Identifying Crop Growth Stages from Solar-Induced Chlorophyll Fluorescence Data in Maize and Winter Wheat from Ground and Satellite Measurements
Abstract
:1. Introduction
2. Materials and Methods
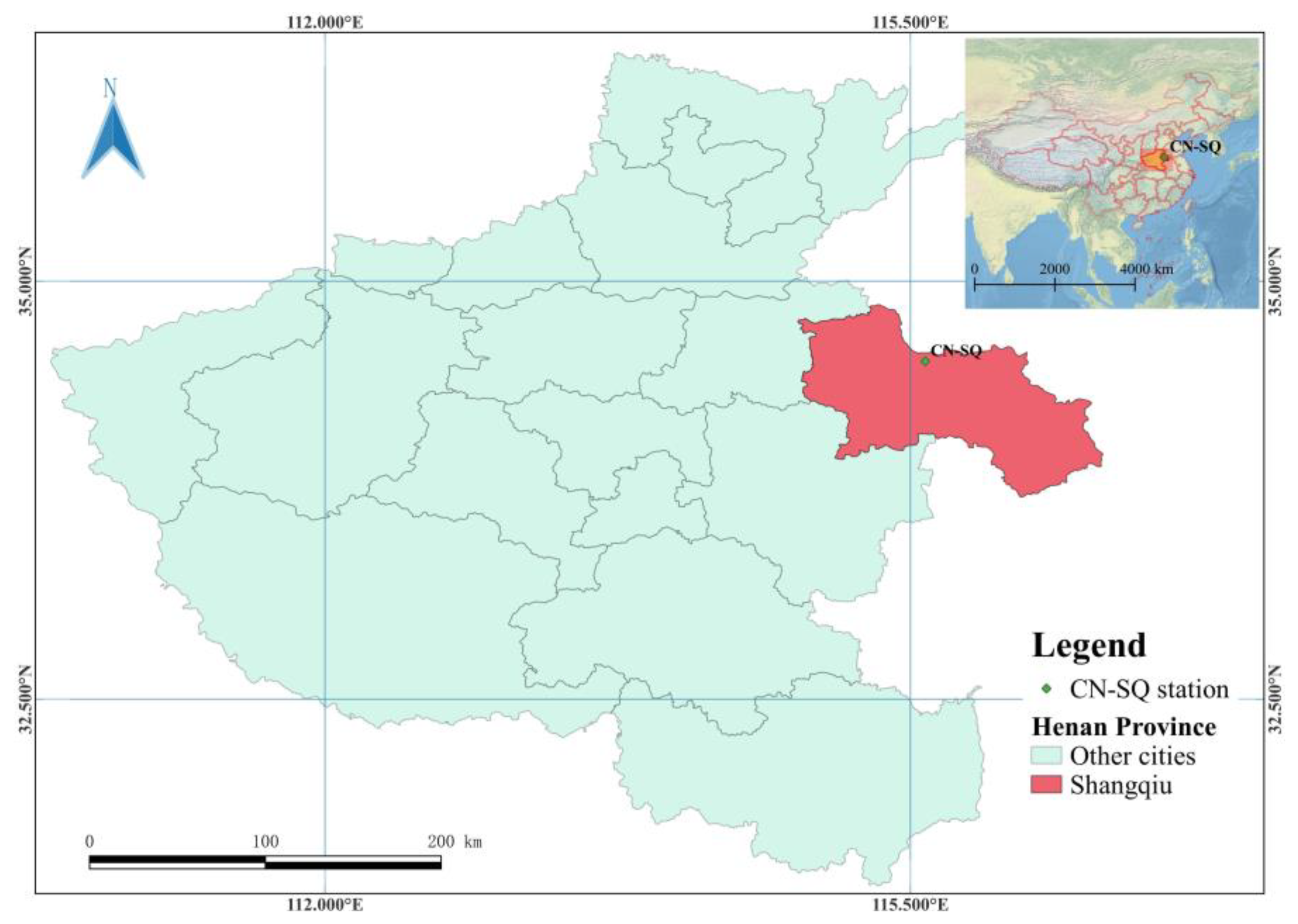
2.1. Study Site
2.2. Observed Crop Growth Stages
2.3. Ground-Based Spectrum Measurements
2.4. Satellite Data
2.4.1. TROPOMI SIF
2.4.2. MODIS EVI
2.5. Methods
2.5.1. Time-Series Phenological Pre-Processing
- Abnormal measurements elimination: To address abnormal measurements, a moving window abnormal elimination method was employed. This approach identifies measurements deviating by more than three times the standard deviation from the average value within an 11-day moving window (slightly longer than the interval of adjacent observed crop growth stages) and subsequently excludes them from the dataset [69].
- Time series smoothing: SIF and EVI time series underwent smoothing using a three-time Savitzky–Golay algorithm with an 11-day moving window [70]. This algorithm serves to attenuate off-season phenological signals while fitting a smoothing curve to the time-series observations.
2.5.2. Time-Series Phenological Modelling
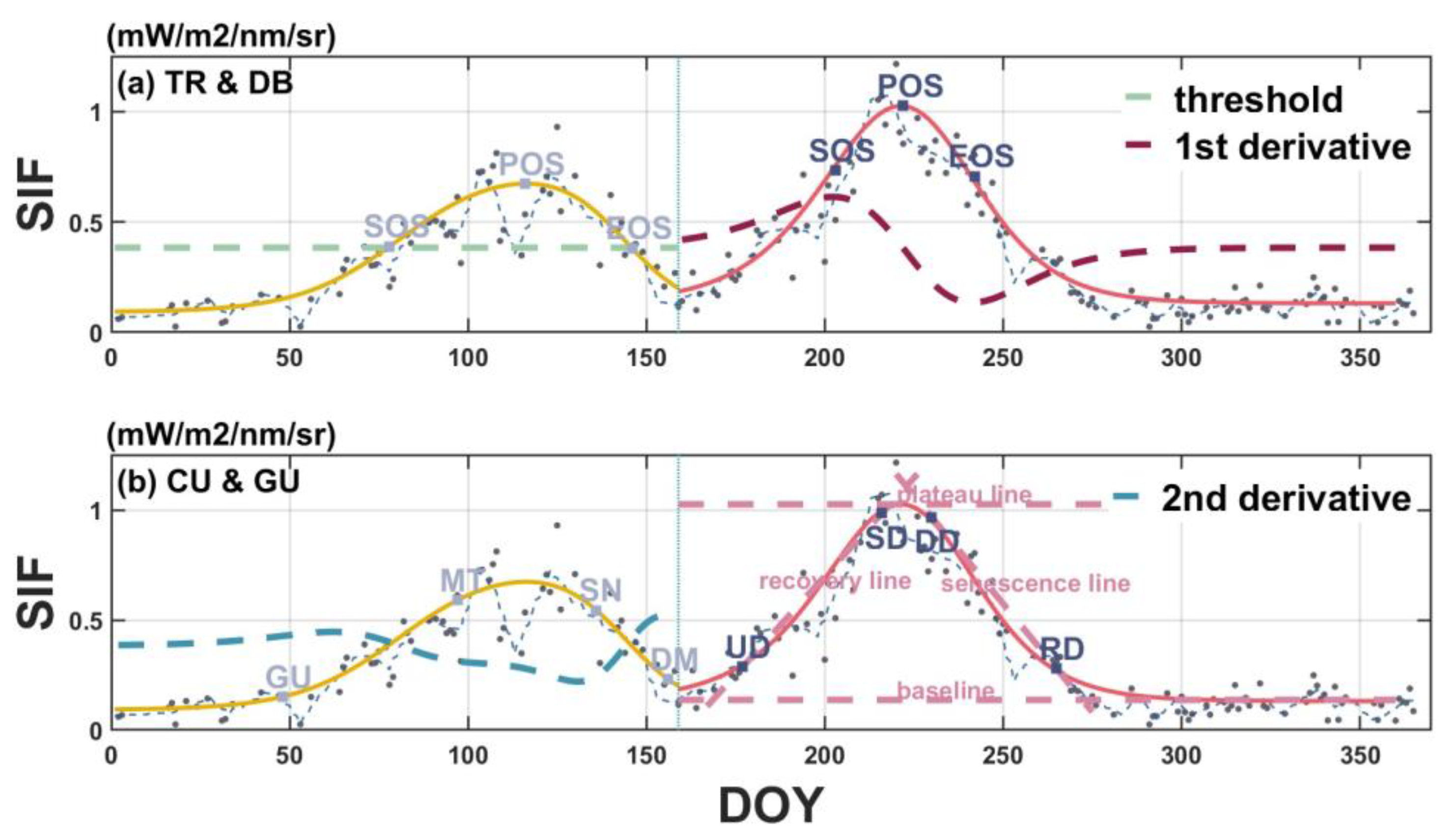
2.5.3. Phenological Transition Characterization
2.6. Accuracy Assessment
3. Results
3.1. Reconciliation of Time-Series Phenological Characteristics with Crop Growth Stages
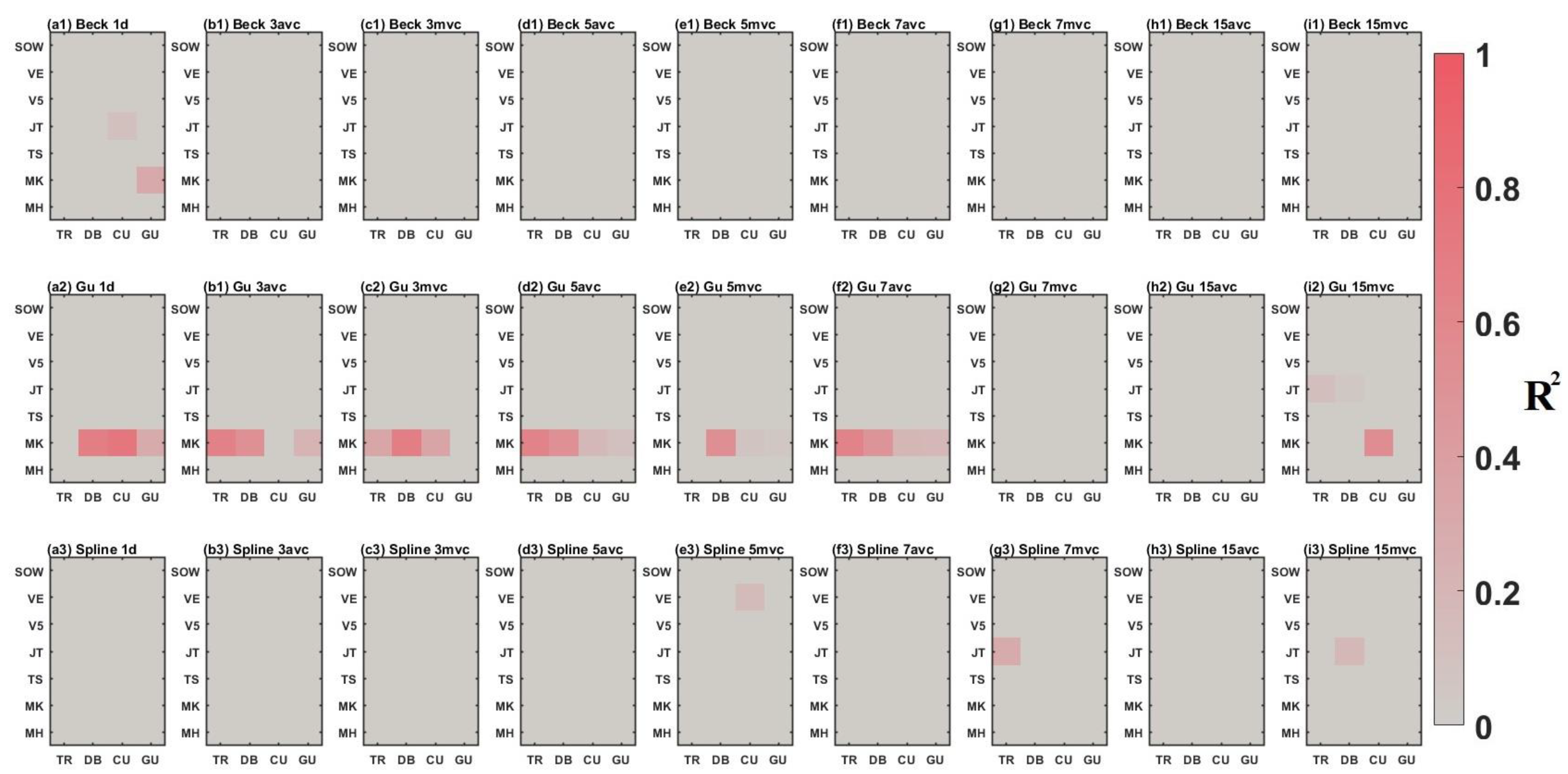
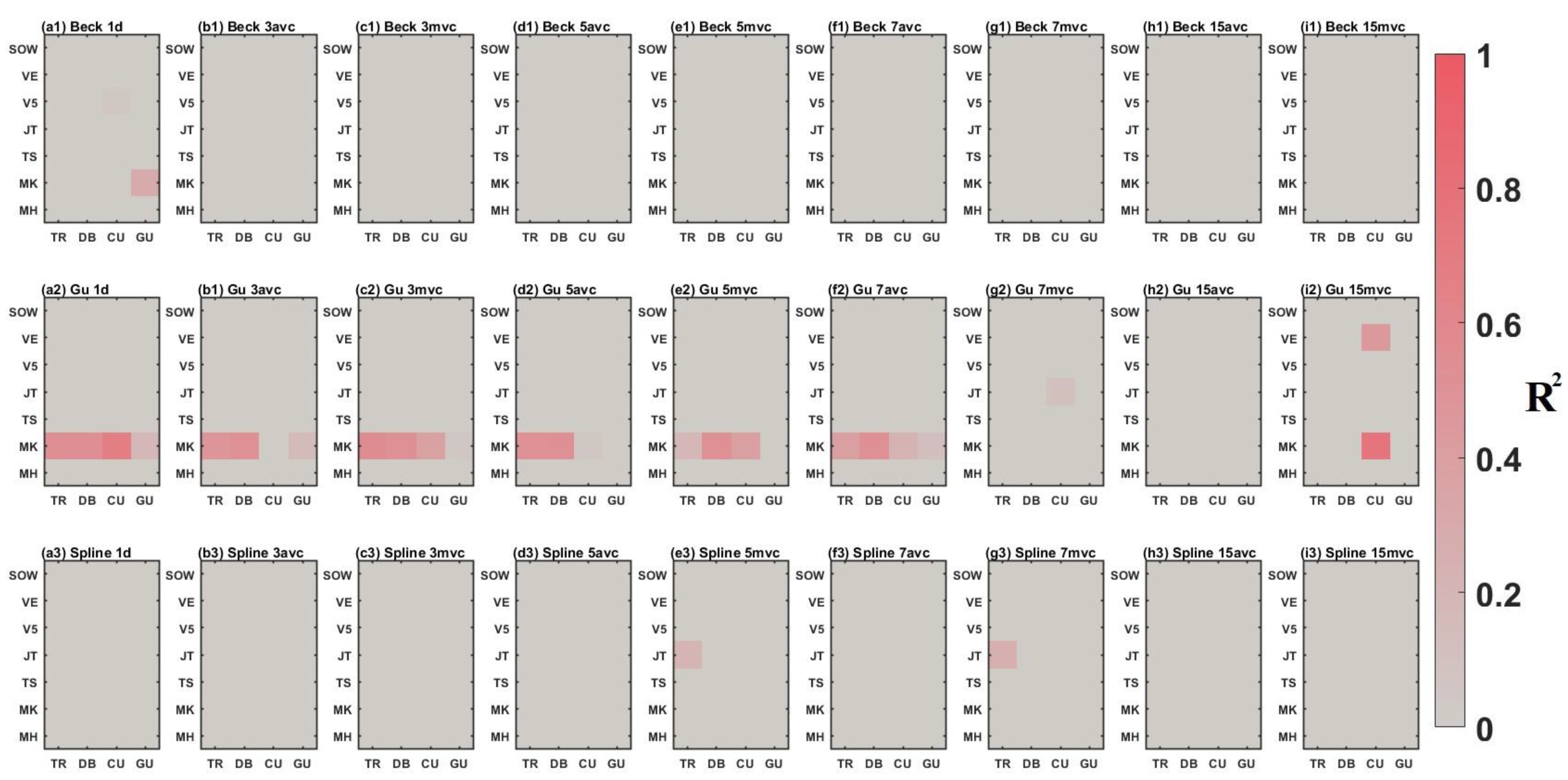
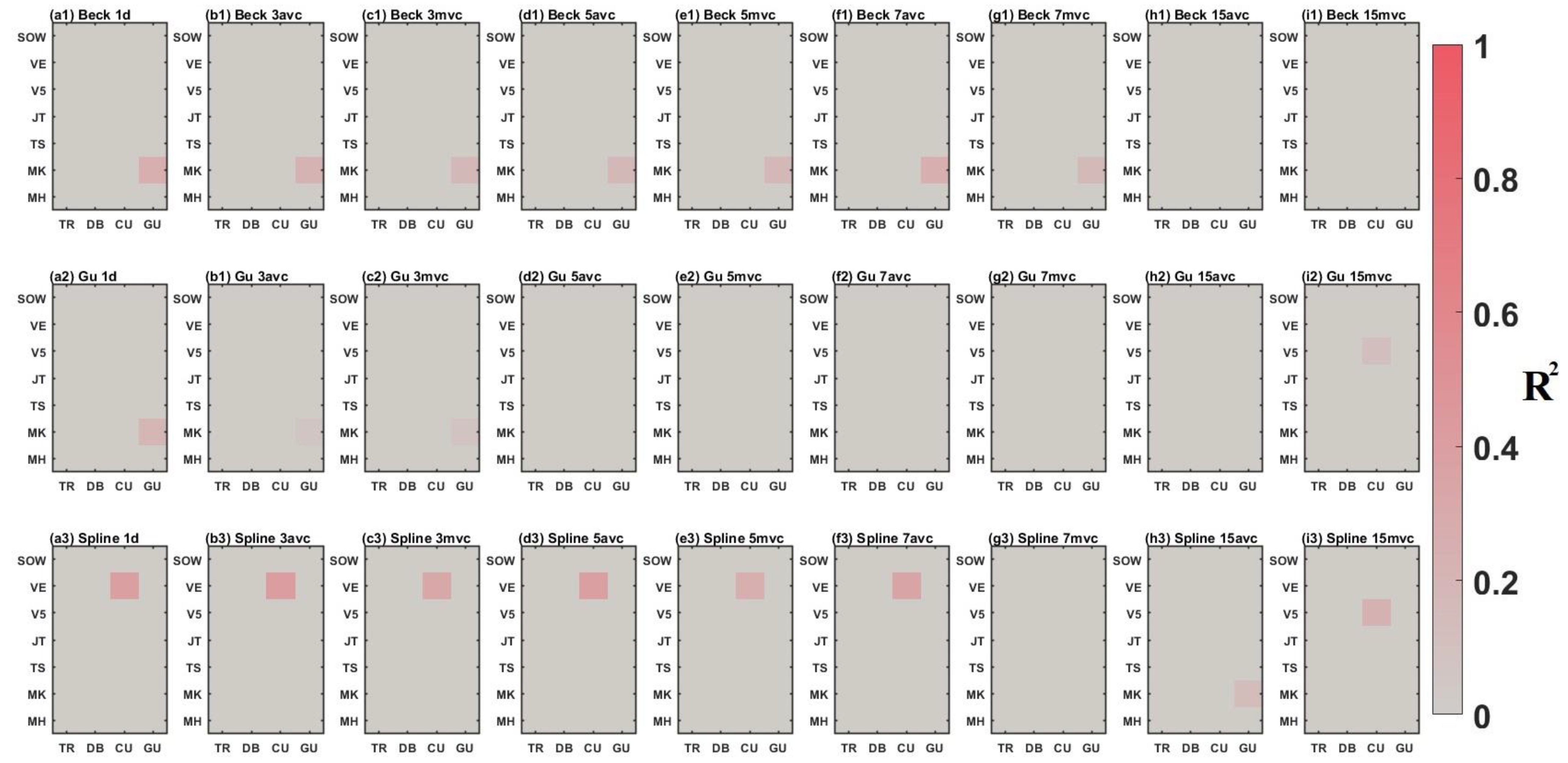
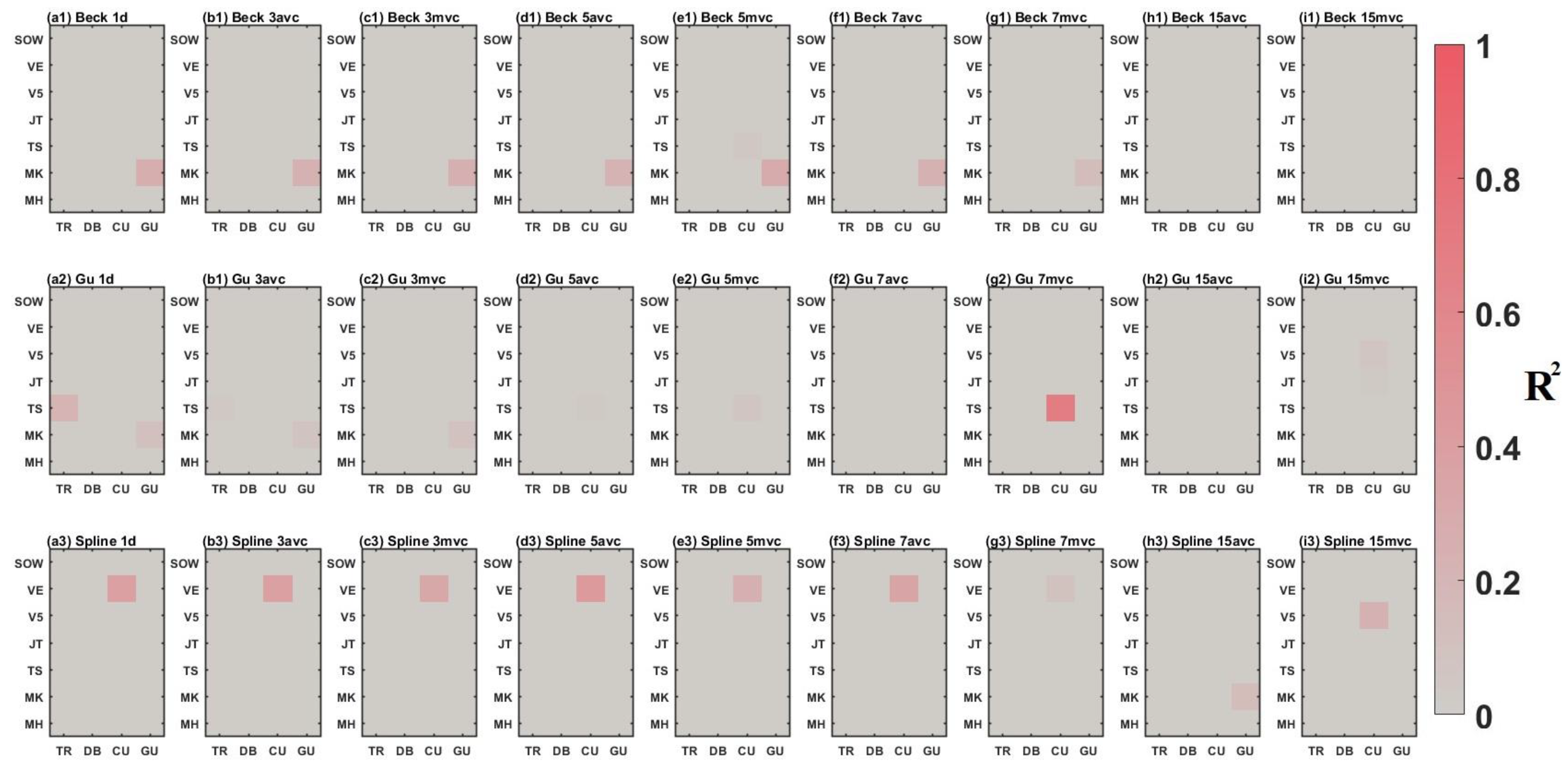
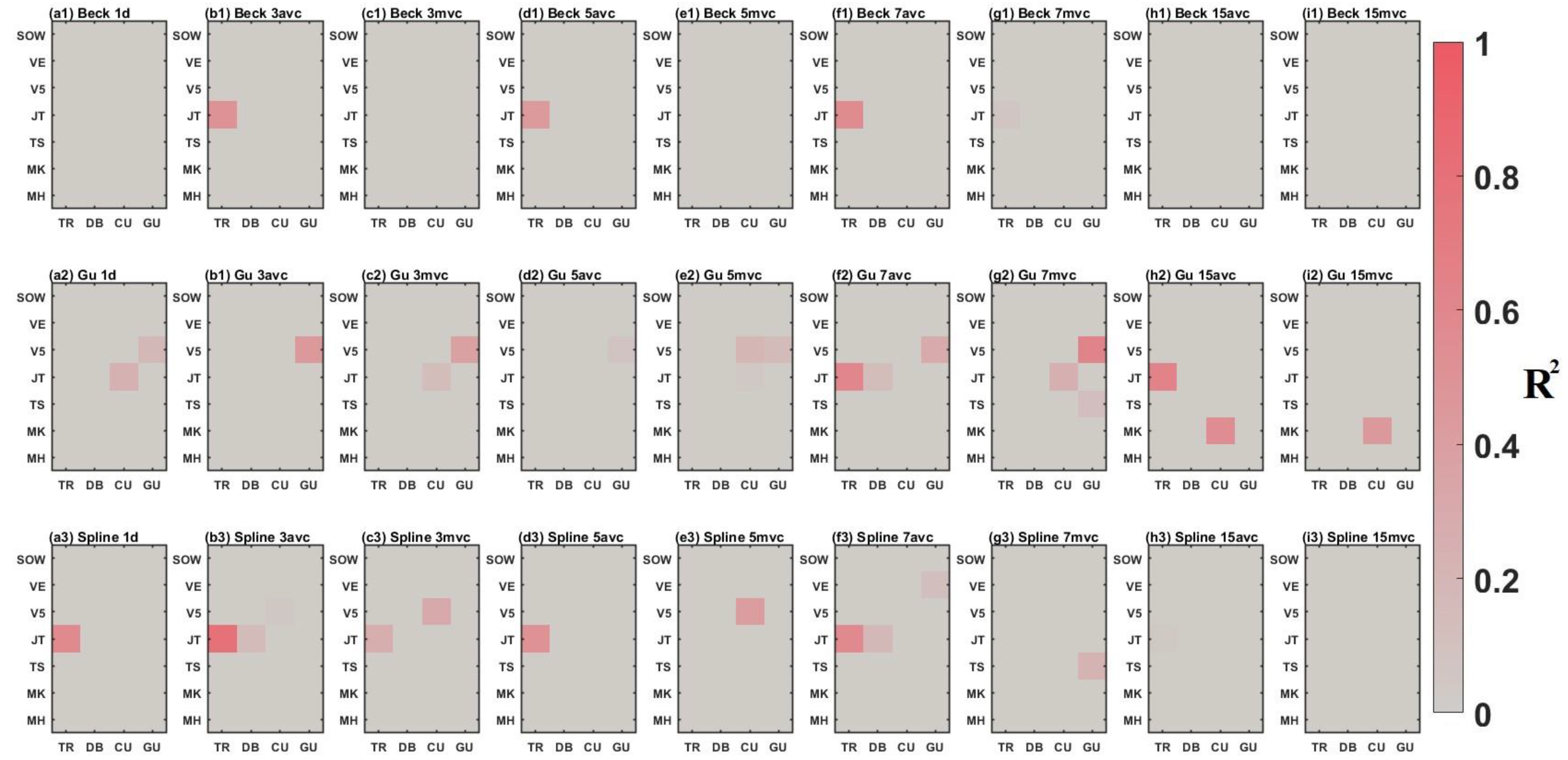
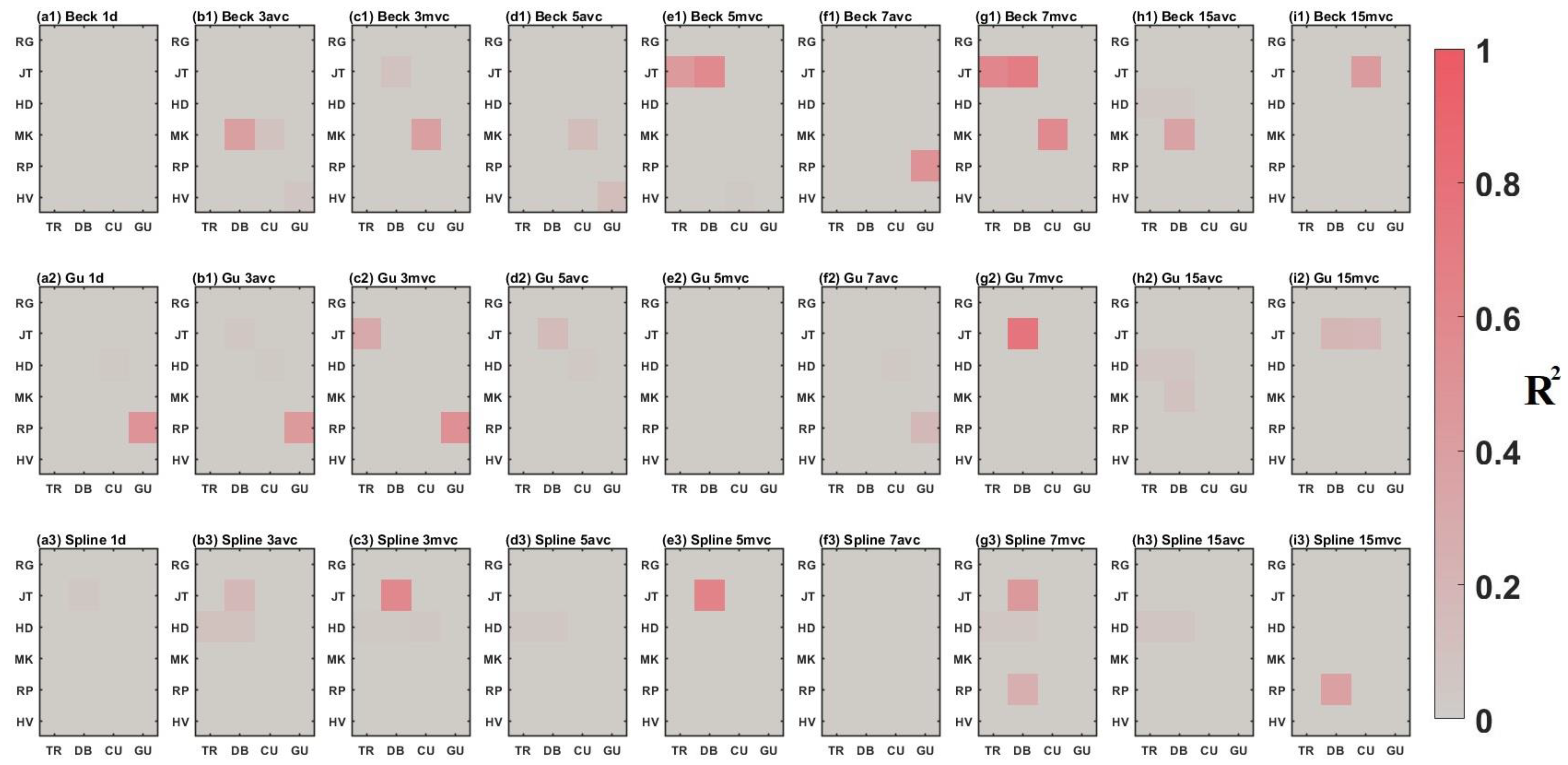
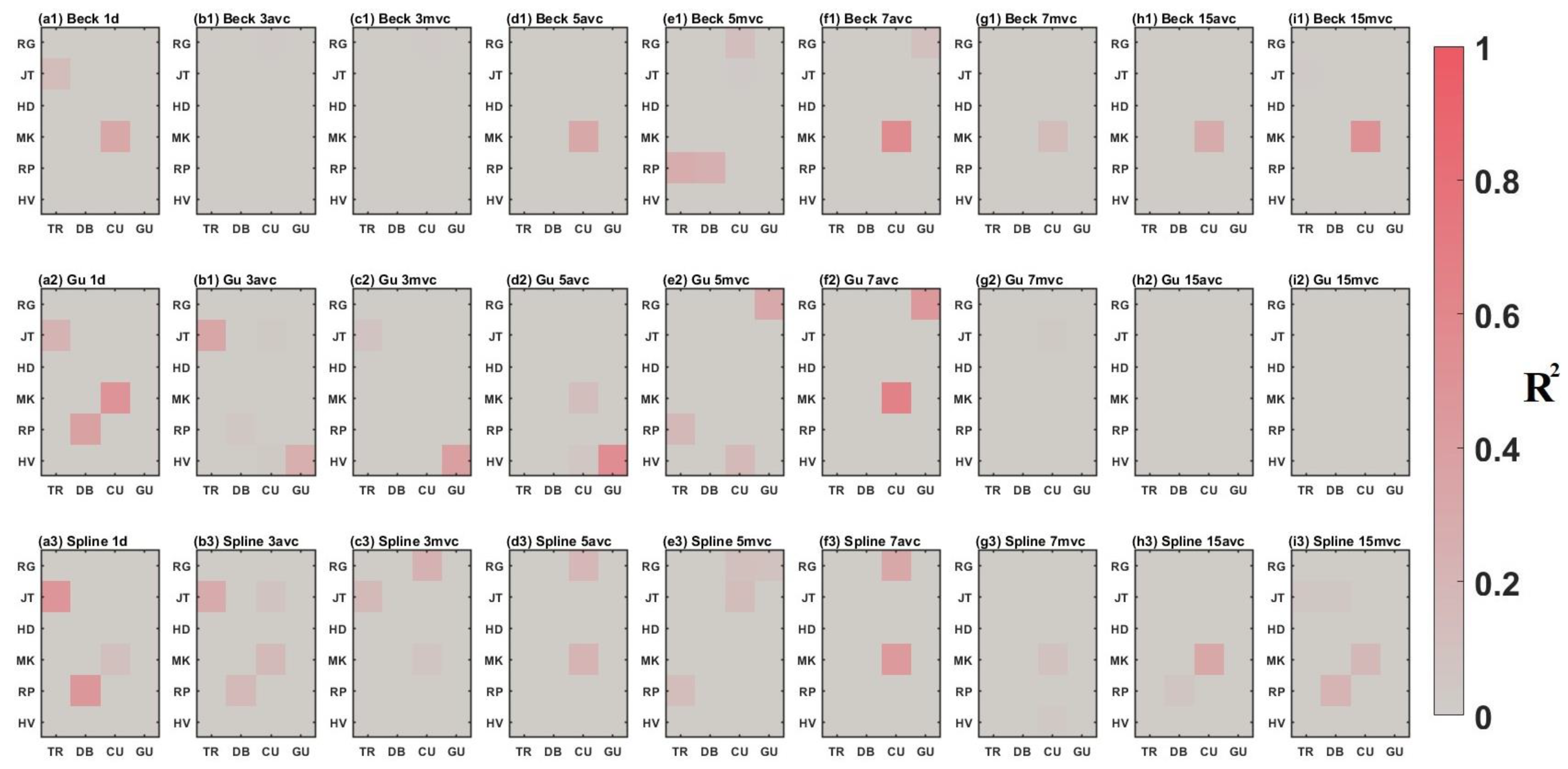
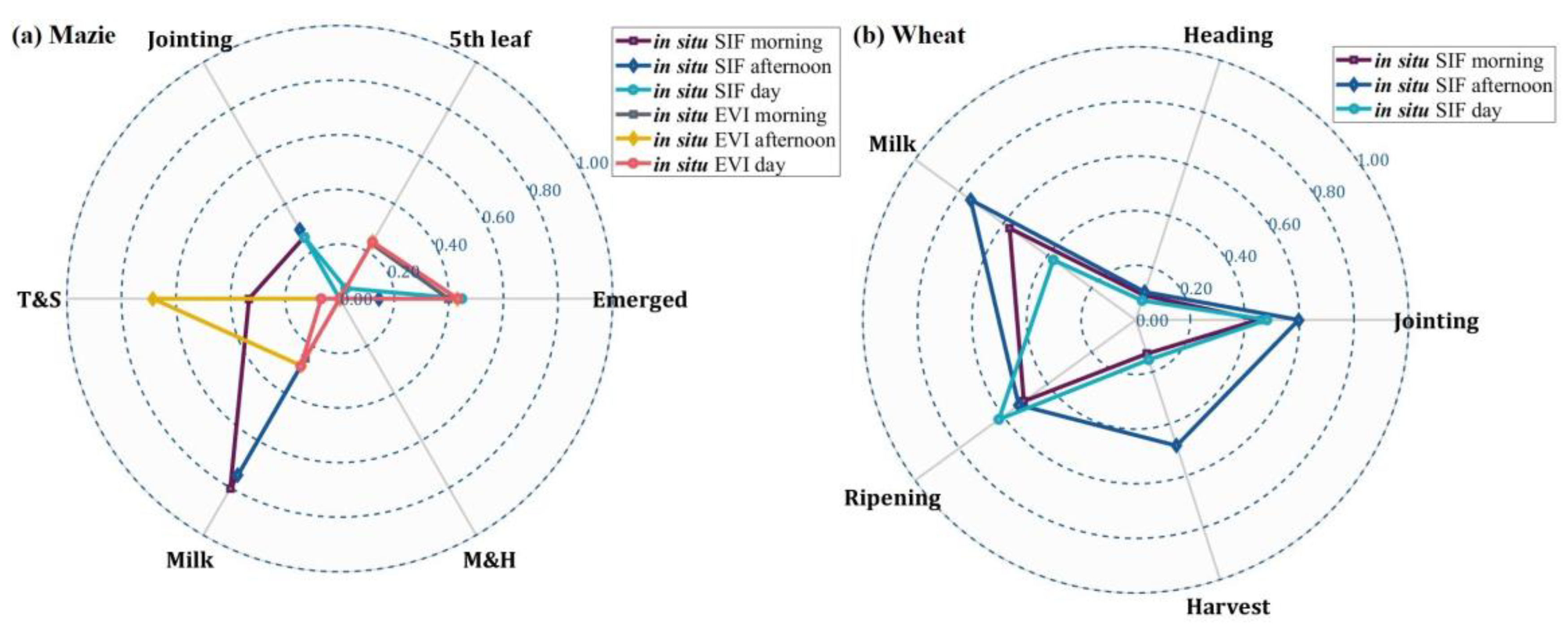
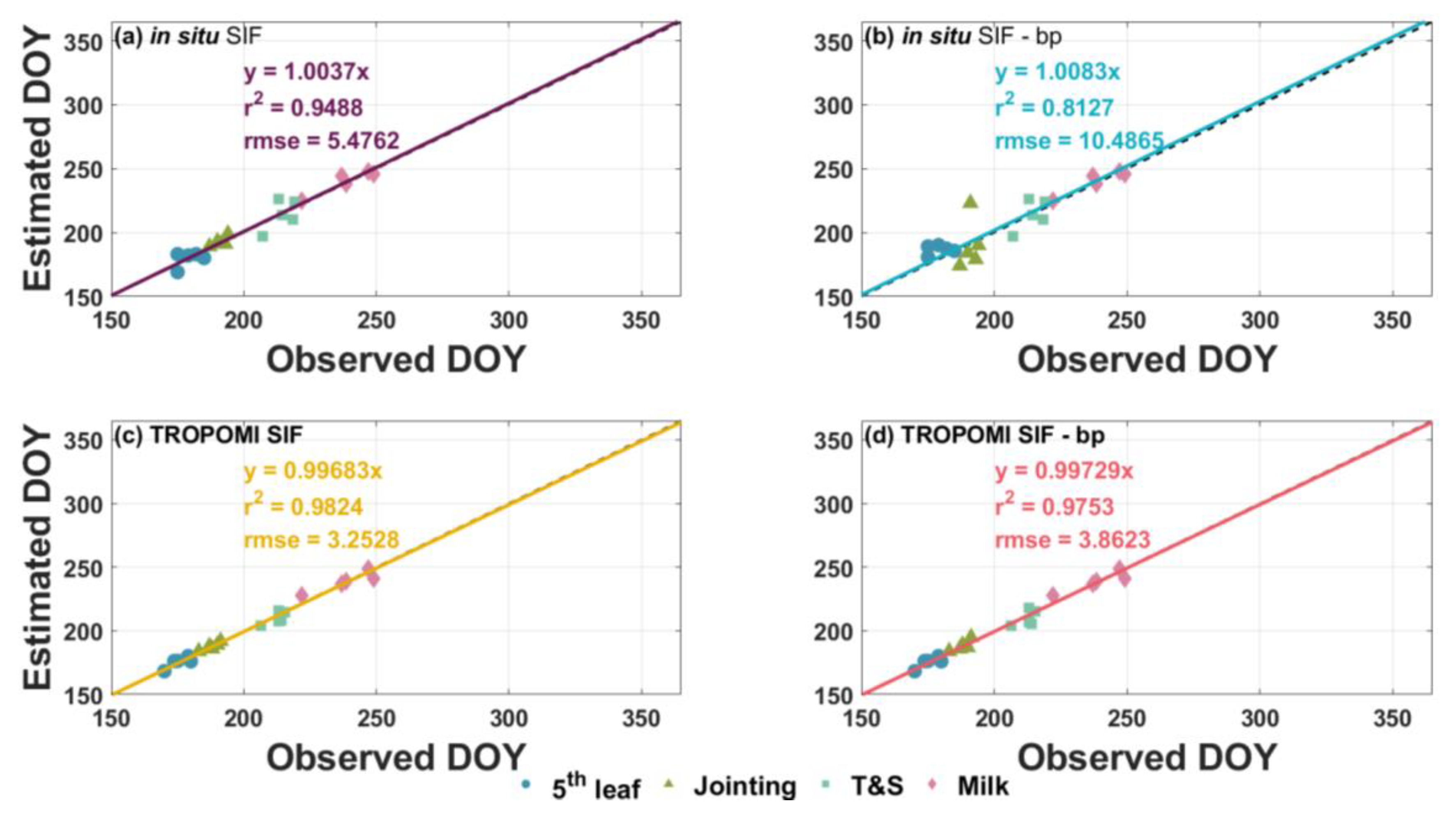
3.2. Comparison between Time-Series Phenological Estimation Portfolios for Crop Growth Stages
3.2.1. SIF vs. EVI
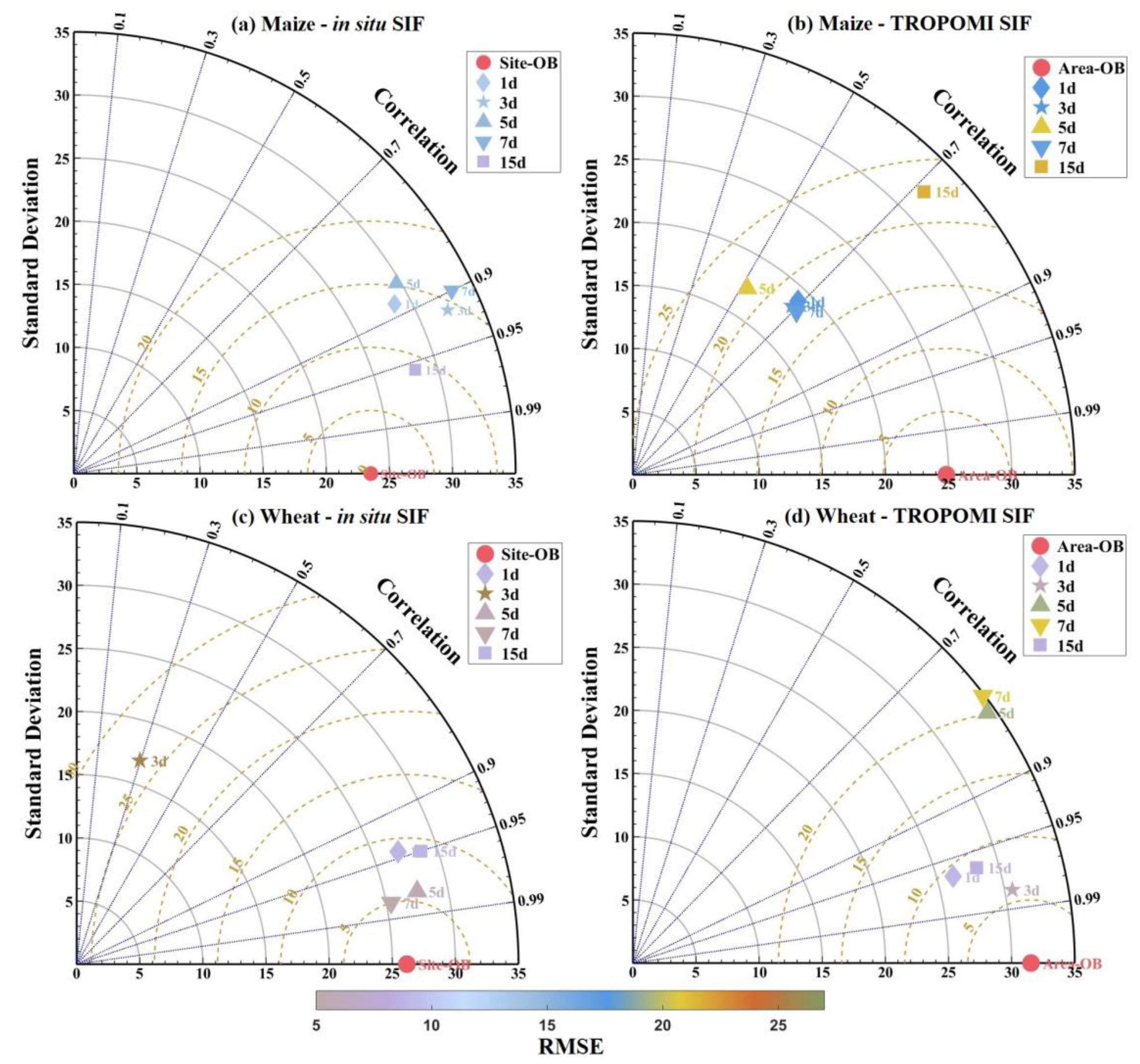
3.2.2. Effect of Data-Measured Time Period on Estimation Accuracy
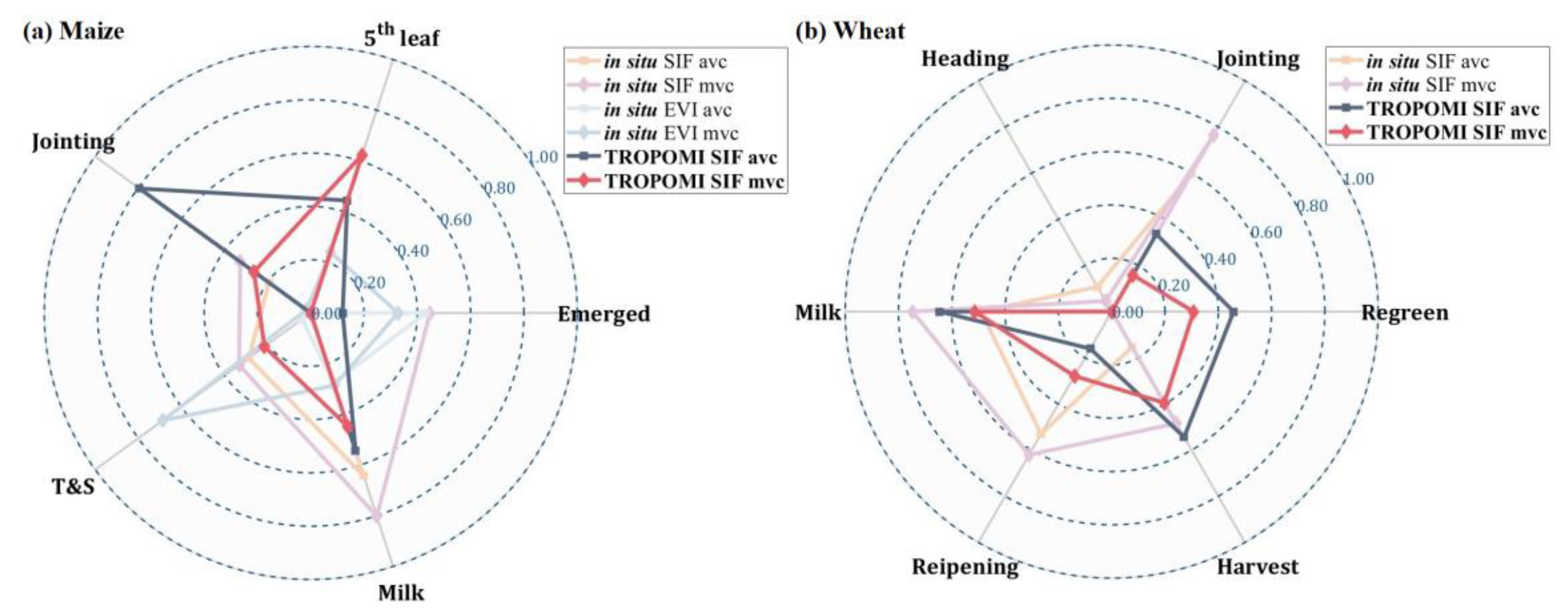
3.2.3. Compositing Methods
3.2.4. Phenological Modeling and Transition Characterization
3.3. Evaluation of Best Time-Series Phenological Estimation
4. Discussion
4.1. Capability of SIF Data in the Crop Growth Stage Estimation Framework
4.2. Evaluation of Elements in The Estimation Portfolio
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A






References
- Field, C.B.; Lobell, D.B.; Peters, H.A.; Chiariello, N.R. Feedbacks of Terrestrial Ecosystems to Climate Change. Annu. Rev. Environ. Resour. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate Change, Phenology, and Phenological Control of Vegetation Feedbacks to the Climate System. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological Sensitivity to Climate across Taxa and Trophic Levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward Mapping Crop Progress at Field Scales through Fusion of Landsat and MODIS Imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef]
- Diao, C. Innovative Pheno-Network Model in Estimating Crop Phenological Stages with Satellite Time Series. ISPRS J. Photogramm. Remote Sens. 2019, 153, 96–109. [Google Scholar] [CrossRef]
- Sakamoto, T.; Wardlow, B.D.; Gitelson, A.A. Detecting Spatiotemporal Changes of Corn Developmental Stages in the U.S. Corn Belt Using MODIS WDRVI Data. IEEE Trans. Geosci. Remote Sens. 2011, 49, 1926–1936. [Google Scholar] [CrossRef]
- Brown, M.E.; de Beurs, K.M.; Marshall, M. Global Phenological Response to Climate Change in Crop Areas Using Satellite Remote Sensing of Vegetation, Humidity and Temperature over 26 years. Remote Sens. Environ. 2012, 126, 174–183. [Google Scholar] [CrossRef]
- Lauer, J. The Effects of Drought and Poor Corn Pollination on Corn. Field Crop. 2012, 28, 493–495. [Google Scholar]
- Koay, E.; Lum, M.S. Effects of Drought Stress and Potassium on the Growth and Yield of Locally Planted Sweet Corn. Int. J. Agric. For. Plant. 2021, 11, 96–103. [Google Scholar]
- Thompson, D.R.; Wehmanen, O.A. Using Landsat Digital Data to Detect Moisture Stress. Photogramm. Eng. Remote Sens. 1979, 45, 201–207. [Google Scholar]
- Boerma, H.R.; Specht, J.E. Front Matter. In Soybeans: Improvement, Production, and Uses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–25. ISBN 978-0-89118-266-5. [Google Scholar]
- Subedi, K.; Ma, B. Corn Crop Production: Growth, Fertilization and Yield. In Corn Crop Production Growth, Fertilization and Yield; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–85. ISBN 978-1-60741-955-6. [Google Scholar]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, C.; Fisher, J.B.; Worden, J.; Badgley, G.; Saatchi, S.S.; Lee, J.-E.; Toon, G.C.; Butz, A.; Jung, M.; Kuze, A.; et al. New Global Observations of the Terrestrial Carbon Cycle from GOSAT: Patterns of Plant Fluorescence with Gross Primary Productivity. Geophys. Res. Lett. 2011, 38, L17706. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.P.; Yoshida, Y.; Corp, L.A.; Middleton, E.M. First Observations of Global and Seasonal Terrestrial Chlorophyll Fluorescence from Space. Biogeosciences 2011, 8, 637–651. [Google Scholar] [CrossRef]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.-E.; et al. Global and Time-Resolved Monitoring of Crop Photosynthesis with Chlorophyll Fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Schimel, D.; Frankenberg, C.; Drewry, D.T.; Fisher, J.B.; Verma, M.; Berry, J.A.; Lee, J.-E.; Joiner, J. Application of Satellite Solar-Induced Chlorophyll Fluorescence to Understanding Large-Scale Variations in Vegetation Phenology and Function over Northern High Latitude Forests. Remote Sens. Environ. 2017, 190, 178–187. [Google Scholar] [CrossRef]
- Yang, K.; Ryu, Y.; Dechant, B.; Berry, J.A.; Hwang, Y.; Jiang, C.; Kang, M.; Min, J.; Kimm, H.; Kornfeld, A.; et al. Sun-Induced Chlorophyll Fluorescence Is More Strongly Related to Absorbed Light than to Photosynthesis at Half-Hourly Resolution in a Rice Paddy. Remote Sens. Environ. 2018, 216, 658–673. [Google Scholar] [CrossRef]
- Kimm, H.; Guan, K.; Jiang, C.; Miao, G.; Wu, G.; Suyker, A.E.; Ainsworth, E.A.; Bernacchi, C.J.; Montes, C.M.; Berry, J.A.; et al. A Physiological Signal Derived from Sun-Induced Chlorophyll Fluorescence Quantifies Crop Physiological Response to Environmental Stresses in the U.S. Corn Belt. Environ. Res. Lett. 2021, 16, 124051. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Chen, J.; Ju, W.; Migliavacca, M.; El-Madany, T. Sensitivity of Estimated Total Canopy SIF Emission to Remotely Sensed LAI and BRDF Products. J. Remote Sens. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Guanter, L.; Frankenberg, C.; Dudhia, A.; Lewis, P.E.; Gómez-Dans, J.; Kuze, A.; Suto, H.; Grainger, R.G. Retrieval and Global Assessment of Terrestrial Chlorophyll Fluorescence from GOSAT Space Measurements. Remote Sens. Environ. 2012, 121, 236–251. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; He, B. Chlorophyll Fluorescence Observed by OCO-2 Is Strongly Related to Gross Primary Productivity Estimated from Flux Towers in Temperate Forests. Remote Sens. Environ. 2018, 204, 659–671. [Google Scholar] [CrossRef]
- Joiner, J.; Guanter, L.; Lindstrot, R.; Voigt, M.; Vasilkov, A.P.; Middleton, E.M.; Huemmrich, K.F.; Yoshida, Y.; Frankenberg, C. Global Monitoring of Terrestrial Chlorophyll Fluorescence from Moderate-Spectral-Resolution near-Infrared Satellite Measurements: Methodology, Simulations, and Application to GOME-2. Atmos. Meas. Tech. 2013, 6, 2803–2823. [Google Scholar] [CrossRef]
- Köhler, P.; Guanter, L.; Joiner, J. A Linear Method for the Retrieval of Sun-Induced Chlorophyll Fluorescence from GOME-2 and SCIAMACHY Data. Atmos. Meas. Tech. 2015, 8, 2589–2608. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Guanter, L.; Middleton, E.M. New Methods for the Retrieval of Chlorophyll Red Fluorescence from Hyperspectral Satellite Instruments: Simulations and Application to GOME-2 and SCIAMACHY. Atmos. Meas. Tech. 2016, 9, 3939–3967. [Google Scholar] [CrossRef]
- Frankenberg, C.; O’Dell, C.; Berry, J.; Guanter, L.; Joiner, J.; Köhler, P.; Pollock, R.; Taylor, T.E. Prospects for Chlorophyll Fluorescence Remote Sensing from the Orbiting Carbon Observatory-2. Remote Sens. Environ. 2014, 147, 1–12. [Google Scholar] [CrossRef]
- Köhler, P.; Frankenberg, C.; Magney, T.S.; Guanter, L.; Joiner, J.; Landgraf, J. Global Retrievals of Solar-Induced Chlorophyll Fluorescence With TROPOMI: First Results and Intersensor Comparison to OCO-2. Geophys. Res. Lett. 2018, 45, 10456–10463. [Google Scholar] [CrossRef]
- Du, S.; Liu, L.; Liu, X.; Zhang, X.; Zhang, X.; Bi, Y.; Zhang, L. Retrieval of Global Terrestrial Solar-Induced Chlorophyll Fluorescence from TanSat Satellite. Sci. Bull. 2018, 63, 1502–1512. [Google Scholar] [CrossRef]
- Yao, L.; Yang, D.; Liu, Y.; Wang, J.; Liu, L.; Du, S.; Cai, Z.; Lu, N.; Lyu, D.; Wang, M.; et al. A New Global Solar-Induced Chlorophyll Fluorescence (SIF) Data Product from TanSat Measurements. Adv. Atmos. Sci. 2021, 38, 341–345. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring Vegetation Phenology Using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F. Cross-Scalar Satellite Phenology from Ground, Landsat, and MODIS Data. Remote Sens. Environ. 2007, 109, 261–273. [Google Scholar] [CrossRef]
- Klosterman, S.T.; Hufkens, K.; Gray, J.M.; Melaas, E.; Sonnentag, O.; Lavine, I.; Mitchell, L.; Norman, R.; Friedl, M.A.; Richardson, A.D. Evaluating Remote Sensing of Deciduous Forest Phenology at Multiple Spatial Scales Using PhenoCam Imagery. Biogeosciences 2014, 11, 4305–4320. [Google Scholar] [CrossRef]
- McNairn, H.; Jiao, X.; Pacheco, A.; Sinha, A.; Tan, W.; Li, Y. Estimating Canola Phenology Using Synthetic Aperture Radar. Remote Sens. Environ. 2018, 219, 196–205. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A Review of Vegetation Phenological Metrics Extraction Using Time-Series, Multispectral Satellite Data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Ma, M.; Veroustraete, F. Reconstructing Pathfinder AVHRR Land NDVI Time-Series Data for the Northwest of China. Adv. Space Res. 2006, 37, 835–840. [Google Scholar] [CrossRef]
- Julitta, T.; Cremonese, E.; Migliavacca, M.; Colombo, R.; Galvagno, M.; Siniscalco, C.; Rossini, M.; Fava, F.; Cogliati, S.; Morra di Cella, U.; et al. Using Digital Camera Images to Analyse Snowmelt and Phenology of a Subalpine Grassland. Agric. For. Meteorol. 2014, 198–199, 116–125. [Google Scholar] [CrossRef]
- Hermance, J.F.; Jacob, R.W.; Bradley, B.A.; Mustard, J.F. Extracting Phenological Signals From Multiyear AVHRR NDVI Time Series: Framework for Applying High-Order Annual Splines With Roughness Damping. IEEE Trans. Geosci. Remote Sens. 2007, 45, 3264–3276. [Google Scholar] [CrossRef]
- Tan, B.; Gao, F.; Wolfe, R.E.; Pedelty, J.A.; Nightingale, J.; Morisette, J.T.; Ederer, G.A. An Enhanced TIMESAT Algorithm for Estimating Vegetation Phenology Metrics From MODIS Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2011, 4, 361–371. [Google Scholar] [CrossRef]
- Moulin, S.; Kergoat, L.; Viovy, N.; Dedieu, G. Global-Scale Assessment of Vegetation Phenology Using NOAA/AVHRR Satellite Measurements. J. Clim. 1997, 10, 1154–1170. [Google Scholar] [CrossRef]
- White, M.A.; De BEURS, K.M.; Didan, K.; Inouye, D.W.; Richardson, A.D.; Jensen, O.P.; O’keefe, J.; Zhang, G.; Nemani, R.R.; Van Leeuwen, W.J.D.; et al. Intercomparison, Interpretation, and Assessment of Spring Phenology in North America Estimated from Remote Sensing for 1982–2006. Glob. Chang. Biol. 2009, 15, 2335–2359. [Google Scholar] [CrossRef]
- Sakamoto, T.; Wardlow, B.D.; Gitelson, A.A.; Verma, S.B.; Suyker, A.E.; Arkebauer, T.J. A Two-Step Filtering Approach for Detecting Maize and Soybean Phenology with Time-Series MODIS Data. Remote Sens. Environ. 2010, 114, 2146–2159. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Asrar, G.R.; Mao, J.; Li, X.; Li, W. Response of Vegetation Phenology to Urbanization in the Conterminous United States. Glob. Chang. Biol. 2017, 23, 2818–2830. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.F.; Gaulton, R.; Barr, S. Assessing Spring Phenology of a Temperate Woodland: A Multiscale Comparison of Ground, Unmanned Aerial Vehicle and Landsat Satellite Observations. Remote Sens. Environ. 2019, 223, 229–242. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Yin, Y.; Walther, S.; Ma, X.; Zhang, Z.; Chen, Y. Improved Vegetation Photosynthetic Phenology Monitoring in the Northern Ecosystems Using Total Canopy Solar-Induced Chlorophyll Fluorescence Derived From TROPOMI. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007369. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Li, J.; Yang, X.; Wu, Y.; Zhang, Z.; Wang, S.; Wang, H.; Zhang, Y. Solar-Induced Chlorophyll Fluorescence and Its Link to Canopy Photosynthesis in Maize from Continuous Ground Measurements. Remote Sens. Environ. 2020, 236, 111420. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, Q.; Chen, J.M.; Porcar-Castell, A.; Guanter, L.; Wu, Y.; Zhang, X.; Wang, H.; Ding, D.; et al. Assessing Bi-Directional Effects on the Diurnal Cycle of Measured Solar-Induced Chlorophyll Fluorescence in Crop Canopies. Agric. For. Meteorol. 2020, 295, 108147. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, L.; Zhang, Y.; Wang, S.; Ju, W.; Zhou, G.; Zhou, L.; Tang, J.; Zhu, X.; et al. ChinaSpec: A Network for Long-Term Ground-Based Measurements of Solar-Induced Fluorescence in China. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006042. [Google Scholar] [CrossRef]
- Hail, R.G.; Nleya, T. iGrow Wheat: Best Management Practices for Wheat Production. Available online: https://extension.sdstate.edu/igrow-wheat-best-management-practices-wheat-production (accessed on 2 October 2023).
- Licht, M. Corn Growth Stages|Integrated Crop Management. Available online: https://crops.extension.iastate.edu/encyclopedia/corn-growth-stages (accessed on 11 July 2023).
- Ye, Y.; Wen, Z.; Yang, H.; Lu, W.; Lu, D. Effects of Post-Silking Water Deficit on the Leaf Photosynthesis and Senescence of Waxy Maize. J. Integr. Agric. 2020, 19, 2216–2228. [Google Scholar] [CrossRef]
- Vennam, R.R.; Poudel, S.; Ramamoorthy, P.; Samiappan, S.; Reddy, K.R.; Bheemanahalli, R. Impact of Soil Moisture Stress during the Silk Emergence and Grain-Filling in Maize. Physiol. Plant. 2023, 175, e14029. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Kataria, S.; Hirve, M.; Prajapati, R. Water Deficit Stress Effects and Responses in Maize. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 129–151. ISBN 978-3-030-06118-0. [Google Scholar]
- Xu, K.; Chai, Q.; Hu, F.; Fan, Z.; Yin, W. N-Fertilizer Postponing Application Improves Dry Matter Translocation and Increases System Productivity of Wheat/Maize Intercropping. Sci. Rep. 2021, 11, 22825. [Google Scholar] [CrossRef]
- Huang, C.; Ma, S.; Gao, Y.; Liu, Z.; Qin, A.; Zhao, B.; Ning, D.; Duan, A.; Liu, X.; Chen, H.; et al. Response of Summer Maize Growth and Water Use to Different Irrigation Regimes. Agronomy 2022, 12, 768. [Google Scholar] [CrossRef]
- MAS Seeds MAS Seeds|Growing Maize|8 Key Growth Stages. Available online: https://www.masseeds.com/nos-dossiers/key-growth-stages-maize (accessed on 1 October 2023).
- Zhang, X.; Huang, C.; Meng, Y.; Liu, X.; Gao, Y.; Liu, Z.; Ma, S.-T. Physiological Mechanism of Waterlogging Stress on Yield of Waxy Maize at the Jointing Stage. Plants 2023, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhu, W.; Rezaei, E.E.; Li, J.; Sun, Z.; Zhang, J. The Optimal Phenological Phase of Maize for Yield Prediction with High-Frequency UAV Remote Sensing. Remote Sens. 2022, 14, 1559. [Google Scholar] [CrossRef]
- Yang, X.; Shi, H.; Stovall, A.; Guan, K.; Miao, G.; Zhang, Y.; Zhang, Y.; Xiao, X.; Ryu, Y.; Lee, J.-E. FluoSpec 2—An Automated Field Spectroscopy System to Monitor Canopy Solar-Induced Fluorescence. Sensors 2018, 18, 2063. [Google Scholar] [CrossRef] [PubMed]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Tsubo, M.; Walker, S. Relationships between Photosynthetically Active Radiation and Clearness Index at Bloemfontein, South Africa. Theor. Appl. Climatol. 2005, 80, 17–25. [Google Scholar] [CrossRef]
- Guanter, L.; Bacour, C.; Schneider, A.; Aben, I.; van Kempen, T.A.; Maignan, F.; Retscher, C.; Köhler, P.; Frankenberg, C.; Joiner, J.; et al. The TROPOSIF Global Sun-Induced Fluorescence Dataset from the Sentinel-5P TROPOMI Mission. Earth Syst. Sci. Data 2021, 13, 5423–5440. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q. Monitoring Interannual Variation in Global Crop Yield Using Long-Term AVHRR and MODIS Observations. ISPRS J. Photogramm. Remote Sens. 2016, 114, 191–205. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.; Wang, Z.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.; Verfaillie, J.; et al. Using Data from Landsat, MODIS, VIIRS and PhenoCams to Monitor the Phenology of California Oak/Grass Savanna and Open Grassland across Spatial Scales. Agric. For. Meteorol. 2017, 237–238, 311–325. [Google Scholar] [CrossRef]
- Suppayasan, P.; Anantakarn, K. Rice Crop Mapping and Gis Analysis for Policy Implementation. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2019, 10, 10A18N. [Google Scholar] [CrossRef]
- Diao, C. Remote Sensing Phenological Monitoring Framework to Characterize Corn and Soybean Physiological Growing Stages. Remote Sens. Environ. 2020, 248, 111960. [Google Scholar] [CrossRef]
- Shekhar, A.; Buchmann, N.; Gharun, M. How Well Do Recently Reconstructed Solar-Induced Fluorescence Datasets Model Gross Primary Productivity? Remote Sens. Environ. 2022, 283, 113282. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Morra di Cella, U.; Richardson, A.D. Phenopix: A R Package for Image-Based Vegetation Phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Migliavacca, M.; Galvagno, M.; Cremonese, E.; Rossini, M.; Meroni, M.; Sonnentag, O.; Cogliati, S.; Manca, G.; Diotri, F.; Busetto, L.; et al. Using Digital Repeat Photography and Eddy Covariance Data to Model Grassland Phenology and Photosynthetic CO2 Uptake. Agric. For. Meteorol. 2011, 151, 1325–1337. [Google Scholar] [CrossRef]
- Luo, J.; Ying, K.; Bai, J. Savitzky–Golay Smoothing and Differentiation Filter for Even Number Data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Kross, A.; Fernandes, R.; Seaquist, J.; Beaubien, E. The Effect of the Temporal Resolution of NDVI Data on Season Onset Dates and Trends across Canadian Broadleaf Forests. Remote Sens. Environ. 2011, 115, 1564–1575. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved Monitoring of Vegetation Dynamics at Very High Latitudes: A New Method Using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.D.; Black, T.A.; Suyker, A.E.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Characterizing the Seasonal Dynamics of Plant Community Photosynthesis Across a Range of Vegetation Types. In Phenology of Ecosystem Processes: Applications in Global Change Research; Noormets, A., Ed.; Springer: New York, NY, USA, 2009; pp. 35–58. ISBN 978-1-4419-0026-5. [Google Scholar]
- White, M.A.; Thornton, P.E.; Running, S.W. A Continental Phenology Model for Monitoring Vegetation Responses to Interannual Climatic Variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar] [CrossRef]
- Yu, H.; Luedeling, E.; Xu, J. Winter and Spring Warming Result in Delayed Spring Phenology on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2010, 107, 22151–22156. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, G.; Cong, N.; Wang, S.; Kong, W.; Piao, S. Increasing Altitudinal Gradient of Spring Vegetation Phenology during the Last Decade on the Qinghai–Tibetan Plateau. Agric. For. Meteorol. 2014, 189–190, 71–80. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.A.; Arkebauer, T.J. MODIS-Based Corn Grain Yield Estimation Model Incorporating Crop Phenology Information. Remote Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Veefkind, J.P.; Aben, I.; McMullan, K.; Förster, H.; de Vries, J.; Otter, G.; Claas, J.; Eskes, H.J.; de Haan, J.F.; Kleipool, Q.; et al. TROPOMI on the ESA Sentinel-5 Precursor: A GMES Mission for Global Observations of the Atmospheric Composition for Climate, Air Quality and Ozone Layer Applications. Remote Sens. Environ. 2012, 120, 70–83. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Nie, C.; Zhang, S.; Wang, G.; Chen, S.; Chen, Z. A Long-Term Reconstructed TROPOMI Solar-Induced Fluorescence Dataset Using Machine Learning Algorithms. Sci. Data 2022, 9, 427. [Google Scholar] [CrossRef]
- Li, X.; Ryu, Y.; Xiao, J.; Dechant, B.; Liu, J.; Li, B.; Jeong, S.; Gentine, P. New-Generation Geostationary Satellite Reveals Widespread Midday Depression in Dryland Photosynthesis during 2020 Western U.S. Heatwave. Sci. Adv. 2023, 9, eadi0775. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy Near-Infrared Reflectance and Terrestrial Photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef]
- Zhao, D.; Hou, Y.; Zhang, Z.; Wu, Y.; Zhang, X.; Wu, L.; Zhu, X.; Zhang, Y. Temporal Resolution of Vegetation Indices and Solar-Induced Chlorophyll Fluorescence Data Affects the Accuracy of Vegetation Phenology Estimation: A Study Using in-Situ Measurements. Ecol. Indic. 2022, 136, 108673. [Google Scholar] [CrossRef]
- Peng, B.; Guan, K.; Chen, M.; Lawrence, D.M.; Pokhrel, Y.; Suyker, A.; Arkebauer, T.; Lu, Y. Improving Maize Growth Processes in the Community Land Model: Implementation and Evaluation. Agric. For. Meteorol. 2018, 250–251, 64–89. [Google Scholar] [CrossRef]





| Crop | Growth Stages | Definition |
|---|---|---|
| Winter wheat | Regreen (RG) | The plant turns green again after the winter. |
| Jointing (JT) | From 1st node detectable to last leaf visible. | |
| Heading (HD) | Head is fully exposed to frost, hail and pests. Plant attains final height. | |
| Milk (MK) | Starch and protein content determination starts, or namely ‘grain filling’. | |
| Ripening (RP) | Kernel hard, difficult to divide by thumbnail. The plant is completely yellow. | |
| Harvest (HV) | The plant has been harvested. | |
| Summer maize | Sowing (SOW) | Seeds have been planted in the soil. |
| Emergence (VE) | Shoot (coleoptile) has emerged from the soil. | |
| 5th leaf (V5) | The 5th leaf collars present. | |
| Jointing (JT) | Between V6 and V9, the first stem of maize grows to the height approximately 2 cm. | |
| Tasseling | Lowest branch of the tassel is visible. | |
| Silking | One or more silks extends outside of husk leaves. | |
| Milk (MK) | Kernels filled with ‘milky’ fluid, or namely ‘grain filling’. | |
| Maturity (MT) | Kernels at maximum dry matter accumulation; a ‘black layer’ will form at kernel base (2–3 days after physiological maturity). | |
| Harvest (HV) | The plant has been harvested. |
| Data | Time Cover | Temporal Resolution | Spatial Resolution | |
|---|---|---|---|---|
| Ground-based data | Ground-measured SIF (In situ SIF) | Maize: 2018–2022 Wheat: 2019, 2021, 2022 | 0.5 h | - |
| Ground-measured EVI (In situ EVI) | Maize: 2019–2022 Wheat: 2019, 2021, 2022 | 0.5 h | - | |
| Satellite data | TROPOMI SIF | 2018–2022 | 1 day | 0.05° |
| MODIS EVI | 2018–2022 | 8/16 days | 0.05° | |
| Threshold | Derivative | Curvature | Gu (ST) | Gu (In Situ) | |
|---|---|---|---|---|---|
| Sowing | - | - | - | - | Upturn |
| Emerged | - | - | Greenup | Upturn | - |
| 5th leaf | - | - | Greenup | Upturn | Stabilization |
| Jointing | SOS | SOS | Greenup | Stabilization | Stabilization |
| Tasseling and silking | POS | POS | Maturity | Stabilization | Downturn |
| Milk | EOS | EOS | Senescence | Downturn | Recession |
| Maturity and harvest | EOS | EOS | Dormancy | Recession | - |
| TR | DB | CU | GU (TROPOMI SIF) | GU (In Situ SIF) | GU (EVI) | |
|---|---|---|---|---|---|---|
| Regreen | - | - | GU | UD | - | UD |
| Jointing | SOS | SOS | GU | - | UD | SD |
| Heading | POS | POS | MT | SD | SD | - |
| Milk | EOS | EOS | SN | DD | DD | RD |
| Ripening | EOS | EOS | DM | - | RD | RD |
| Harvest | - | - | DM | RD | RD | - |
| Crop | Data | Growth Stage | Measured-Time | Compositing Method | Characterization Method |
|---|---|---|---|---|---|
| Maize | In situ SIF | V5 | Morning | 5d-MVC | GU |
| JT | Morning | 5d-MVC | CU | ||
| T&S | Morning | 5d-MVC | TB | ||
| MK | Morning | 5d-MVC | CU | ||
| TROPOMI SIF | V5 | - | 7d-MVC | GU | |
| JT | - | 15d-AVC | TB | ||
| T&S | - | 7d-MVC | GU | ||
| MK | - | 15d-AVC | CU | ||
| Winter wheat | In situ SIF | JT | Afternoon | 7d-MVC | TB |
| HD | Afternoon | 7d-MVC | TB | ||
| MK | Afternoon | 7d-MVC | CU | ||
| TROPOMI SIF | JT | - | 1d | DB | |
| HD | - | 1d | TB | ||
| MK | - | 1d | CU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Wu, Y.; Wu, L.; Pei, L.; Zhang, Z.; Ding, D.; Wang, G.; Li, Z.; Zhang, Y. Identifying Crop Growth Stages from Solar-Induced Chlorophyll Fluorescence Data in Maize and Winter Wheat from Ground and Satellite Measurements. Remote Sens. 2023, 15, 5689. https://doi.org/10.3390/rs15245689
Hou Y, Wu Y, Wu L, Pei L, Zhang Z, Ding D, Wang G, Li Z, Zhang Y. Identifying Crop Growth Stages from Solar-Induced Chlorophyll Fluorescence Data in Maize and Winter Wheat from Ground and Satellite Measurements. Remote Sensing. 2023; 15(24):5689. https://doi.org/10.3390/rs15245689
Chicago/Turabian StyleHou, Yuqing, Yunfei Wu, Linsheng Wu, Lei Pei, Zhaoying Zhang, Dawei Ding, Guangshuai Wang, Zhongyang Li, and Yongguang Zhang. 2023. "Identifying Crop Growth Stages from Solar-Induced Chlorophyll Fluorescence Data in Maize and Winter Wheat from Ground and Satellite Measurements" Remote Sensing 15, no. 24: 5689. https://doi.org/10.3390/rs15245689







