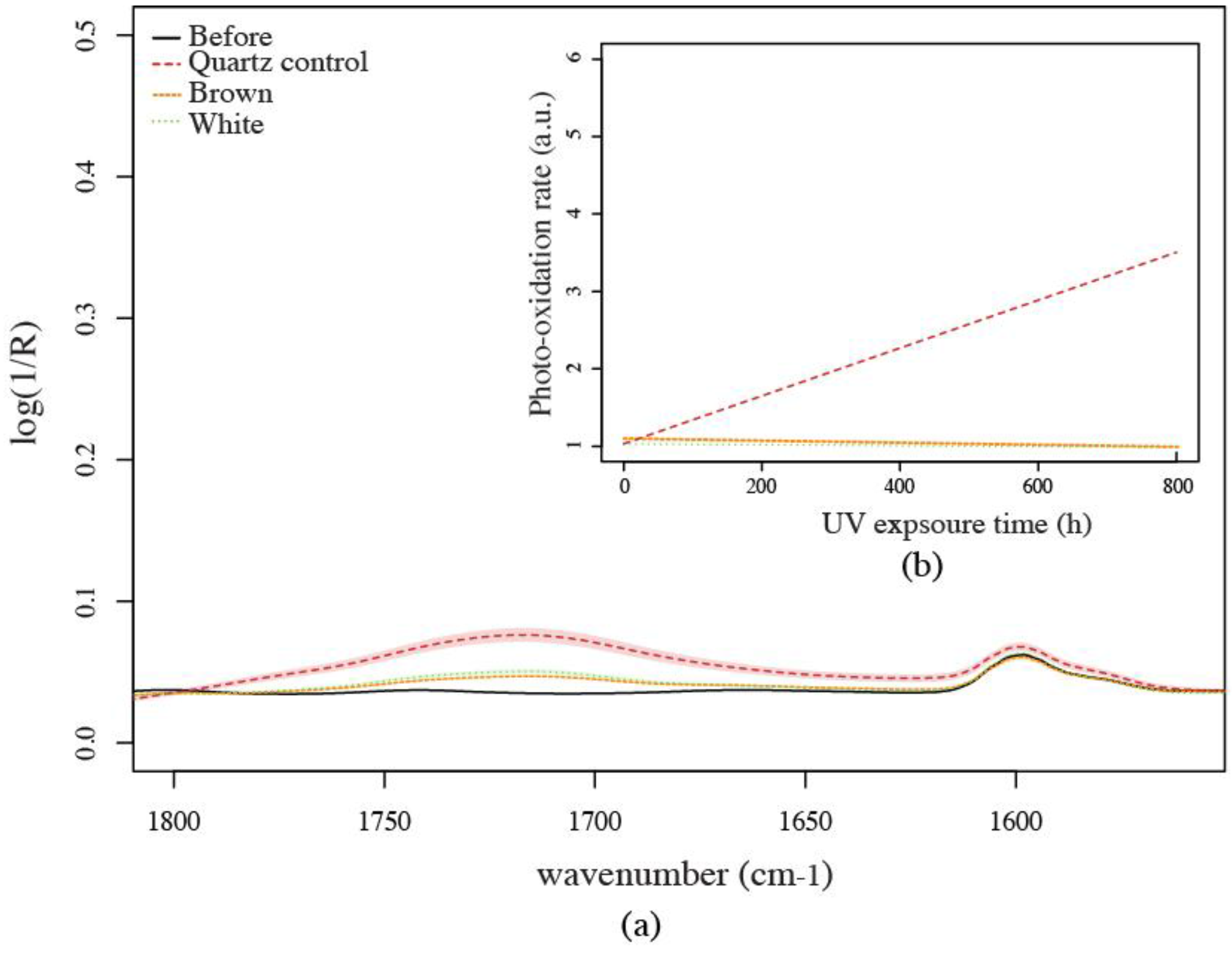
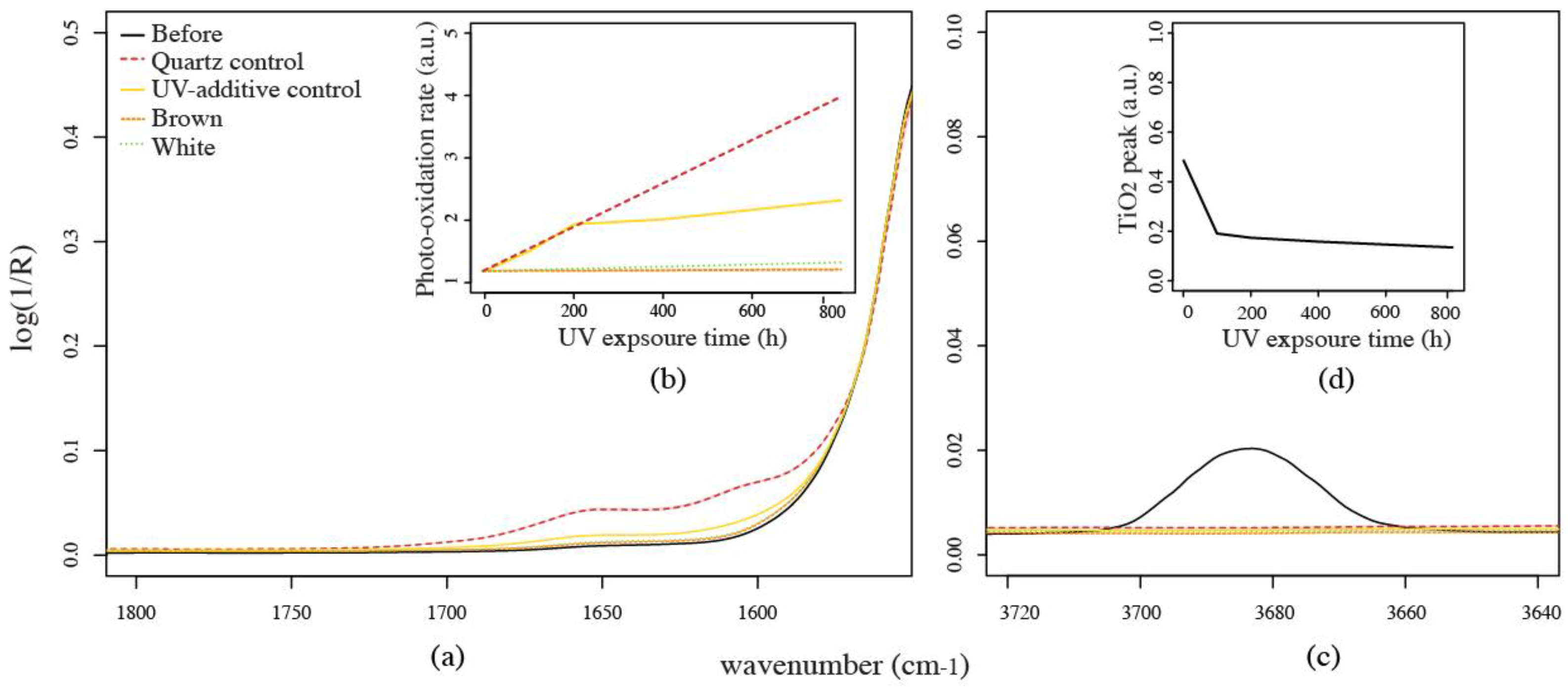
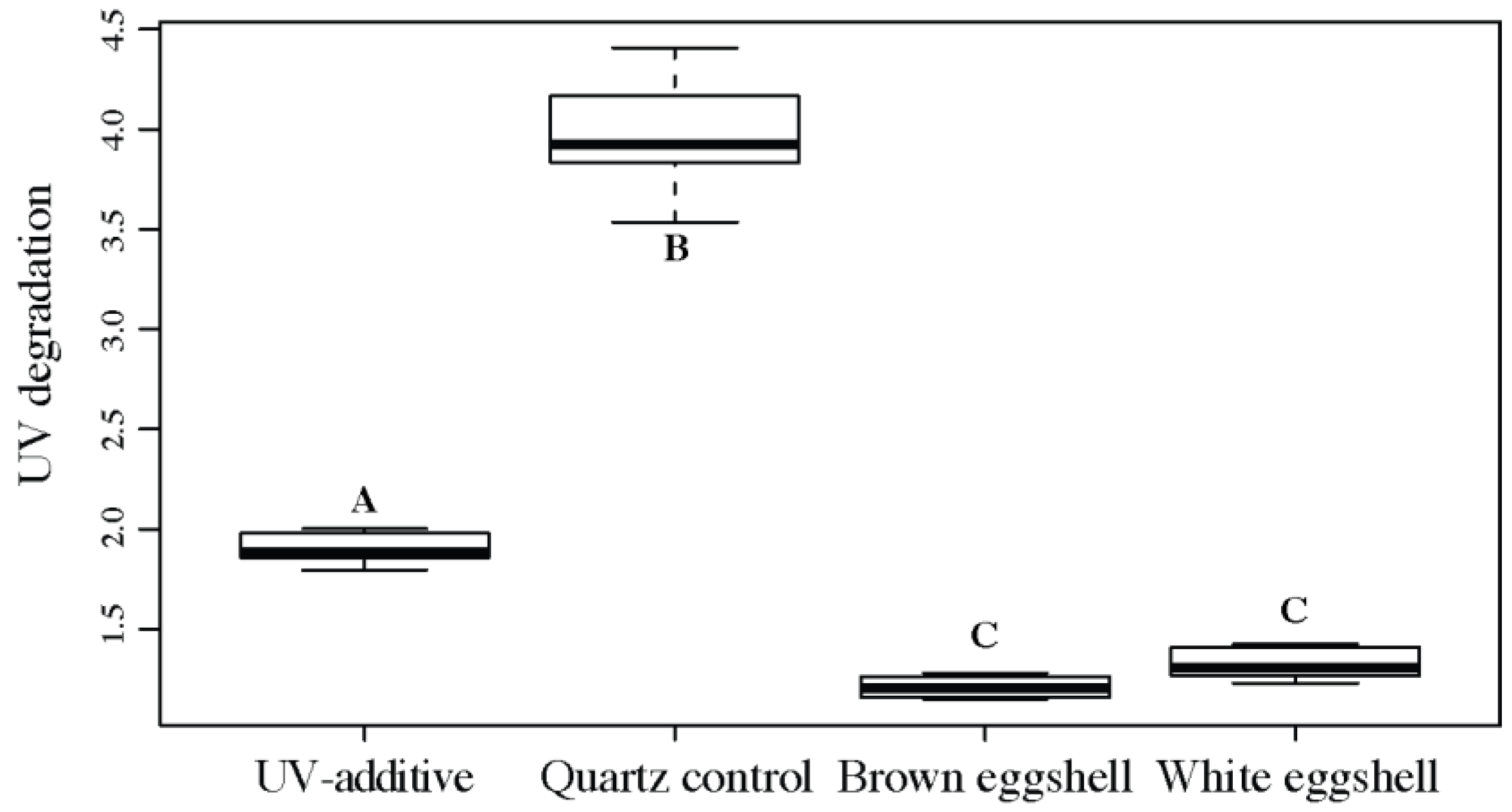
3.1.4. Fourier-Transform Infrared Spectroscopy
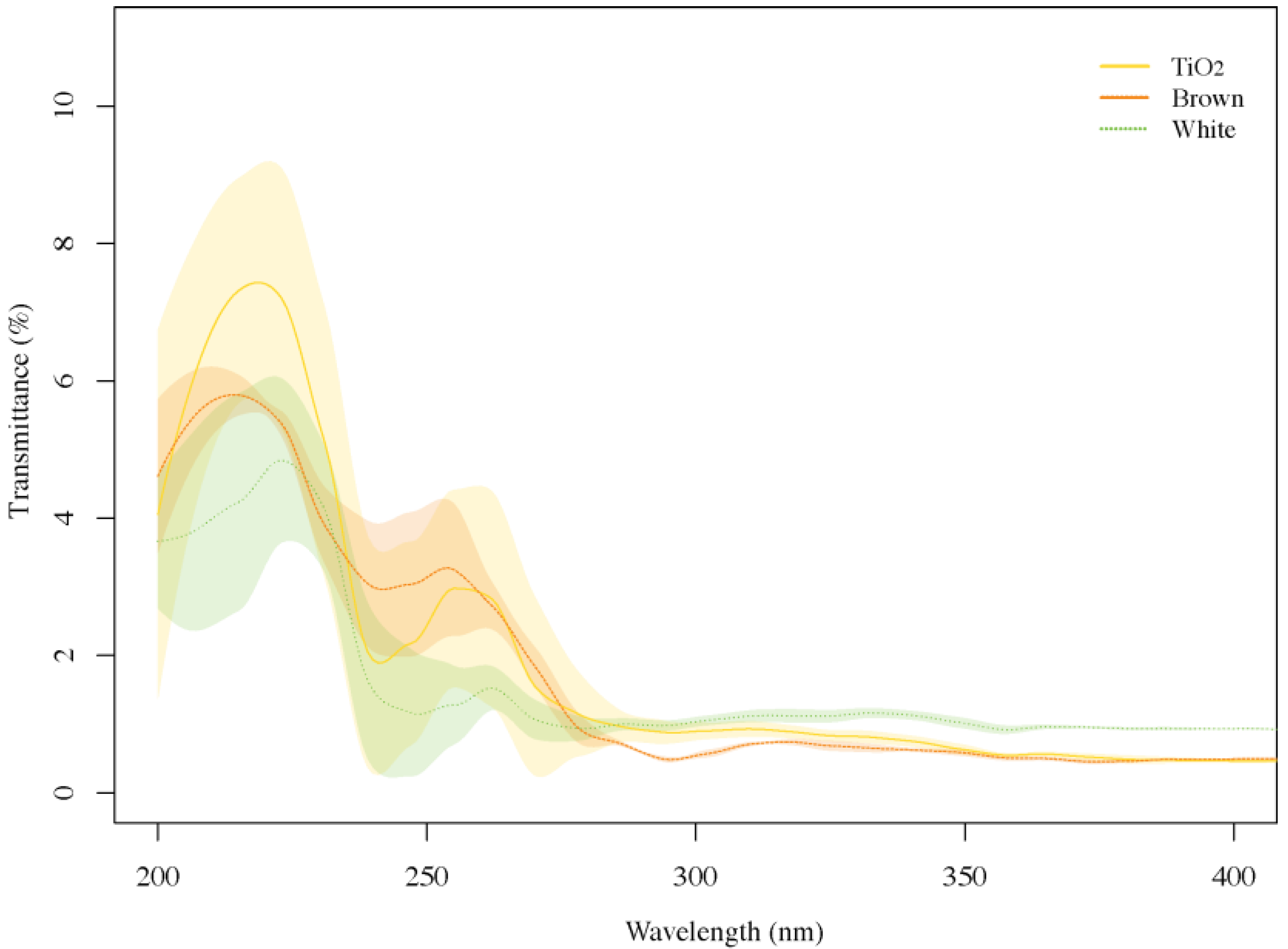
3.2. UV Transmittance through Eggshell and TiO2 Particle Suspension Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brooks, E.R. Critical Color: The Use of Color in Nature for Energy Performance and Its Application to Building Skins. Ph.D. Thesis, University of Washington, Seattle, WA, USA, September 2012. [Google Scholar]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Madronich, S.; Flocke, S. Theoretical Estimation of Biologically Effective UV Radiation at the Earth’s Surface. In Solar Ultraviolet Radiation; Zerefos, C.S., Bais, A.F., Eds.; NATO ASI Series; Springer: Berlin/Heidelberg, Germany, 1997; pp. 23–48. [Google Scholar]
- Andrady, A.L.; Hamid, S.H.; Hu, X.; Torikai, A. Effects of increased solar ultraviolet radiation on materials. J. Photochem. Photobiol. B 1998, 46, 96–103. [Google Scholar] [CrossRef]
- Berdahl, P.; Akbari, H.; Levinson, R.; Miller, W.A. Weathering of roofing materials—An overview. Constr. Build. Mater. 2008, 22, 423–433. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, S.; Pan, N. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Völz, H.G.; Kischkewitz, J.; Woditsch, P.; Westerhaus, A.; Griebler, W.-D.; De Liedekerke, M.; Buxbaum, G.; Printzen, H.; Mansmann, M.; Räde, D.; et al. Inorganic Pigments. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Gázquez, M.J.; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A Review of the Production Cycle of Titanium Dioxide Pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef]
- Buxbaum, G. Industrial Inorganic Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Chiarello, G.L.; Selli, E.; Guarino, M. Effects of TiO2 based photocatalytic paint on concentrations and emissions of pollutants and on animal performance in a swine weaning unit. J. Environ. Manag. 2012, 96, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Fecheyr-Lippens, D.C.; Igic, B.; D’Alba, L.; Hanley, D.; Verdes, A.; Holford, M.; Waterhouse, G.I.; Grim, T.; Hauber, M.E.; Shawkey, M.D. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol. Open 2015, 4, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kamkum, P.; Atiwongsangthong, N.; Muanghlua, R.; Vittayakorn, N. Application of chicken eggshell waste as a starting material for synthesizing calcium niobate (Ca4Nb2O9) powder. Ceram. Int. 2015, 41 (Suppl. S1), S69–S75. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Hsieh, J.S.; Zou, P.; Kokoszka, J. Utilization of calcium carbonate particles from eggshell waste as coating pigments for ink-jet printing paper. Bioresour. Technol. 2009, 100, 6416–6421. [Google Scholar] [CrossRef] [PubMed]
- Fedor, G.R.; Brennan, P.J. Comparison between natural weathering and fluorescent UV exposures: UVA-340 lamp test results. ASTM Spec. Tech. Publ. 1996, 1294, 91–105. [Google Scholar]
- Kohan, M.I. Nylon Plastics Handbook; Hanser: New York, NY, USA, 1995; Volume 378. [Google Scholar]
- Kopitkovas, G.; Lippert, T.; David, C.; Wokaun, A.; Gobrecht, J. Surface micromachining of UV transparent materials. Thin Solid Films 2004, 453, 31–35. [Google Scholar] [CrossRef]
- Jelle, B.P. Accelerated climate ageing of building materials, components and structures in the laboratory. J. Mater. Sci. 2012, 47, 6475–6496. [Google Scholar] [CrossRef]
- Grossman, D.M. Correlation Questions & Answers. 1984. Available online: http://life.q-lab.cn/documents/public/7e877944-fcfa-41e6-9a52-3de77c8a6d6d.pdf (accessed on 2 February 2016).
- Wen, Z.; Hu, X.; Shen, D. The FTIR studies of photo-oxidative degradation of polypropylene. Chin. J. Polym. Sci. 1988, 6, 285–288. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 17 December 2016).
- Lahti, D.C.; Ardia, D.R.; Dudley, R.; Bronstein, J.L. Shedding Light on Bird Egg Color: Pigment as Parasol and the Dark Car Effect. Am. Nat. 2016, 187, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Thanki, P.N.; Singh, R.P. Photo-oxidative degradation of nylon 66 under accelerated weathering. Polymer 1998, 39, 6363–6367. [Google Scholar] [CrossRef]
- Hirose, F.; Kuribayashi, K.; Suzuki, T.; Narita, Y.; Kimura, Y.; Niwano, M. UV Treatment Effect on TiO2 Electrodes in Dye-Sensitized Solar Cells with N719 Sensitizer Investigated by Infrared Absorption Spectroscopy. Electrochem. Solid-State Lett. 2008, 11, A109–A111. [Google Scholar]
- Zan, L.; Tian, L.; Liu, Z.; Peng, Z. A new polystyrene—TiO2 nanocomposite film and its photocatalytic degradation. Appl. Catal. Gen. 2004, 264, 237–242. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, Z.; Liao, Z.F.; Mai, Y.L.; Zhang, M.Q. Roles of anatase and rutile TiO2 nanoparticles in photooxidation of polyurethane. Polym. Test. 2007, 26, 202–208. [Google Scholar] [CrossRef]
- Walton, H.V.; Cotterill, O.J.; Vandepopuliere, J.M. Composition of Shell Waste from Egg Breaking Plants. Poult. Sci. 1973, 52, 1836–1841. [Google Scholar] [CrossRef]
- Than, M.M.; Lawanprasert, P.; Jateleela, S. Utilization of eggshell powder as excipient in fast and sustained release acetaminophen tablets. Mahidol Univ. J. Pharm. Sci. 2012, 39, 32–38. [Google Scholar]
- Tegethoff, F.W.; Rohleder, J.; Kroker, E. Calcium Carbonate: From the Cretaceous Period into the 21st Century; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Mann, S. Biomineralization: Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Suzuki, M.; Nagasawa, H.; Kogure, T. Microstructural control of calcite via incorporation of intracrystalline organic molecules in shells. J. Cryst. Growth 2013, 381, 114–120. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Chrétien, M.N.; Maretti, L.; Scaiano, J.C. TiO2-promoted mineralization of organic sunscreens in water suspension and sodium dodecyl sulfate micelles. Photochem. Photobiol. Sci. 2003, 2, 487–492. [Google Scholar] [CrossRef]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fecheyr-Lippens, D.; Nallapaneni, A.; Shawkey, M.D. Exploring the Use of Unprocessed Waste Chicken Eggshells for UV-Protective Applications. Sustainability 2017, 9, 232. https://doi.org/10.3390/su9020232
Fecheyr-Lippens D, Nallapaneni A, Shawkey MD. Exploring the Use of Unprocessed Waste Chicken Eggshells for UV-Protective Applications. Sustainability. 2017; 9(2):232. https://doi.org/10.3390/su9020232
Chicago/Turabian StyleFecheyr-Lippens, Daphne, Asritha Nallapaneni, and Matthew D. Shawkey. 2017. "Exploring the Use of Unprocessed Waste Chicken Eggshells for UV-Protective Applications" Sustainability 9, no. 2: 232. https://doi.org/10.3390/su9020232




