Treelines—Approaches at Different Scales
Abstract
:1. Introduction
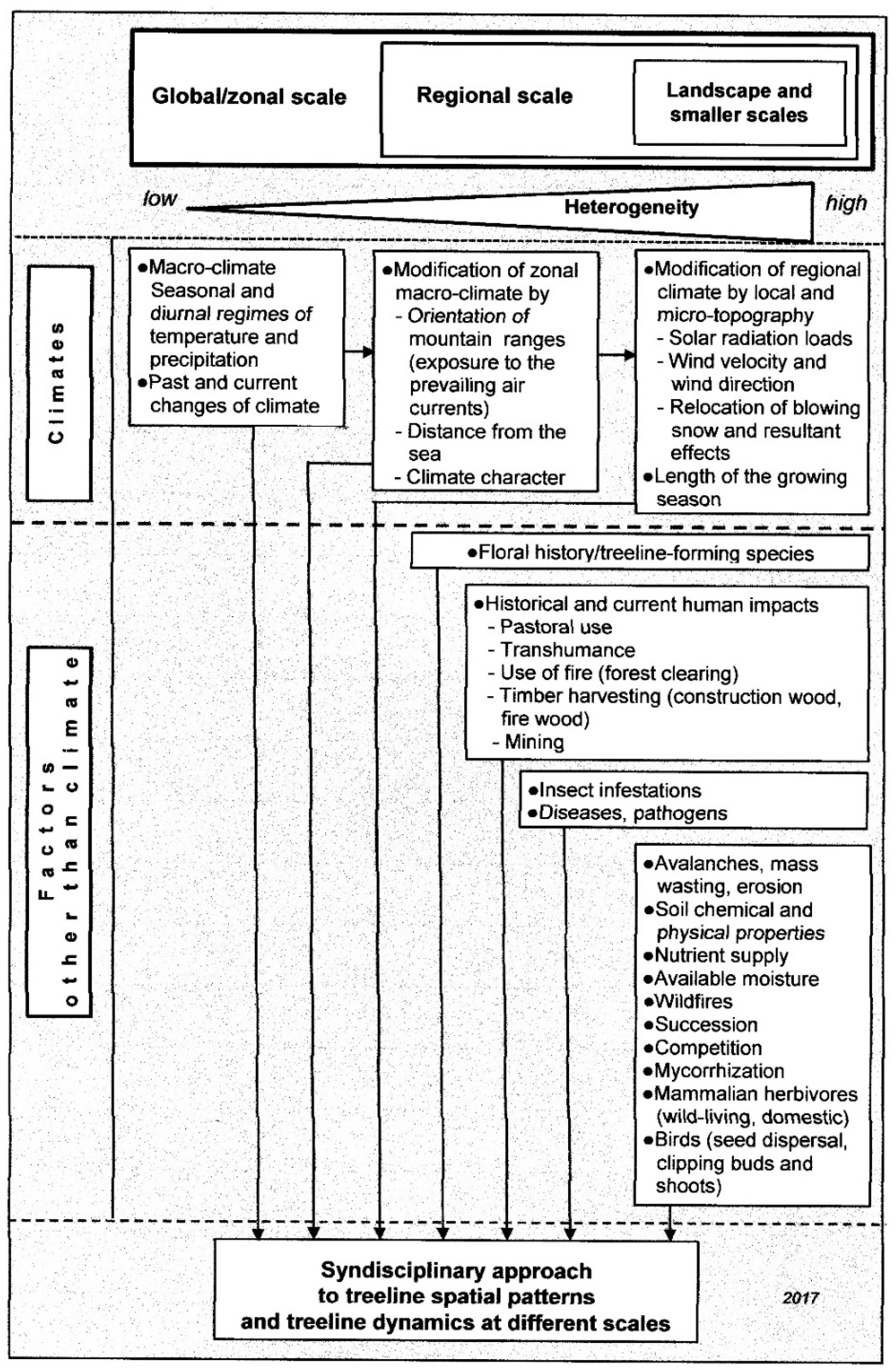
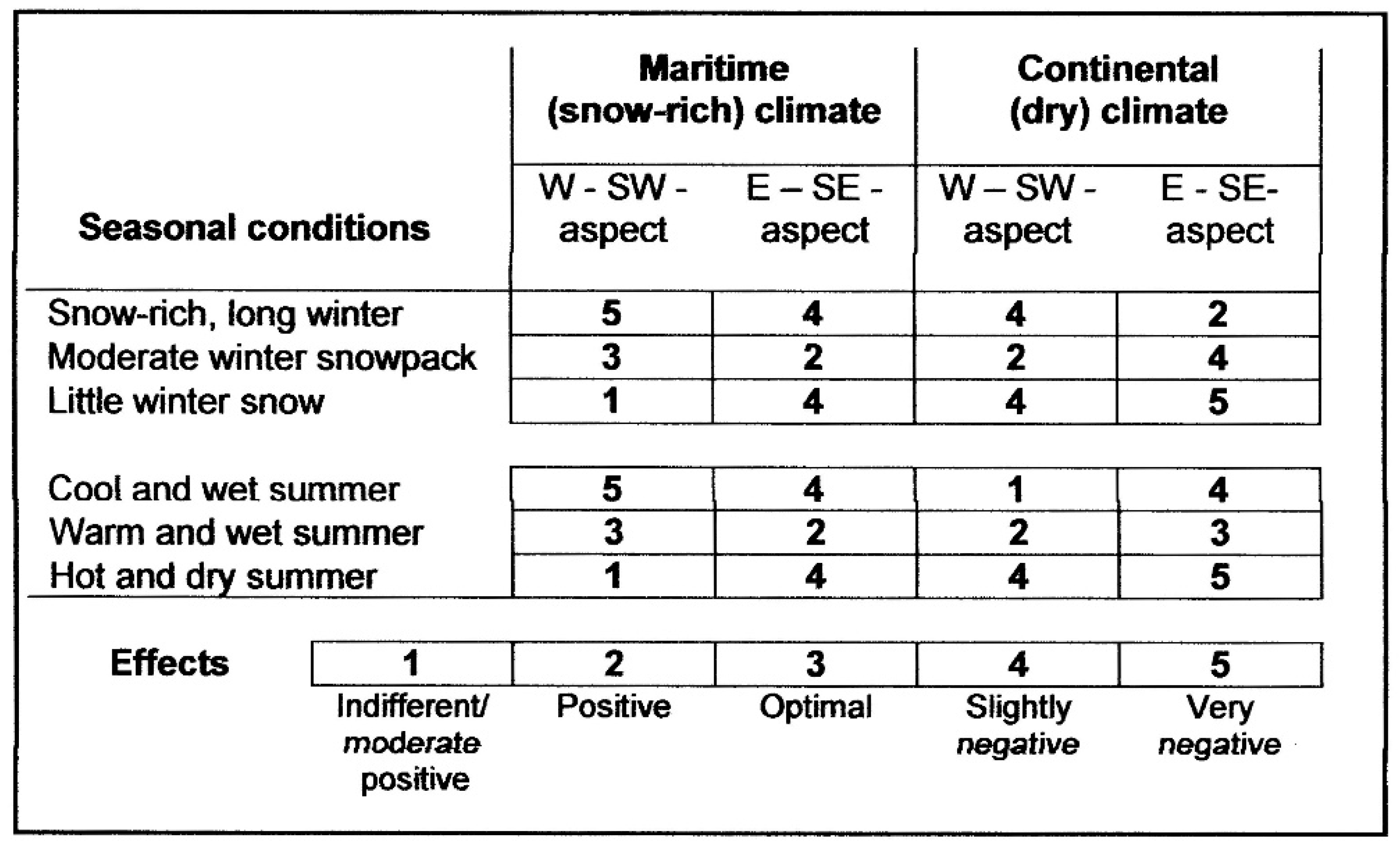
2. Traits and Causation of Treeline at Coarse (Global/Zonal) Scales
3. Treeline at Landscape (Regional), Local, and Microscales
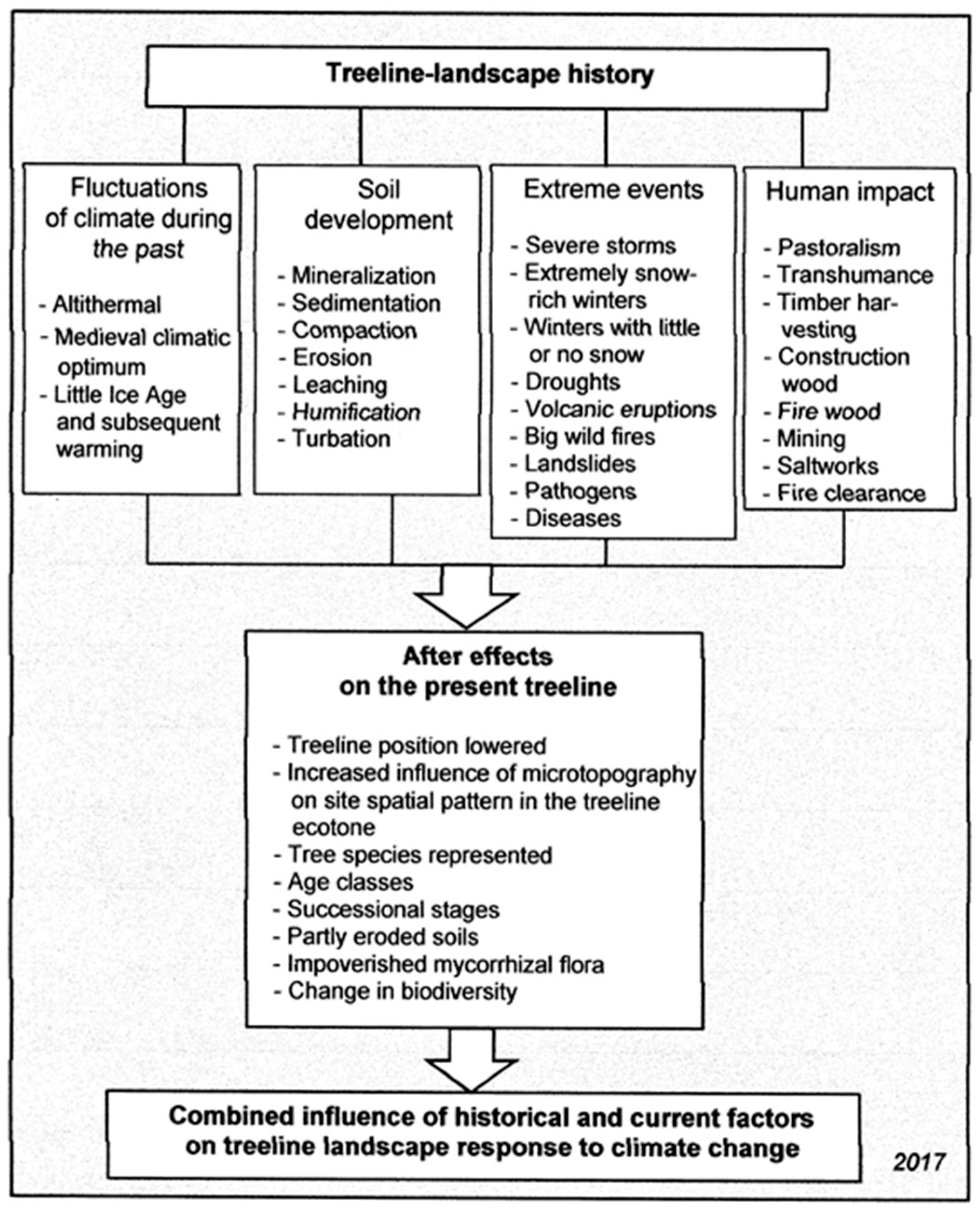
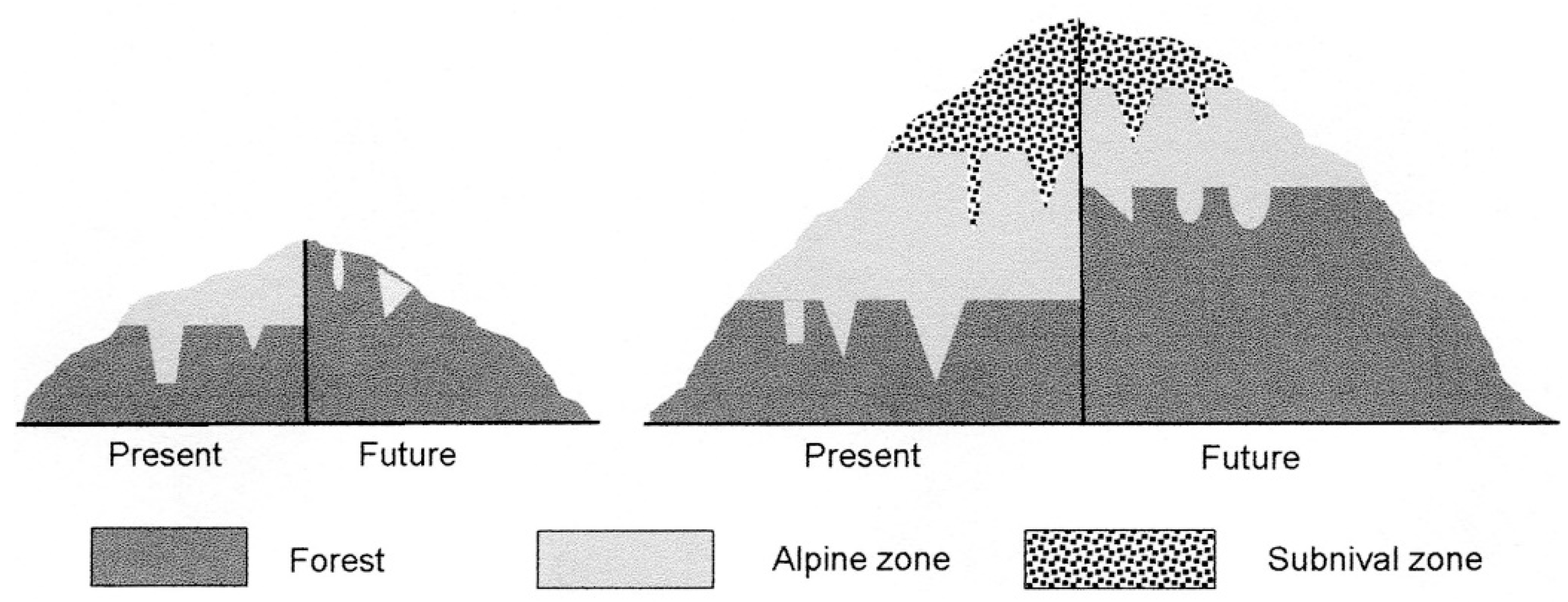
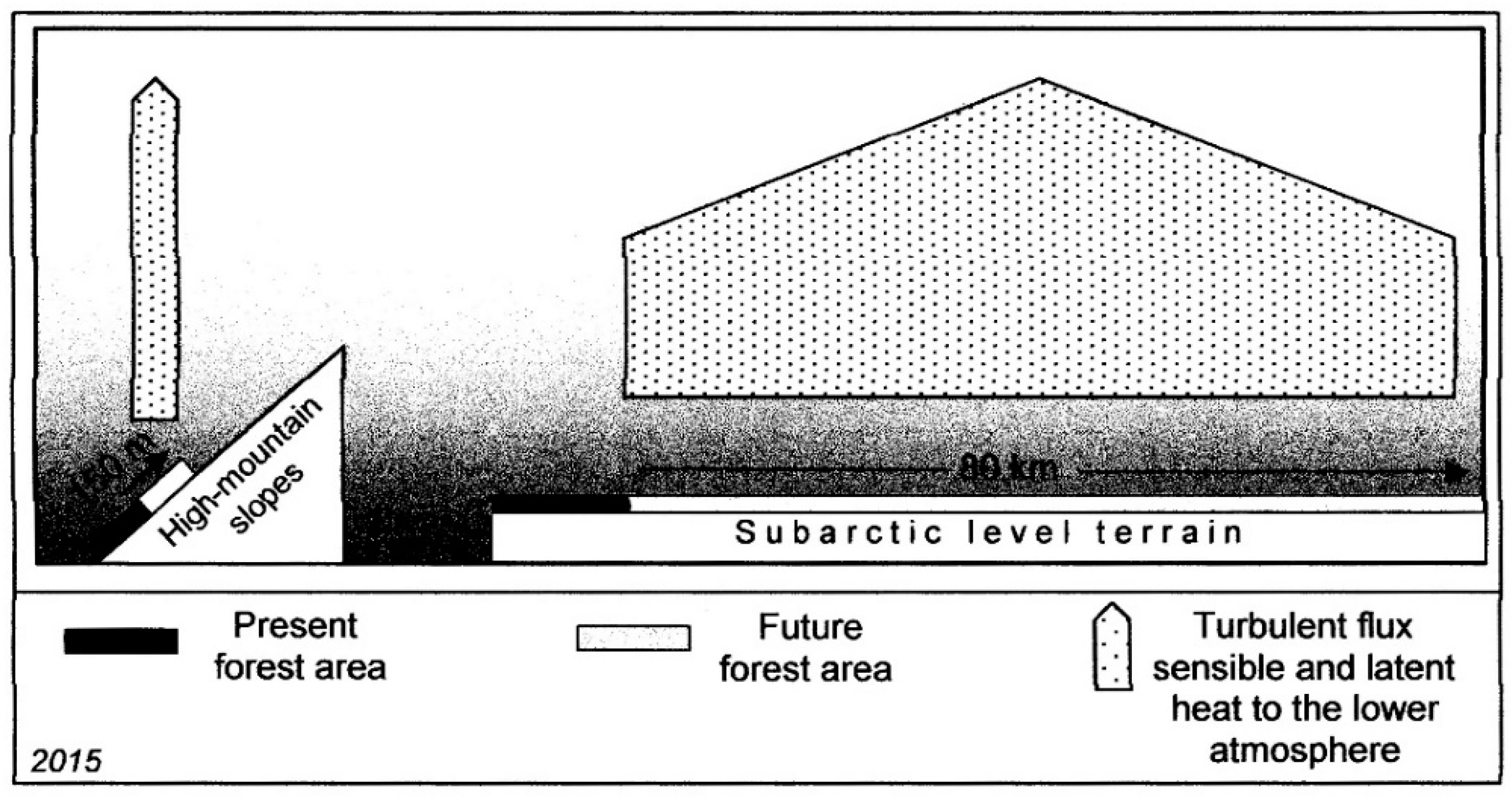
4. Treeline Dynamics at Different Spatial and Temporal Scales
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meentemeyer, V.; Box, E.O. Scale effects in landscape studies. In Landscape Heterogeneity and Disturbance; Turner, M.G., Ed.; Springer: New York, NY, USA, 1987; Volume 64, pp. 15–34. [Google Scholar]
- Keskitalo, E.C.H.; Horstkotte, T.; Kivinen, S.; Forbes, B.; Käyhkö, J. ‘Generality of mis-fit’? The real-life difficulty of matching scales in an interconnected world. Ambio 2016, 45, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Kullman, L. Modern climate change and shifting ecological states of the subalpine/alpine landscape in the Swedish Scandes. Geoöko 2007, 28, 187–221. [Google Scholar]
- Holtmeier, F.-K.; Broll, G. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Glob. Ecol. Biogeogr. 2005, 14, 395–410. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Mountain Timberlines: Ecology, Patchiness, and Dynamics, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Kullman, L.; Öberg, L. Post-Little Ice Age tree line rise by climate warming in the Swedish Scandes: A landscape ecological perspective. J. Ecol. 2009, 97, 415–429. [Google Scholar] [CrossRef]
- Butler, D.R.; Malanson, G.P.; Walsh, S.J.; Fagre, D.B. Influences of geomorphology and geology on alpine treeline in the American West—More important than climatic influences? Phys. Geogr. 2007, 28, 434–450. [Google Scholar] [CrossRef]
- Harsh, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.P.; Kipfmueller, F.F. Multi-scale influences of slope aspect and spatial pattern on ecological dynamics at upper treeline in the Southern Rocky Mountains, USA. Arct. Antarct. Alp. Res. 2010, 42, 45–56. [Google Scholar] [CrossRef]
- Whitesides, C.J.; Butler, D.R. Adequacies and deficiencies of alpine and subalpine treeline studies in the national parks of the Western United States. Prog. Phys. Geogr. 2010, 35, 19–42. [Google Scholar] [CrossRef]
- Case, B.S.; Duncan, R.O. A novel framework for disentangling the scale-dependent influences of abiotic factors on alpine treeline position. Ecography 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Case, B.S.; Buckley, H.L. Local-scale topoclimate effects on treeline elevations: A country-wide investigation of New Zealand’s southern beech treelines. PeerJ 2015, 3, e1334. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Malanson, G.P.; Walsh, S.J. Multiscale relationships between alpine treeline elevation and hypothesized environmental controls in the western United States. Ann. Assoc. Am. Geogr. 2015, 105, 437–453. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Ferrarini, A. Braking effects of climate and topography on global change-induced upslope forest expansion. Int. J. Biometeorol. 2016, 61, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Compostella, C.; Caccianiga, M. A comparison between different treeline types shows contrasting response to climate fluctuations. Plant Biosyst. 2016, 151, 436–449. [Google Scholar] [CrossRef]
- Malanson, G.P.; Brown, D.G.; Butler, D.R.; Cairns, D.M.; Fagre, D.B.; Walsh, S.J. Ecotone dynamics: Invasibility of alpine tundra by tree species from the subalpine forest. In The Changing Alpine Treeline: The Example of Glacier National Park, MT, USA; Butler, D., Malanson, G., Walsh, S., Fagre, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 12, pp. 35–61. [Google Scholar]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treeline. Functional Ecology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Tranquillini, W. Physiological Ecology of the Alpine Timberline—Tree Existence at High Altitudes with Special Reference to the European Alps, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979; Volume 31. [Google Scholar]
- Wilson, C.; Grace, J.; Allen, S.; Slack, F. Temperature and stature: A study of temperatures in montane vegetation. Funct. Ecol. 1987, 1, 405–413. [Google Scholar] [CrossRef]
- Grace, J.; Allen, S.; Wilson, C. Climate and meristem temperature of plant communities near the tree-line. Oecologia 1989, 79, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G. Climate at the upper timberline. In Trees at Their Upper Limit. Treelife Limitation at the Alpine Timberline, 1st ed.; Wieser, G., Tausz, M., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 5, pp. 19–36. [Google Scholar]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Evaluating thermal treeline indicators based on air and soil temperature using air-to-soil temperature transfer model. Ecol. Model. 2008, 213, 345–355. [Google Scholar] [CrossRef]
- Odland, A. Effect of latitude and mountain height on the timberline (Betula pubescens ssp. czerepanovii) elevation along the central Scandinavian mountain range. Fennia 2015, 193, 260–270. [Google Scholar]
- Kašpar, J.; Treml, V. Thermal charcteristics of alpine treelines in Central Europe north of the Alps. Clim. Res. 2016, 68, 1–12. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Geoökologische Beobachtungen und Studien an der Subarktischen und Alpinen Waldgrenze in Vergleichender Sicht (Nördliches Fennoskandien/Zentralalpen), 1st ed.; Steiner: Wiesbaden, Germany, 1974. [Google Scholar]
- Pereg, D.; Payette, S. Development of black spruce growth forms at treeline. Plant Ecol. 1998, 138, 137–147. [Google Scholar] [CrossRef]
- Kullman, L. One century of treeline change and stability—An illustrated view from the Swedish Scandes. Landsc. Online 2010, 17, 1–31. [Google Scholar] [CrossRef]
- Kihlmann, A.O. Pflanzenbiologische Studien aus Russisch-Lappland; Weilin & Göös: Helsinki, Finland, 1890. [Google Scholar]
- Roder, K. Die Polare Waldgrenze. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 1895. [Google Scholar]
- Wegener, A. Das Wesen der Baungrenze. Meteorol. Z. 1923, 40, 371–372. [Google Scholar]
- Holtmeier, F.-K. What does the term “krummholz” really mean? Observations with special reference to the Alps and the Colorado Front Range. Mt. Res. Dev. 1981, 1, 253–260. [Google Scholar] [CrossRef]
- Gamache, I.; Payette, S. Height growth response of treeline black spruce to recent climate warming across the forest-tundra ecotone of eastern Canada. J. Ecol. 2004, 92, 835–845. [Google Scholar] [CrossRef]
- Devi, N.; Hagedorn, F.; Moiseev, P.; Bugmann, H.; Shiyatov, S.; Mazepa, V.; Rigling, A. Expanding forests and changing growth forms of Siberian larch at the Polar Urals treeline during the 20th century. Glob. Chang. Biol. 2008, 14, 1581–1592. [Google Scholar] [CrossRef]
- Öberg, L. Treeline Dynamics in Short and Long Term Perspectives—Observational and Historical Evidence from the Southern Swedish Scandes. Ph.D. Thesis, Mid Sweden University, Sundsvall, Sweden, 15 March 2013. [Google Scholar]
- Petrov, I.A.; Kharuk, V.I.; Dsvinskaya, M.L.; Im, S.T. Reaction of coniferous trees in the Kuznetsk Alatau alpine forest-tundra ecotone to climate change. Contemp. Probl. Ecol. 2015, 8, 423–430. [Google Scholar] [CrossRef]
- Kapralov, D.S.; Shiyatov, S.G.; Moiseev, P.A.; Fomin, V.V. Changes in the composition, structure, and altitudinal distribution of low forests at the upper limit of their growth in the northern Ural Mountains. Russ. J. Ecol. 2006, 37, 367–372. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Altitudinal and polar treelines in the northern hemisphere—causes and response to climate change. Polarforschung 2010, 79, 139–153. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Die klimatische Waldgrenze—Linie oder Übergangssaum (Ökoton)? Ein Diskussionsbeitrag unter besonderer Berücksichtigung der Waldgrenze in den mittleren und hohen Breiten der Nordhalbkugel. Erdkunde 1985, 30, 271–285. [Google Scholar]
- Didier, L.; Brun, J.-J. Limite supraforestière et changements environnementaux: Pour une approche pluriscalaire et spatialisée des ècoystèmes d’ altitude. Geogr. Phys. Quat. 1998, 52, 1–9. [Google Scholar] [CrossRef]
- Dial, R.J.; Berg, E.E.; Timm, K.; McMahon, A.; Geck, J. Changes in the alpine forest-tundra ecotone commensurate with recent warming in southcentral Alaska: Evidence from orthophotos and field plots. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Ninot, J.M.; Battlori, W.; Carillo, E.; Carreras, J.; Ferré, A.; Gutiérrez, E. Timberline structure and limited tree recruitment in the Catalan Pyrenees. Plant Ecol. Divers. 2008, 1, 47–57. [Google Scholar] [CrossRef]
- Grafius, D.R.; Malanson, G.P.; Weiss, D.J. Secondary controls of alpine treeline elevations in the western USA. Phys. Geogr. 2014, 33, 146–164. [Google Scholar] [CrossRef]
- Kullman, L. Norway spruce (Picea abies (L.) Karst.) treeline ecotone performance since the mid-1970s in the Swedish Scandes—Evidence of stability and minor change from repeat surveys and photography. Geoöko 2015, 36, 23–53. [Google Scholar]
- Shresta, K.B.; Hofgaard, A.; Vandvik, U. Tree-growth response to climatic variability in two climatically contrasting treeline ecotone areas, central Himalaya, Nepal. Can. J. For. Res. 2015, 45, 1643–1653. [Google Scholar] [CrossRef]
- Vanière, B.; Blarquez, O.; Rius, D.; Doyen, E.; Brücher, T.; Colombaroli, D.; Connor, S.; Feurdean, A.; Hickler, T.; Kaltenrieder, P.; et al. 7000-year human legacy of elevation-dependent European fire regimes. Quat. Sci. Rev. 2016, 132, 206–212. [Google Scholar] [CrossRef]
- Alftine, K.J.; Malanson, G.P. Directional positive feedback and pattern at an alpine tree line. J. Veg. Sci. 2004, 15, 3–12. [Google Scholar] [CrossRef]
- Kullman, L. Wind-conditioned 20th century decline of birch treeline vegetation in the Swedish Scandes. Arctic 2005, 58, 286–294. [Google Scholar] [CrossRef]
- Resler, L.M. Geomorphic controls of spatial pattern and process at alpine treeline. Prof. Geogr. 2006, 5, 124–138. [Google Scholar] [CrossRef]
- Broll, G.; Holtmeier, F.-K.; Anschlag, K.; Brauckmann, H.-J.; Wald, S.; Drees, B. Landscape mosaic in the treeline ecotone on Mt. Rodjanoaivi, subarctic Finland. Fennia 2007, 185, 89–105. [Google Scholar]
- Batllori, E. Regional Assessment of Recent Pinus Uncinata Alpine Treeline Dynamics in the Pyrenees. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2008. [Google Scholar]
- Malanson, G.P.; Resler, M.M.; Bader, M.Y.; Holtmeier, F.-K.; Butler, D.R.; Weiss, D.J.; Daniels, L.D.; Fagre, D.B. Mountain treelines: A roadmap for research orientation. Arct. Antarc. Alp. Res. 2011, 43, 167–177. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Landform influences on treeline patchiness and dynamics in a changing climate. Phys. Geogr. 2012, 35, 430–437. [Google Scholar] [CrossRef]
- Moyes, A.B.; Germino, M.J.; Kueppers, L. Moisture rivals temperature in limiting photosenthesis by trees establishing beyond their cold-edge range limit under ambient and warmed conditions. New Phytol. 2015, 207, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Gaire, N.P.; Koirala, M.; Bhuju, D.R.; Carrer, M. Site- and species specific treeline responses to climatic variability in eastern Nepal Himalaya. Dendrochronologia 2016, 41, 44–56. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Bowman, W.D.; Humphries, H.; Seastedt, T.; Suding, K. Changes in alpine vegetation of 21 years: Are patterns across a heterogeneous landscape consistent with predictions? Ecosphere 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Smith, W.K.; Germino, M.J.; Johnson, D.M.; Reinhardt, K. The altitude of alpine treeline: A bellwether of climate change effects. Bot. Rev. 2009, 75, 163–190. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. The influence of tree islands and microtopography on pedoecological conditions in the forest-alpine tundra ecotone on Niwot Ridge, Colorado Front Range, USA. Arct. Antarc. Alp. Res. 1992, 24, 216–228. [Google Scholar] [CrossRef]
- Stöhr, D. Soils—Heterogeneity at a microscale. In Trees at Their Upper Limit. Treelife Limitation at the Alpine Timberline; Wieser, G., Tausz, M., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Valtera, M.; Šamonil, P.; Svoboda, M.; Janda, P. Effects of topography and forest stand dynamics on soil morphology in three natural mountain forests. Plant Soil 2015, 392, 57–69. [Google Scholar] [CrossRef]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clément, J.-C.; Fajardo, A.; Lavorel, S.; Sunsdqvist, M.K.; Bahn, M.; Chisholm, C.; et al. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.M.M.; Jeffrees, C.E.; Rees, W.G. Paludification and forest retreat in northern oceanic environments. Ann. Bot. 2003, 91, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Holtmeier, F.-K. Relocation of snow and its effects in the treeline ecotone—With special regard to the Rocky Mountains, the Alps and Northern Europe. Die Erde 2005, 4, 334–374. [Google Scholar]
- Holtmeier, F.-K.; Broll, G. Feedbacks of clonal groups and tree clusters at treeline: Implications for treeline dynamics. Clim. Res. 2017. [Google Scholar] [CrossRef]
- Grace, J. Tree lines. Philos. Trans. R. Soc. B 1989, 234, 233–245. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Growth and carbon relations of tree line forming conifers at constant vs. variable low soil temperature. J. Ecol. 2009, 97, 57–66. [Google Scholar] [CrossRef]
- Anschlag, K.; Broll, G.; Holtmeier, F.-K. Mountain birch seedlings in the treeline ecotone, Subarctic Finland. Variation in above- and below-ground growth in relation to microtopography. Arct. Antarc. Alp. Res. 2008, 40, 609–616. [Google Scholar] [CrossRef]
- Bader, M.Y.; van Geloof, I.; Rietkerk, M. High solar radiation hinders tree regeneration above the alpine treeline in Ecuador. Plant Ecol. 2008, 191, 33–45. [Google Scholar] [CrossRef]
- Germino, M.J.; Smith, W.K. Differences in microsite, plant form, and low-temperature photoinhibition of photosynthesis in alpine plants. Arct. Antarc. Alp. Res. 2000, 32, 388–396. [Google Scholar] [CrossRef]
- Sutinen, M.-L.; Ritari, A.; Holappa, T.; Kujala, K. Seasonal changes in soil temperature and in the frost hardiness of Scots pine roots under subarctic conditions. In Proceedings of the International Symposium on Physics, Chemistry, and Ecology of Seasonally Frozen Soils, Fairbanks, AK, USA, 10–12 June 1997; Islande, I.K., Wright, E.A., Radtke, J.K., Shamatt, B.S., Groeneveld, O.H., Hinzman, L.B., Eds.; U.S. Army Cold Regions Research and Engineering Laboratory: Hannover, NH, USA, 1997; pp. 513–517. [Google Scholar]
- Weih, M.; Karlsson, P.S. Low winter temperature affects summertime nutrient uptake capacity and growth rate of mountain birch seedlings in the Subarctic, Swedish Lapland. Arct. Antarct. Alp. Res. 2002, 34, 434–439. [Google Scholar] [CrossRef]
- Sutinen, R.; Vajda, A.; Hänninen, P.; Sutinen, M.-L. Significance of snowpack for root-zone water and temperature cycles in subarctic Lapland. Arct. Antarct. Alp. Res. 2009, 41, 373–380. [Google Scholar] [CrossRef]
- Caccianiga, M.; Payette, S. Recent advance of white spruce (Picea glauca) in the coastal tundra of the eastern shore of Hudson Bay (Québec, Canada). J. Biogeogr. 2006, 33, 2120–2135. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Ablegerbildung im Hochlagenwald und an der oberen Waldgrenze in der Front Range, Colorado. Mitt. Dtsch. Dendrol. Ges. 1999, 84, 39–61. [Google Scholar]
- Wiegand, T.; Camarero, J.J.; Rüdiger, N.; Gutiérrez, E. Abrupt population changes in treeline ecotones along smooth gradients. J. Ecol. 2006, 94, 880–892. [Google Scholar] [CrossRef]
- Lingua, E.; Cherubini, P.; Motta, R.; Nola, P. Spatial structure along an altitudinal gradient in the Italian central Alps suggests competition and facilitation among coniferous species. J. Veg. Sci. 2008, 19, 425–436. [Google Scholar] [CrossRef]
- Wang, Y.; Camarero, J.J.; Lio, T.; Liang, E. Spatial pattern of Smith fir alpine treelines on the south-eastern Tibetan Plateau support that contingent local conditions drive recent treeline pattern. Plant Ecol. Divers. 2012, 5, 311–321. [Google Scholar] [CrossRef]
- Camarero, J.J.; Linares, J.C.; García-Cervigón, A.I.; Batllori, E.; Martínez, J.; Gutiérrez, E. Back to the future: The responses of alpine treelines to climate warming are constrained by the current ecotone structure. Ecosystems 2016, 1–18. [Google Scholar] [CrossRef]
- Barbeito, I.; Dawes, M.A.; Rixen, C.; Senn, J.; Bebi, P. Factors driving mortality and growth at treeline: A 30-year experiment of 92.000 conifers. Ecology 2012, 93, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Holtmeier, F.-K. Animals’ Influence on the Landscape and Ecological Importance; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2015. [Google Scholar]
- Paulsen, J.; Körner, C. A climate-based model to predict treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Hofgaard, A. Inter-relationships between treeline position, species diversity, land use and climate change in the central Scandes Mountains of Norway. Glob. Ecol. Biogeogr. Lett. 1997, 6, 419–429. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G.; Müterthies, A.; Anschlag, K. Regeneration of trees in the treeline ecotone, northern Finnish Lapland. Fennia 2003, 181, 103–128. [Google Scholar]
- Dalen, L.; Hofgaard, A. Differential regional treeline dynamics in the Scandes Mountains. Arct. Antarct. Alp. Res. 2005, 37, 284–296. [Google Scholar] [CrossRef]
- Batllori, E.; Camarero, J.J.; Ninot, J.M.; Gutiérrez, E. Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line responses to climate warming. Glob. Ecol. Biogeogr. 2009, 18, 460–472. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Response of Scots pine (Pinus sylvestris) to warming climate at its altitudinal limit in northernmost Subarctic Finland. Arctic 2011, 64, 269–280. [Google Scholar] [CrossRef]
- Hofgaard, A.; Tømmervik, H.; Rees, G.; Hansen, F. Latitudinal forest advance in northern Norway since the early 20th century. J. Biogeogr. 2013, 40, 938–949. [Google Scholar] [CrossRef]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhari, R.P.; Gerlitz, L.; Heyken, H.; Lange, J.; Müller, M.; Scholten, T.; et al. Do Himalayan treelines respond to recent climate change? An evaluation of sensitivity indicators. Earth Syst. Dyn. 2015, 6, 245–265. [Google Scholar] [CrossRef]
- Skre, O.; Gjelsvik, S. Physiological changes in seeds of Norway spruce, Picea abies (L.) Karst., during ripening and their ecological importance. Collect. Nord. 1983, 47, 123–131. [Google Scholar]
- Hutchins, H.E. Role of various animals in dispersal and establishment of whitebark pine in the Rocky Mountains, USA. In Proceedings of the International Workshop on Subalpine Stone Pines and Their Environment: The Status of Our Knowledge, St. Moritz, Switzerland, 5–11 September 1992; U.S. Dept. of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1994; pp. 163–171. [Google Scholar]
- Walker, X.; Henry, G.H.R.; McLeod, K.; Hofgaard, A. Reproduction and seedling establishment of Picea glauca across the northernmost forest-tundra region in Canada. Glob. Chang. Biol. 2012, 18, 3202–3211. [Google Scholar] [CrossRef]
- Juntunen, V.; Neuvonen, S. Natural regeneration of Scots pine and Norway spruce close to the timberline in northern Finland. Silva. Fenn. 2006, 40, 443–458. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Impact of wild herbivorous mammals and birds on the altitudinal and northern treeline ecotones. Landsc. Online 2012, 30, 1–28. [Google Scholar] [CrossRef]
- Moen, J.; Cairns, P.M.; Lafon, C.W. Factors structuring the treeline ecotone in Fennoscandia. Plant Ecol. Divers. 2008, 1, 77–87. [Google Scholar] [CrossRef]
- Treml, V.; Šenfeldr, M.; Chuman, T.; Ponocná, T.; Demková, K. Twentieh century treeline ecotone advance in the Sudetes Mountains (Central Europe) was induced by agricultural land abandonment rather than climate change. J. Veg. Sci. 2016, 27, 1207–1221. [Google Scholar] [CrossRef]
- Yao, J.S.; Feng, J.G.; Chen, B.X.; Shi, P.L.; Zhang, J.L.; Fang, J.P.; Wang, Z.K.; Yao, S.C.; Ding, L.B. Controls of seed quantity and quality of seedling recruitment of Smith fir along altitudinal gradient in southeastern Tibetan Plateau. J. Mt. Sci. 2016, 13, 811–821. [Google Scholar] [CrossRef]
- Troll, C. The upper timberlines in different climatic zones. Arct Alp. Res. 1973, 5, 3–18. [Google Scholar] [CrossRef]
- Horvat, I.; Glavac, V.; Ellenberg, H. Vegetation Südeuropas, 1st ed.; Fischer-Verlag: Stuttgart, Germany, 1974. [Google Scholar]
- Wardle, P. Alpine timberlines. In Arctic and Alpine Environments, 1st ed.; Ives, J., Barry, R., Eds.; Methuen: London, UK, 1974; pp. 371–402. [Google Scholar]
- Höllermann, P.W. Geoecological aspects oft the upper timberline in Tenerife, Canary Islands. Arct. Alp. Res. 1978, 19, 365–382. [Google Scholar] [CrossRef]
- Leuschner, C.; Schulte, M. Microclimatological investigations in the tropical alpine scrub of Maui, Hawaii: Evidence for a drought-induced alpine timberline. Pac. Sci. 1991, 45, 152–168. [Google Scholar]
- Brandes, R. Waldgrenzen griechischer Hochgebirge. Unter besonderer Berücksichtigung des Taygetos, Südpeloponnes (Walddynamik, Tannensterben, Dendrochronologie), 1st ed.; Fränkische Geographische Gesellschaft: Erlangen, Germany, 2007. [Google Scholar]
- Grunewald, K.; Scheithauer, J. Untersuchungen an der alpinen Waldgrenze im Piringebirge (Bulgarien). Geoöko 2008, 29, 1–32. [Google Scholar]
- Gonzáles de Andrés, E.; Camarero, J.J.; Büntgen, U. Complex climate constraints of upper treeline formation in the Pyrenees. Trees 2015, 29, 941–952. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Anthelme, F.; Harter, D.E.V.; Jentsch, A.; Lotter, E.; Steinbauer, M.J.; Beierkuhnlein, C. Patterns of island treeline elevation—A global perspective. Ecography 2015, 39, 427–436. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Smith, J.M.; Paritsis, J.; Veblen, T.T.; Chapman, T. Permanent plots show accelerating mortality in subalpine forests of the Colorado Front Range from 1982–2013. For. Ecol. Manag. 2015, 341, 8–17. [Google Scholar] [CrossRef]
- Winkler, D.F.; Chapin, K.J.; Kueppers, L.M. Soil moisture mediate alpine life form and community productivity responses to warming. Ecology 2016, 97, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Van Migroet, H.; Aysell, M.T.; Johnson, A.D. Soil microclimate and chemistry of spruce-fir tree islands in Northern Utah. Soil Sci. Soc. Am. J. 2000, 64, 1515–1525. [Google Scholar] [CrossRef]
- Dolanc, C.R.; Westfall, R.D.; Safford, H.D.; Thorne, J.H.; Schwartz, M.W. Growth-climate relationships for six subalpine tree species in a Mediterranean climate. Can. J. For. Res. 2013, 43, 1114–1126. [Google Scholar] [CrossRef]
- Gamache, I.; Payette, S. Latitudinal response of subarctic tree lines to recent climate change in Eastern Canada. J. Biogeogr. 2005, 32, 849–862. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Wind as an ecological agent at treelines in North America, the Alps, and in the European Subarctic. Phys. Geogr. 2010, 31, 203–233. [Google Scholar] [CrossRef]
- Temperli, C.; Veblen, T.T.; Hart, S.J.; Kulakowski, D.; Tepley, A. Interactions among spruce beetle disturbance, climate change and forest dynamics captured by a forest Landscape model. Ecosphere 2015, 6, 1–20. [Google Scholar] [CrossRef]
- Löffler, J.; Lundberg, A.; Rössler, O.; Bräuning, A.; Jung, G.; Pape, R.; Wundram, D. The alpine treeline under changing land use and changing climate: Approach and preliminary results from continental Norway. Nor. J. Geogr. 2004, 58, 183–193. [Google Scholar] [CrossRef]
- Battlori, E.; Camarero, J.J.; Gutiérrez, E. Current regeneration patterns at the treeline in the Pyrenees indicate similar recruitment processes irrespective of the past disturbance regime. J. Biogeogr. 2010, 37, 1938–1950. [Google Scholar]
- Mamet, S.D.; Cairns, D.M.; Brook, R.K.; Kershaw, G.P. Modeling the spatial distribution of subarctic forest in northern Manitoba using GIS-based terrain and climate data. Phys. Geogr. 2015, 36, 93–112. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Treeline advance—Driving processes and adverse factors. Landsc. Online 2007, 1, 1–33. [Google Scholar] [CrossRef]
- Kaczka, R.; Czaijka, B.; Łajczka, A.; Swargryzk, J.; Nicia, P. The timberline as result of the interactions among forest, abiotic environment and human activity in Babia Góra Mt., Western Carpathians. Geogr. Pol. 2015, 88, 177–191. [Google Scholar] [CrossRef]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J. Veg. Sci. 2007, 18, 571–582. [Google Scholar] [CrossRef]
- Rössler, O.; Löffler, J. Uncertainties of treeline alterations due to climatic change during the past century in the central Norwegian Scandes. Geoöko 2007, 27, 104–114. [Google Scholar]
- Staland, H.; Salmonsson, J.; Hörnberg, G. A thousand years of human impact in the northern Scandinavian mountain range: Long-lasting effects on forest lines and vegetation. Holocene 2010, 21, 379–391. [Google Scholar] [CrossRef]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-use legacies rather than climate change are driving the recent upward shift of the mountain tree line in the Pyrenees. Glob. Ecol. Biogeogr. 2015, 25, 263–273. [Google Scholar] [CrossRef]
- Schickhoff, U. The upper timberline in the Himalayas, Hindu Kush and Karakorum: A review of geographical and ecological aspects. In Mountain Ecosystems. Studies in Treeline Ecology, 1st ed.; Broll, G., Keplin, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Solberg, B.O.; Hofgaard, A.; Hytteborn, H. Shifts in radial growth responses of coastal Picea abies induced by climatic change during the 20th century, central Norway. Ecoscience 2002, 9, 79–88. [Google Scholar] [CrossRef]
- Tuovinen, M.; McCarrol, D.; Gudd, H.; Jalkanen, R.; Los, S. Spatial and temporal stability of the climate signal in northern Fennoscandian pine tree-ring width and maximum density. Boreas 2009, 38, 1–12. [Google Scholar] [CrossRef]
- Mathisen, I.E.; Hofgaard, A. Recent height and diameter growth in Scots pine (Pinus sylvestris L.) along the Arctic margin: The importance of growing season versus non-growing season climate factors. Plant Ecol. Divers. 2011, 4, 1–11. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Human impacts on high altitude forests and upper timberline with special reference to the Alps. In Human Impacts and Management of Mountain Forests; Fujimori, T., Kimura, M., Eds.; Forestry and Forest Products Research Institute: Ibaraki, Japan, 1987; pp. 9–20. [Google Scholar]
- McIntire, E.J.B.; Piper, F.I.; Fajardo, A. Wind exposure and light exposure, more than elevation-related temperature limit tree line seedling abundance on three continents. J. Ecol. 2016, 104, 1379–1390. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, Q.-B.; Deng, X.; Mäkinen, H. Fine scale distribution of treeline trees and the nurse plant facilitation on the eastern Tibetan Plateau. Ecol. Indic. 2016, 66, 251–258. [Google Scholar] [CrossRef]
- Elliott, G.P.; Cowell, C.M. Slope aspect mediates fine-scale tree establishment pattern at upper treeline during wet and dry periods of the 20th century. Arct. Antarc. Alp. Res. 2015, 47, 681–692. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Die ökologische Funktion des Tannenhähers im Zirben-Lärchenwald und an der Waldgrenze im Oberengadin. J. Ornithol. 1966, 4, 337–345. [Google Scholar] [CrossRef]
- Neuvonen, S.; Bylund, H.; Tömmervik, H. Forest defoliation risk in birch forest by insects under different climate and land use scenarios in northern Europe. In Plant Ecology, Herbivory, and Human Impact in Nordic Mountain Birch Forests, 1st ed.; Wielgolaski, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 180, pp. 125–138. [Google Scholar]
- Schütz, H.-U. Pocket gopher—Actor under the stage. Studies on Niwot Ridge, Colorado Front Range, USA. In Mountain Ecosystems. Studies in Treeline Ecology, 1st ed.; Broll, G., Keplin, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 153–180. [Google Scholar]
- Cairns, D.; Moen, J.; Young, A. Influences of animal activity on treeline position and pattern: Implications for treeline responses to climate change. Phys. Geogr. 2007, 28, 419–433. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Björk, R.G.; Molau, U. Establishment of boreal forest species in alpine dwarf-shrub heath in subarctic Sweden. Plant Ecol. Divers. 2008, 1, 67–75. [Google Scholar] [CrossRef]
- Hofgaard, A.; Løkken, J.O.; Dalen, L.; Hytteborn, H. Comparing warming and grazing effects on birch growth in an alpine environment—A 10-year-experiment. Plant Ecol. Divers. 2010, 3, 19–27. [Google Scholar] [CrossRef]
- Munier, A.; Hermanutz, L.; Jacobs, J.D.; Lewis, K. The interacting effects of temperature, ground disturbance and herbivory on seedling establishment: Implications for treeline advance with climate warming. Plant Ecol. 2010, 210, 19–30. [Google Scholar] [CrossRef]
- Aune, S.; Hofgaard, A.; Söderström, L. Contrasting climate and land-use driven tree encroachment pattern of subarctic tundra in northern Norway and the Kola Peninsula. Can. J. For. Res. 2011, 41, 437–449. [Google Scholar] [CrossRef]
- Bowman, W.D.; Theodose, T.A.; Schardt, J.C.; Conant, R.T. Constraints of nutrient availability on primary production in two alpine tundra communities. Ecology 1993, 74, 2085–2097. [Google Scholar] [CrossRef]
- Butler, D.R. The impact of climate change on patterns of zoogeomorphological influence: Examples from the Rocky Mountains of the Western USA. Geomorphology 2012, 157, 183–191. [Google Scholar] [CrossRef]
- Greenwood, S.; Jump, A.S. Consequences of treeline shifts for the diversity of high altitude ecosystems. Arct. Antarc. Alp. Res. 2014, 46, 829–840. [Google Scholar] [CrossRef]
- Harding, R.; Khury, P.; Christensen, T.R.; Sykes, M.T.; Dankers, R.; Van der Linden, S. Climate feedbacks at the tundra-taiga interface. Ambio 2002, 12, 47–55. [Google Scholar]
- Tape, K.; Sturm, M.; Racine, C. The evidence of shrub expansion in northern Alaska and the Pan-Arctic. Glob. Chang. Biol. 2006, 12, 686–702. [Google Scholar] [CrossRef]
- Wookey, P.A.; Aerts, R.; Bardgett, R.D.; Baptist, F.; Bråthen, K.; Cornelissen, J.C.; Gough, L.; Hartley, I.P.; Hopkins, D.W.; Lavorel, S.; et al. Ecosystem feedbacks and cascade processes: Understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob. Chang. Biol. 2008, 15, 1153–1172. [Google Scholar] [CrossRef]
- MacDonald, G.M.; Kremenetski, K.V.; Beilman, D.W. Climate change and the northern Russian treeline ecotone. Philos. Trans. R. Soc. B 2008, 363, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, I.E. Structure, dynamics and regeneration capacity at the subarctic forest-tundra ecotone of northern Norway and Kola Peninsula, NW Russia. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2013. [Google Scholar]
- Callaghan, T.V.; Crawford, R.M.M.; Eronen, M.; Hofgaard, A.; Payette, S.; Rees, W.G.; Skre, O.; Sveinbjörnsson, B.; Vlassova, T.K.; Werkman, B.R. The dynamics of the tundra-taiga boundary: An overview and suggested coordination and integrated approach to research. Ambio 2002, 12, 3–5. [Google Scholar]
- De Wit, H.A.; Bryn, A.; Hofgaard, A.; Karstensen, J.; Kvalevåg, M.M.; Peters, G.P. Climate warming feedback from mountain birch forest expansion: Reduced albedo dominates carbon uptake. Glob. Chang. Biol. 2014, 20, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.T.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef]
- Danby, R.; Hik, D.S. Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Glob. Chang. Biol. 2007, 13, 437–451. [Google Scholar] [CrossRef]
- Stueve, M.; Isaacs, R.E.; Tyrell, L.E.; Densmore, R.V. Spatial variability of biotic and abiotic tree establishment constraints across a tundra ecotone in the Alaska Range. Ecology 2011, 92, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Bugmann, H.; Zierl, B.; Schumacher, S. Projecting the impacts of climate change on mountain forests and landscapes. In Global Change and Mountain Regions: An Overview of Current Knowledge, 1st ed.; Huber, U., Bugmann, H., Reasoner, M., Eds.; Springer: Dordecht, The Netherlands, 2005; pp. 477–487. [Google Scholar]
- Lantz, T.C.; Marsh, P.; Kokelj, S.V. Recent shrub proliferation in the Mackenzie Delta uplands and microclimatic implications. Ecosystem 2012, 16, 40–59. [Google Scholar] [CrossRef]
- Tremblay, B.; Lévesque, E.; Boudreau, S. Recent expansion of erect shrubs in the Low Arctic: Evidence from Eastern Nunavik. Environ. Res. Lett. 2012, 7. [Google Scholar] [CrossRef]
- Frost, G.V.; Epstein, H.E. Tall shrubs and tree expansion in Siberian tundra ecotones since the 1960s. Glob. Chang. Biol. 2014, 20, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Montesano, P.M.; Sun, G.; Dubayah, R.Q.; Ranson, K.J. Spaceborne potential for examining taiga-tundra ecotone forms and vulnerability. Biogeosciences 2016, 13, 3847–3861. [Google Scholar] [CrossRef]
- Baker, W.L.; Weisberg, D.J. Landscape analysis of the forest-tundra ecotone in Rocky Mountain National Park, Colorado. Prof. Geogr. 1995, 47, 361–375. [Google Scholar] [CrossRef]
- Rees, G.; Brown, I.; Mikkola, K.; Virtanen, T.; Werkman, B. How can the dynamics of the tundra-taiga boundary be remotely monitored? Ambio 2002, 12, 56–62. [Google Scholar]
- Roush, W.; Munroe, J.S.; Fagre, D.B. Development of a spatial analysis method using ground-based repeat photography to detect changes in the alpine treeline ecotone, Glacier National Park, MT, USA. Arct. Antarc. Alp. Res. 2007, 39, 297–308. [Google Scholar] [CrossRef]
- Rundqvist, S.; Hedenås, H.; Sandström, A.; Emanuelsson, U.; Eriksson, H.; Jonesson, C.; Callaghan, T.V. Tree and shrub expansion over the past 34 years at the treeline near Abisko, Sweden. Ambio 2011, 40, 683–692. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtmeier, F.-K.; Broll, G. Treelines—Approaches at Different Scales. Sustainability 2017, 9, 808. https://doi.org/10.3390/su9050808
Holtmeier F-K, Broll G. Treelines—Approaches at Different Scales. Sustainability. 2017; 9(5):808. https://doi.org/10.3390/su9050808
Chicago/Turabian StyleHoltmeier, Friedrich-Karl, and Gabriele Broll. 2017. "Treelines—Approaches at Different Scales" Sustainability 9, no. 5: 808. https://doi.org/10.3390/su9050808
APA StyleHoltmeier, F.-K., & Broll, G. (2017). Treelines—Approaches at Different Scales. Sustainability, 9(5), 808. https://doi.org/10.3390/su9050808





