Quantitative Estimation of Soil Salinity Using UAV-Borne Hyperspectral and Satellite Multispectral Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. EMI Measurements
2.3. Soil Sampling and Laboratory Measurement
2.4. Remote Sensing Data Processing
2.5. Soil Salinity Prediction Using RF
3. Results
3.1. Soil Salinity Content and Variation
3.2. Prediction Accuracy of RF Regression Models
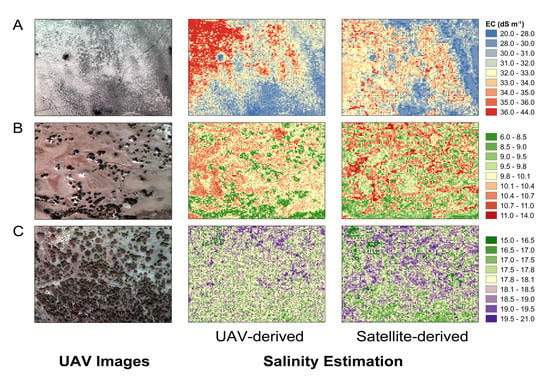
3.3. Soil Salinity Maps Derived from UAV and GF-2 Data
4. Discussion
4.1. Comparison of RF Regression Models Based on UAV and GF-2
4.2. Soil Salinity under Various Vegetation Cover Conditions
4.3. Evaluation of the Variable Importance for Hyperspectral Soil Salinity Modeling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dehni, A.; Lounis, M. Remote sensing techniques for salt affected soil mapping: Application to the oran region of algeria. Procedia Eng. 2012, 33, 188–198. [Google Scholar] [CrossRef]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; CAB International: Wallingford, UK, 1995. [Google Scholar]
- Corwin, D. Past, Present, and Future Trends of Soil Electrical Conductivity Measurement Using Geophysical Methods; FAO: Rome, Italy, 2008. [Google Scholar]
- Ding, J.; Yu, D. Monitoring and evaluating spatial variability of soil salinity in dry and wet seasons in the werigan–kuqa oasis, china, using remote sensing and electromagnetic induction instruments. Geoderma 2014, 235–236, 316–322. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Shi, Z.; Li, H. Mapping spatial variability of soil salinity in a coastal paddy field based on electromagnetic sensors. PLoS ONE 2015, 10, e0127996. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Ballantyne, A.K.; Cameron, D.R.; Read, D.W.L. Measurement of apparent electrical conductivity of soils by an electromagnetic induction probe to aid salinity surveys. Soil Sci. Soc. Am. J. 1979, 43, 810–812. [Google Scholar] [CrossRef]
- McNeill, J.D. Rapid, accurate mapping of soil salinity by electromagnetic ground conductivity meters. In Advances in Measurement of Soil Physical Properties: Bringing Theory into Practice; Topp, G.C., Reynolds, W.D., Green, R.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1992; pp. 209–229. [Google Scholar]
- Corwin, D.L.; Lesch, S.M. Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, Z.; Li, H.Y.; Triantafilis, J. Application of digital soil mapping methods for identifying salinity management classes based on a study on coastal central china. Soil Use Manag. 2013, 29, 445–456. [Google Scholar] [CrossRef]
- Triantafilis, J.; Odeh, I.O.A.; McBratney, A.B. Five geostatistical models to predict soil salinity from electromagnetic induction data across irrigated cotton. Soil Sci. Soc. Am. J. 2001, 65, 869–878. [Google Scholar] [CrossRef]
- Csillag, F.; Pasztor, L.; Biehl, L.L. Spectral band selection for the characterization of salinity status of soils. Remote Sens. Environ. 1993, 43, 231–242. [Google Scholar] [CrossRef]
- Eldeiry, A.A.; Garcia, L.A. Detecting soil salinity in alfalfa fields using spatial modeling and remote sensing. Soil Sci. Soc. Am. J. 2008, 72, 201–211. [Google Scholar] [CrossRef]
- Kalra, N.K.; Joshi, D.C. Potentiality of landsat, spot and irs satellite imagery, for recognition of salt affected soils in indian arid zone. Int. J. Remote Sens. 1996, 17, 3001–3014. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Metternicht, G.; Goldshleger, N.; Mor, E.; Mirlas, V.; Basson, U. Review of Remote Sensing-Based Methods to Assess Soil Salinity; Taylor & Francis Group: Abingdon, UK, 2009; pp. 39–60. [Google Scholar]
- Metternicht, G.I.; Zinck, J.A. Remote sensing of soil salinity: Potentials and constraints. Remote Sens. Environ. 2003, 85, 1–20. [Google Scholar] [CrossRef]
- Dwivedi, R.S.; Sreenivas, K. Image transforms as a tool for the study of soil salinity and alkalinity dynamics. Int. J. Remote Sens. 1998, 19, 605–619. [Google Scholar] [CrossRef]
- Hick, P.T.; Russell, W.G.R. Some spectral considerations for remote-sensing of soil-salinity. Aust. J. Soil Res. 1990, 28, 417–431. [Google Scholar] [CrossRef]
- Mougenot, B.; Pouget, M.; Epema, G.F. Remote sensing of salt affected soils. Remote Sens. Rev. 1993, 7, 241–259. [Google Scholar] [CrossRef]
- Verma, K.S.; Saxena, R.K.; Barthwal, A.K.; Deshmukh, S.N. Remote-sensing technique for mapping salt-affected soils. Int. J. Remote Sens. 1994, 15, 1901–1914. [Google Scholar] [CrossRef]
- Dehaan, R.L.; Taylor, G.R. Field-derived spectra of salinized soils and vegetation as indicators of irrigation-induced soil salinization. Remote Sens. Environ. 2002, 80, 406–417. [Google Scholar] [CrossRef]
- Douaoui, A.E.K.; Nicolas, H.; Walter, C. Detecting salinity hazards within a semiarid context by means of combining soil and remote-sensing data. Geoderma 2006, 134, 217–230. [Google Scholar] [CrossRef]
- Eldeiry, A.A.; Garcia, L.A. Comparison of ordinary kriging, regression kriging, and cokriging techniques to estimate soil salinity using landsat images. J. Irrig. Drain. Eng. Asce 2010, 136, 355–364. [Google Scholar] [CrossRef]
- Fernandez-Buces, N.; Siebe, C.; Cram, S.; Palacio, J.L. Mapping soil salinity using a combined spectral response index for bare soil and vegetation: A case study in the former lake texcoco, mexico. J. Arid Environ. 2006, 65, 644–667. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Patkin, K.; Banin, A.; Karnieli, A. Mapping of several soil properties using dais-7915 hyperspectral scanner data—A case study over clayey soils in israel. Int. J. Remote Sens. 2002, 23, 1043–1062. [Google Scholar] [CrossRef]
- Cloutis, E.A. Hyperspectral geological remote sensing: Evaluation of analytical techniques. Int. J. Remote Sens. 1996, 17, 2215–2242. [Google Scholar] [CrossRef]
- Ghosh, G.; Kumar, S.; Saha, S.K. Hyperspectral satellite data in mapping salt-affected soils using linear spectral unmixing analysis. J. Indian Soc. Remote Sens. 2012, 40, 129–136. [Google Scholar] [CrossRef]
- Weng, Y.; Qi, H.; Fang, H.; Zhao, F.; Lu, Y. Plsr-based hyperspectral remote sensing retrieval of soil salinity of chaka-gonghe basin in qinghai province. Acta Pedol. Sin. 2010, 47, 1255–1263. [Google Scholar]
- Farifteh, J.; Van der Meer, F.D.; Atzberger, C.; Carranza, E.J.M. Quantitative analysis of salt-affected soil reflectance spectra: A comparison of two adaptive methods (plsr and ann). Remote Sens. Environ. 2007, 110, 59–78. [Google Scholar] [CrossRef]
- Pang, G.; Wang, T.; Liao, J.; Li, S. Quantitative model based on field-derived spectral characteristics to estimate soil salinity in minqin county, china. Soil Sci. Soc. Am. J. 2014, 78, 546–555. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Fereres Castiel, E.; Suárez Barranco, M.D.; Zarco-Tejada, P.J. Thermal and narrow-band multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. Ieee Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Gong, J. Uav remote sensing for urban vegetation mapping using random forest and texture analysis. Remote Sens. 2015, 7, 1074–1094. [Google Scholar] [CrossRef]
- Aasen, H.; Burkart, A.; Bolten, A.; Bareth, G. Generating 3d hyperspectral information with lightweight uav snapshot cameras for vegetation monitoring: From camera calibration to quality assurance. Isprs J. Photogramm. Remote Sens. 2015, 108, 245–259. [Google Scholar] [CrossRef]
- de Castro, A.I.; Torres-Sanchez, J.; Pena, J.M.; Jimenez-Brenes, F.M.; Csillik, O.; Lopez-Granados, F. An automatic random forest-obia algorithm for early weed mapping between and within crop rows using uav imagery. Remote Sens. 2018, 10, 285. [Google Scholar] [CrossRef]
- Lelong, C.; Burger, P.; Jubelin, G.; Roux, B.; Labbé, S.; Baret, F. Assessment of unmanned aerial vehicles imagery for quantitative monitoring of wheat crop in small plots. Sensors 2008, 8, 3557. [Google Scholar] [CrossRef] [PubMed]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Franceschini, M.H.D.; Kramer, H.; van Loo, E.N.; Jaramillo Roman, V.; Finkers, R. Uav based soil salinity assessment of cropland. Geoderma 2019, 338, 502–512. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Droogers, P.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on citrus physiology assessed by uav remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in china. Plant Soil 2011, 342, 495–526. [Google Scholar] [CrossRef]
- Heil, K.; Schmidhalter, U. Comparison of the em38 and em38-mk2 electromagnetic induction-based sensors for spatial soil analysis at field scale. Comput. Electron. Agric. 2015, 110, 267–280. [Google Scholar] [CrossRef]
- Peng, J.; Ji, W.; Ma, Z.; Li, S.; Chen, S.; Zhou, L.; Shi, Z. Predicting total dissolved salts and soluble ion concentrations in agricultural soils using portable visible near-infrared and mid-infrared spectrometers. Biosyst. Eng. 2016, 152, 94–103. [Google Scholar] [CrossRef]
- Roosjen, P.; Suomalainen, J.; Bartholomeus, H.; Kooistra, L.; Clevers, J. Mapping reflectance anisotropy of a potato canopy using aerial images acquired with an unmanned aerial vehicle. Remote Sens. 2017, 9, 417. [Google Scholar] [CrossRef]
- Adler-Golden, S.M.; Matthew, M.W.; Bernstein, L.S.; Levine, R.Y.; Berk, A.; Richtsmeier, S.C.; Acharya, P.K.; Anderson, G.P.; Felde, J.W.; Gardner, J.A.; et al. Atmospheric correction for shortwave spectral imagery based on modtran4. SPIE 1999, 3753, 61–69. [Google Scholar]
- Peng, J.; Biswas, A.; Jiang, Q.S.; Zhao, R.Y.; Hu, J.; Hu, B.F.; Shi, Z. Estimating soil salinity from remote sensing and terrain data in southern xinjiang province, china. Geoderma 2019, 337, 1309–1319. [Google Scholar] [CrossRef]
- Ho, T.K. The random subspace method for constructing decision forests. Ieee Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cutler, A.; Cutler, D.R.; Stevens, J.R. Random forests. In Ensemble Machine Learning: Methods and Applications; Zhang, C., Ma, Y., Eds.; Springer US: Boston, MA, USA, 2012; pp. 157–175. [Google Scholar]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Mutanga, O.; Adam, E.; Cho, M.A. High density biomass estimation for wetland vegetation using worldview-2 imagery and random forest regression algorithm. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 399–406. [Google Scholar] [CrossRef]
- Were, K.; Bui, D.T.; Dick, Ø.B.; Singh, B.R. A comparative assessment of support vector regression, artificial neural networks, and random forests for predicting and mapping soil organic carbon stocks across an afromontane landscape. Ecol. Indic. 2015, 52, 394–403. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomforest. R News 2002, 2, 18–22. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Forkuor, G.; Hounkpatin, O.K.L.; Welp, G.; Thiel, M. High resolution mapping of soil properties using remote sensing variables in south-western burkina faso: A comparison of machine learning and multiple linear regression models. PLoS ONE 2017, 12, e0170478. [Google Scholar] [CrossRef]
- Zhu, R.; Zeng, D.; Kosorok, M.R. Reinforcement learning trees. J. Am. Stat. Assoc. 2015, 110, 1770–1784. [Google Scholar] [CrossRef]
- Lawrence, I.K.L. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- Zhou, Y.; Hartemink, A.E.; Shi, Z.; Liang, Z.; Lu, Y. Land use and climate change effects on soil organic carbon in north and northeast china. Sci. Total Environ. 2019, 647, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Farifteh, J.; van der Meer, F.; van der Meijde, M.; Atzberger, C. Spectral characteristics of salt-affected soils: A laboratory experiment. Geoderma 2008, 145, 196–206. [Google Scholar] [CrossRef]
- Mashimbye, Z.E.; Cho, M.A.; Nell, J.P.; De Clercq, W.P.; Van Niekerk, A.; Turner, D.P. Model-based integrated methods for quantitative estimation of soil salinity from hyperspectral remote sensing data: A case study of selected south african soils. Pedosphere 2012, 22, 640–649. [Google Scholar] [CrossRef]
- Ji, W.J.; Li, S.; Chen, S.C.; Shi, Z.; Rossel, R.A.V.; Mouazen, A.M. Prediction of soil attributes using the chinese soil spectral library and standardized spectra recorded at field conditions. Soil Tillage Res. 2016, 155, 492–500. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef] [Green Version]
- Honkavaara, E.; Saari, H.; Kaivosoja, J.; Pölönen, I.; Hakala, T.; Litkey, P.; Mäkynen, J.; Pesonen, L. Processing and assessment of spectrometric, stereoscopic imagery collected using a lightweight uav spectral camera for precision agriculture. Remote Sens. 2013, 5, 5006–5039. [Google Scholar] [CrossRef]
- Lorenz, S.; Salehi, S.; Kirsch, M.; Zimmermann, R.; Unger, G.; Vest Sørensen, E.; Gloaguen, R. Radiometric correction and 3d integration of long-range ground-based hyperspectral imagery for mineral exploration of vertical outcrops. Remote Sens. 2018, 10, 176. [Google Scholar] [CrossRef]
- Le Rest, K.; Pinaud, D.; Monestiez, P.; Chadoeuf, J.; Bretagnolle, V. Spatial leave-one-out cross-validation for variable selection in the presence of spatial autocorrelation. Glob. Ecol. Biogeogr. 2014, 23, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.A.O.; Li, Y.A.N.; Xu, G.; Zou, T. Ecophysiological response and morphological adjustment of two central asian desert shrubs towards variation in summer precipitation. Plantcell Environ. 2007, 30, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes*. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.T.; Qi, J.G.; Gao, Y.; Ouyang, Z.T.; Zeng, S.L.; Zhao, B. Detecting soil salinity with modis time series vi data. Ecol. Indic. 2015, 52, 480–489. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Aldakheel, Y.Y. Assessing soil salinity using soil salinity and vegetation indices derived from ikonos high-spatial resolution imageries: Applications in a date palm dominated region. Geoderma 2014, 230, 1–8. [Google Scholar] [CrossRef]
- Sidike, A.; Zhao, S.; Wen, Y. Estimating soil salinity in pingluo county of china using quickbird data and soil reflectance spectra. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 156–175. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Tao, J.; Weng, Y. Soil salinity retrieval from advanced multi-spectral sensor with partial least square regression. Remote Sens. 2015, 7, 488–511. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zeng, S.L.; Gao, Y.; Ouyang, Z.T.; Li, B.; Fang, C.M.; Zhao, B. Using hyperspectral vegetation indices as a proxy to monitor soil salinity. Ecol. Indic. 2011, 11, 1552–1562. [Google Scholar] [CrossRef]




| Sensor | No. of Bands | Spectral Range (μm) | Spatial Resolution | Platform |
|---|---|---|---|---|
| Hyperspectral Imager | 62 | Visible and NIR | 0.1 m | UAV |
| B1~62: 0.50–0.89 | (flight height: 154 m) | |||
| GF-2 | 5 | Visible and NIR | 1 m (Panchromatic)/ 4 m (Multispectral) | Satellite |
| Band1: 0.45–0.52 (Blue) | ||||
| Band2: 0.52–0.59 (Green) | ||||
| Band3: 0.63–0.69 (Red) | ||||
| Band4: 0.77–0.89 (NIR) | ||||
| Panchromatic: 0.45–0.90 |
| Field | Conductivity | Descriptive Statistics (ECa, mS m−1;EC1:5, dS m−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | Median | Std.Dev. | CV | ||
| A | ECah | 30 | 571.15 | 955.72 | 765.05 | 766.72 | 119.02 | 16% |
| ECav | 30 | 598.15 | 1065.57 | 846.74 | 865.22 | 144.53 | 17% | |
| EC1:5 | 30 | 20.25 | 54.90 | 37.64 | 35.80 | 9.21 | 25% | |
| B | ECah | 30 | 450.20 | 1092.15 | 830.47 | 903.09 | 200.58 | 24% |
| ECav | 30 | 585.67 | 1035.90 | 824.51 | 779.02 | 154.27 | 19% | |
| EC1:5 | 30 | 7.20 | 14.68 | 11.73 | 11.91 | 2.38 | 20% | |
| C | ECah | 30 | 695.86 | 1126.99 | 890.15 | 861.09 | 136.54 | 15% |
| ECav | 30 | 560.17 | 955.56 | 778.00 | 782.09 | 118.92 | 15% | |
| EC1:5 | 30 | 9.64 | 19.64 | 14.11 | 14.50 | 2.94 | 21% | |
| Field | Descriptive Statistics (EC1:5, dS m−1) | ||||||
|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | Median | Std.Dev. | CV | |
| A | 1500 | 18.81 | 47.14 | 31.54 | 31.22 | 4.17 | 13% |
| B | 1500 | 5.04 | 15.20 | 9.89 | 10.00 | 1.64 | 17% |
| C | 1500 | 11.98 | 25.94 | 18.13 | 18.37 | 2.10 | 12% |
| Data Set | Source | A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | RPD | RMSE | CC | RPD | RMSE | CC | RPD | RMSE | ||
| (dS m−1) | (dS m−1) | (dS m−1) | ||||||||
| Training (n = 1000) | UAV | 0.96 | 3.92 | 1.05 | 0.94 | 3.29 | 0.49 | 0.81 | 1.91 | 1.07 |
| GF-2 | 0.93 | 2.93 | 1.40 | 0.92 | 2.75 | 0.58 | 0.74 | 1.67 | 1.22 | |
| Resampled UAV | 0.95 | 3.22 | 1.28 | 0.92 | 2.72 | 0.59 | 0.70 | 1.55 | 1.31 | |
| Validation (n = 500) | UAV | 0.94 | 2.98 | 1.40 | 0.86 | 2.15 | 0.74 | 0.56 | 1.29 | 1.59 |
| GF-2 | 0.88 | 2.23 | 1.87 | 0.84 | 2.00 | 0.80 | 0.44 | 1.20 | 1.71 | |
| Resampled UAV | 0.89 | 2.35 | 1.78 | 0.81 | 1.85 | 0.86 | 0.40 | 1.12 | 1.83 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Peng, J.; Zhou, Y.; Xu, D.; Zhao, R.; Jiang, Q.; Fu, T.; Wang, F.; Shi, Z. Quantitative Estimation of Soil Salinity Using UAV-Borne Hyperspectral and Satellite Multispectral Images. Remote Sens. 2019, 11, 736. https://doi.org/10.3390/rs11070736
Hu J, Peng J, Zhou Y, Xu D, Zhao R, Jiang Q, Fu T, Wang F, Shi Z. Quantitative Estimation of Soil Salinity Using UAV-Borne Hyperspectral and Satellite Multispectral Images. Remote Sensing. 2019; 11(7):736. https://doi.org/10.3390/rs11070736
Chicago/Turabian StyleHu, Jie, Jie Peng, Yin Zhou, Dongyun Xu, Ruiying Zhao, Qingsong Jiang, Tingting Fu, Fei Wang, and Zhou Shi. 2019. "Quantitative Estimation of Soil Salinity Using UAV-Borne Hyperspectral and Satellite Multispectral Images" Remote Sensing 11, no. 7: 736. https://doi.org/10.3390/rs11070736