Red Palm Weevil Detection in Date Palm Using Temporal UAV Imagery
Abstract
:1. Introduction
2. Materials and Methods
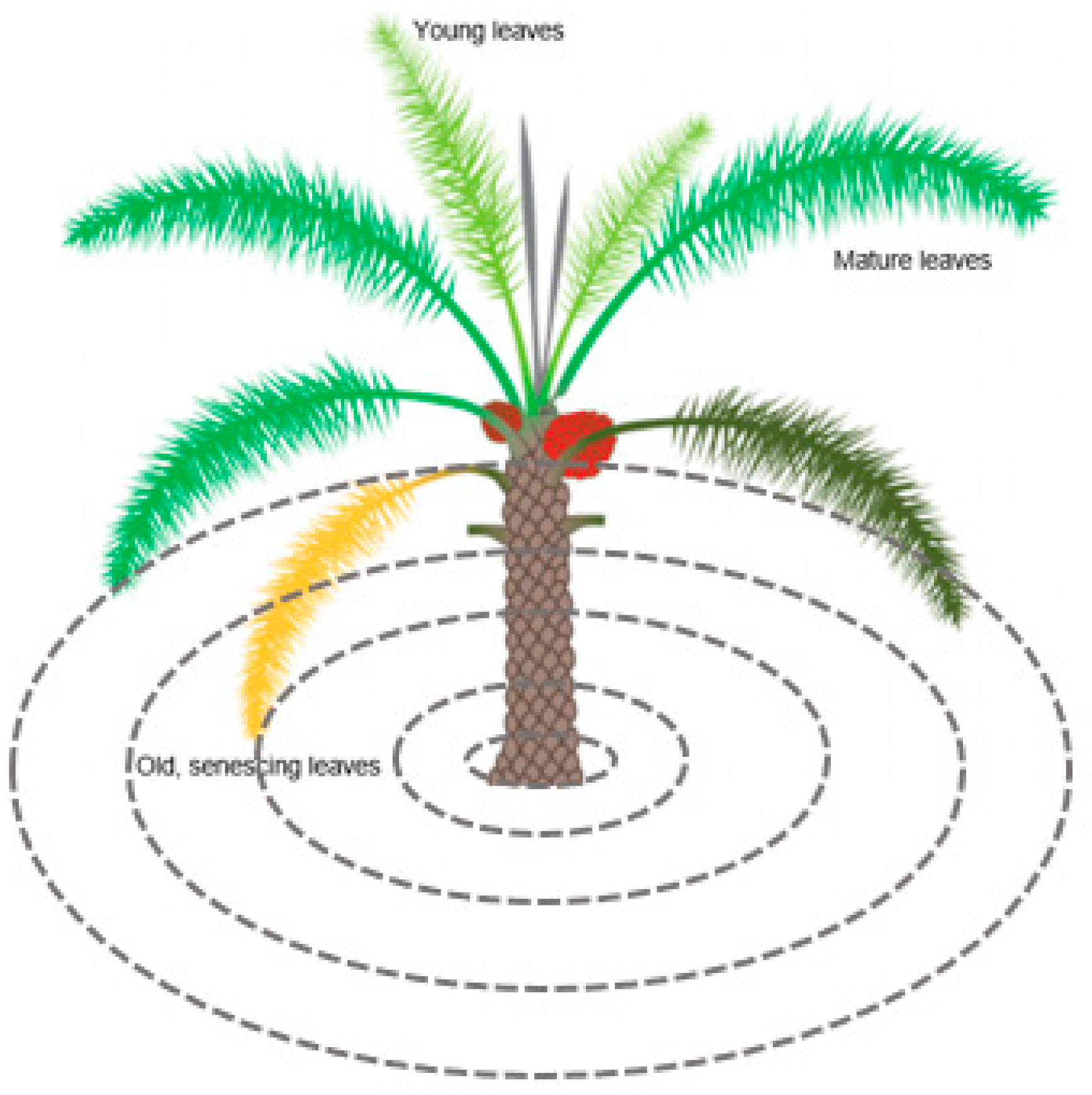
2.1. Study Area and Experimental Set-Up
2.2. Dataset
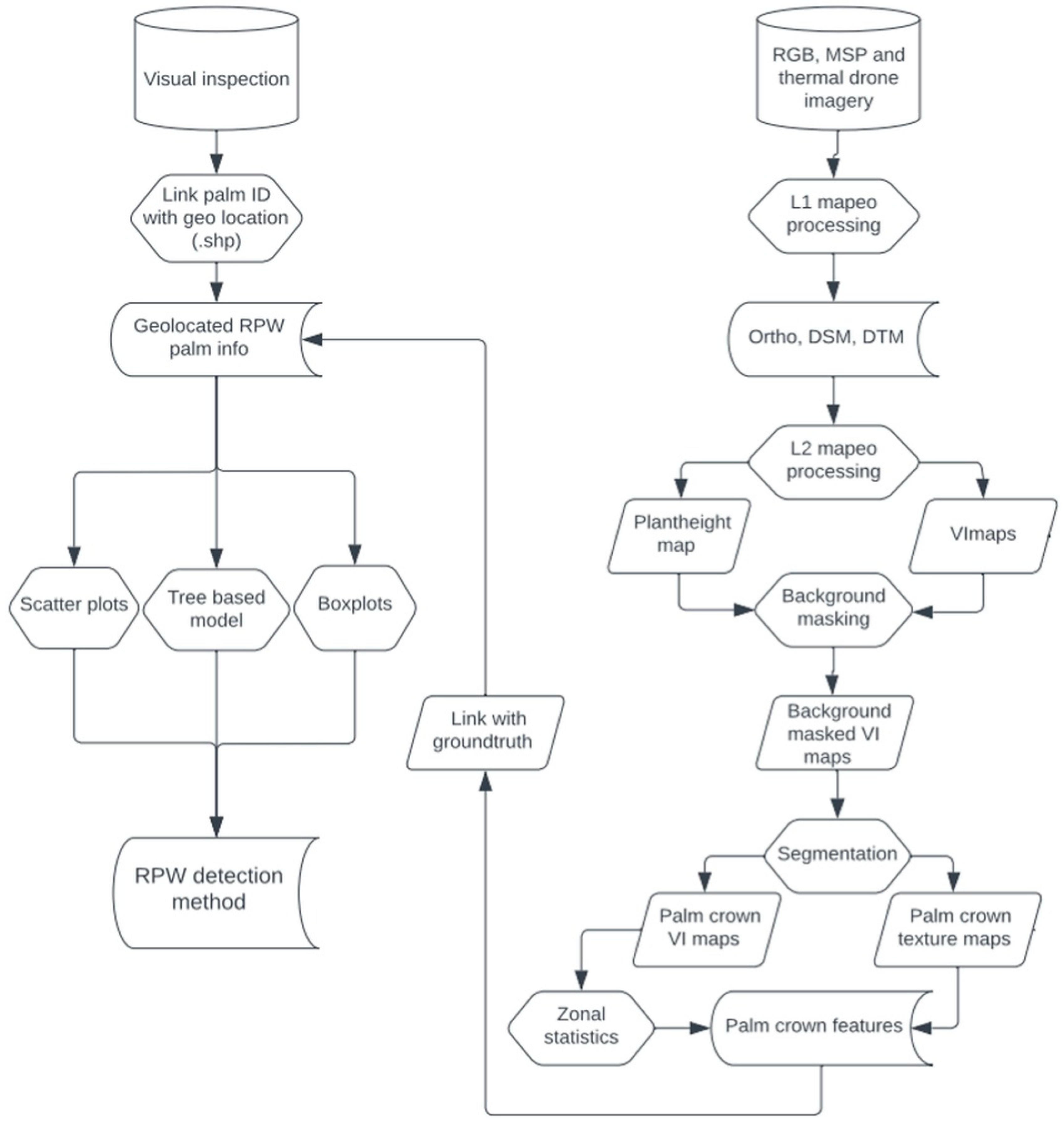
2.3. Methodology
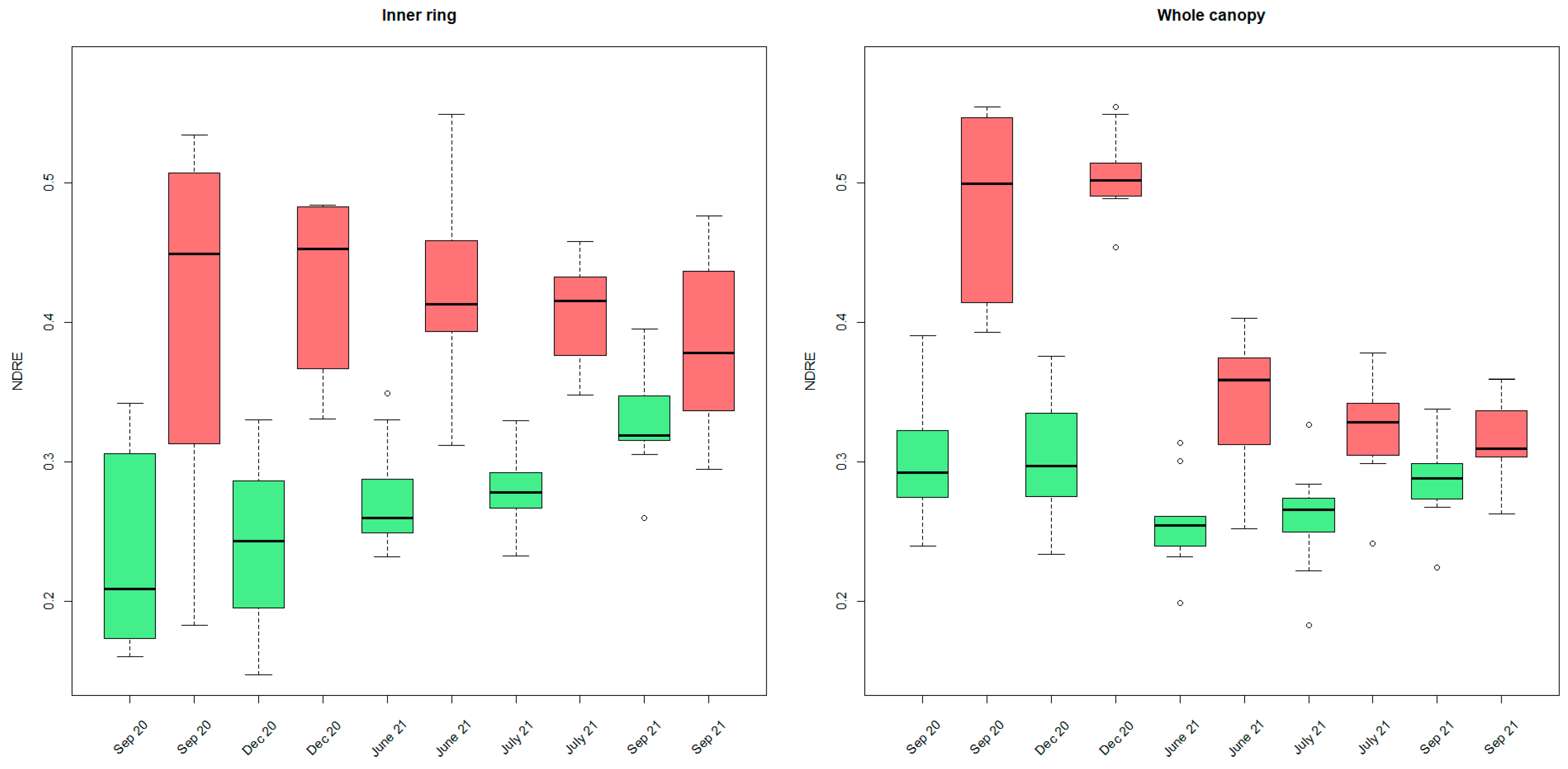
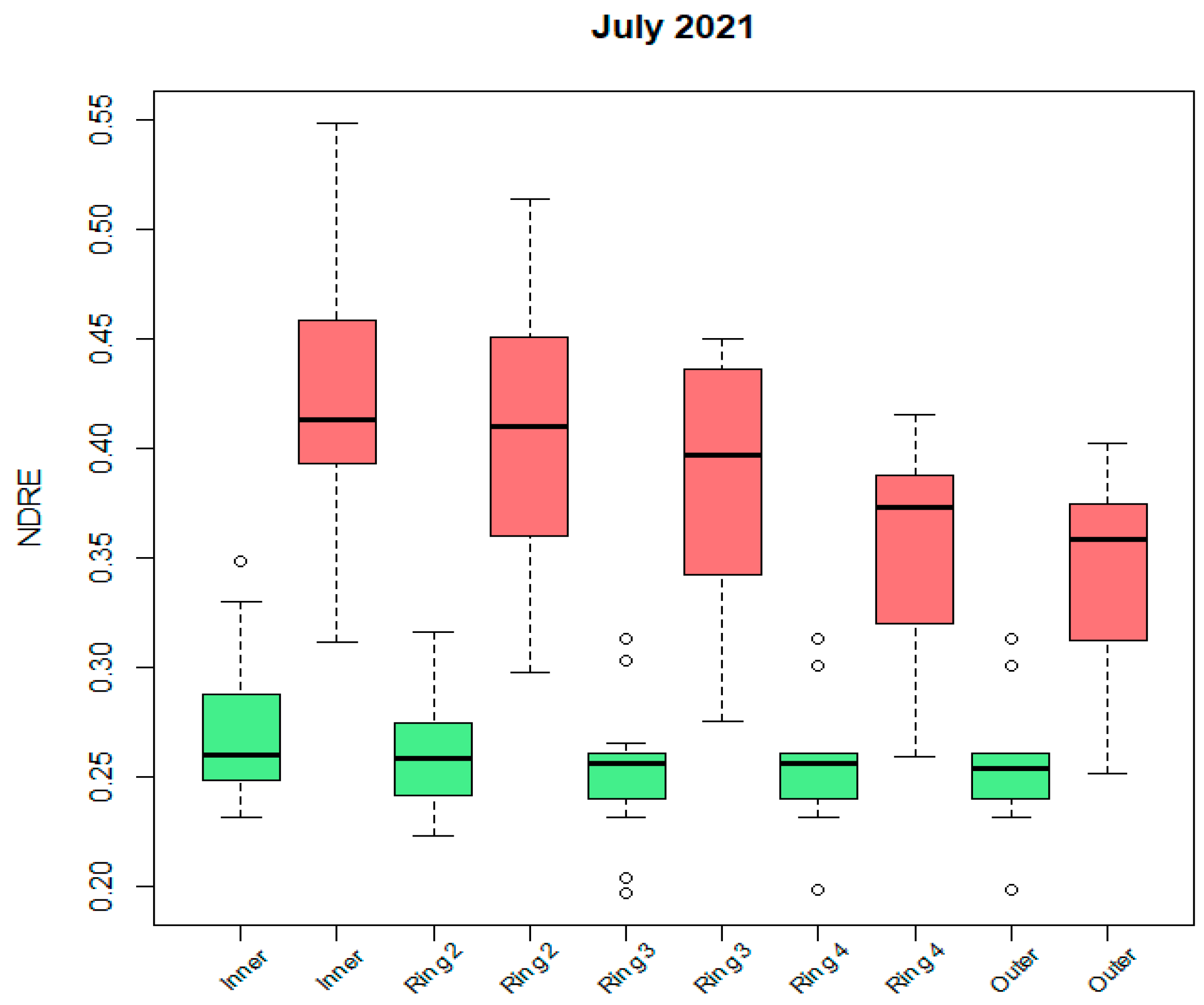
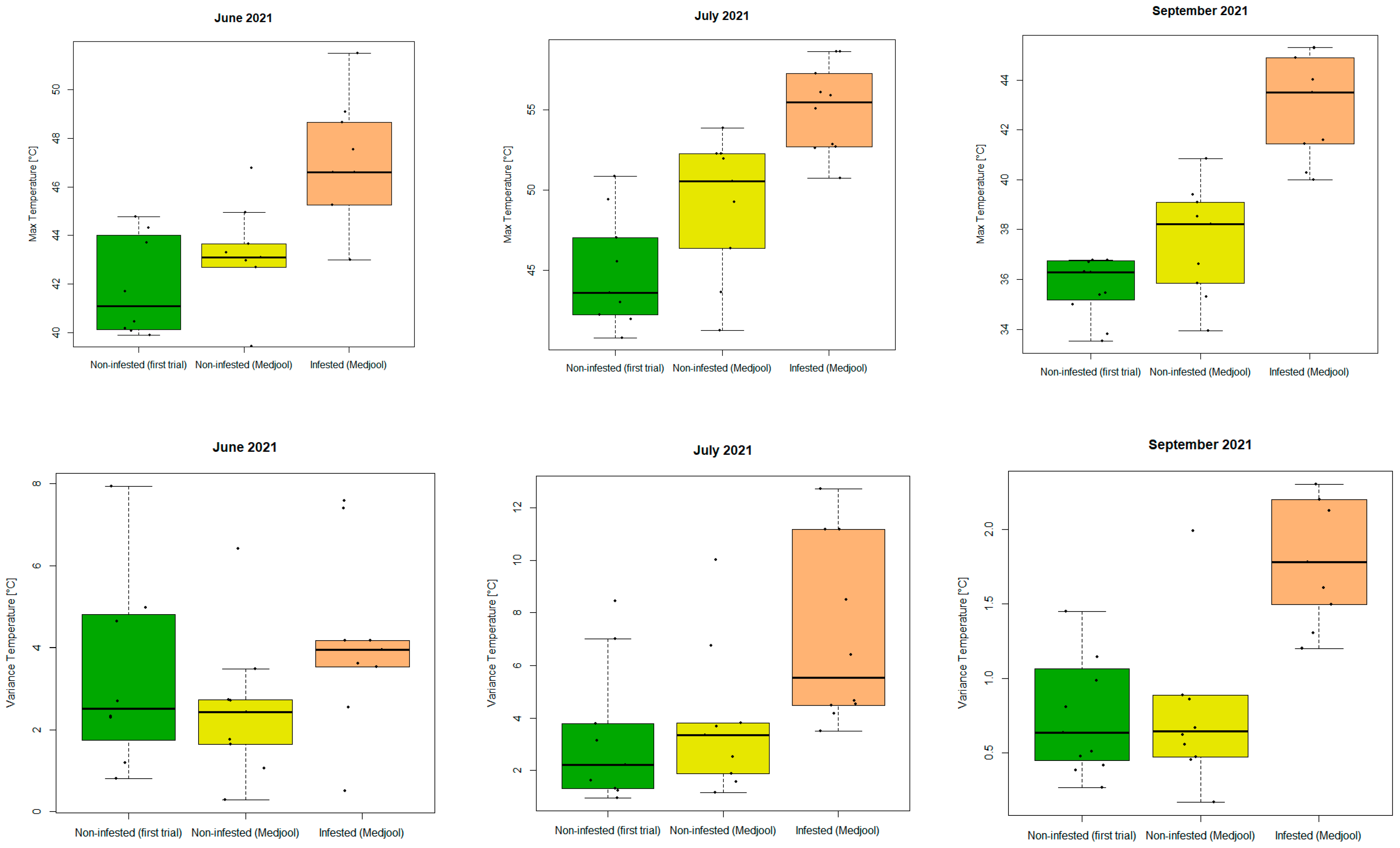
3. Results
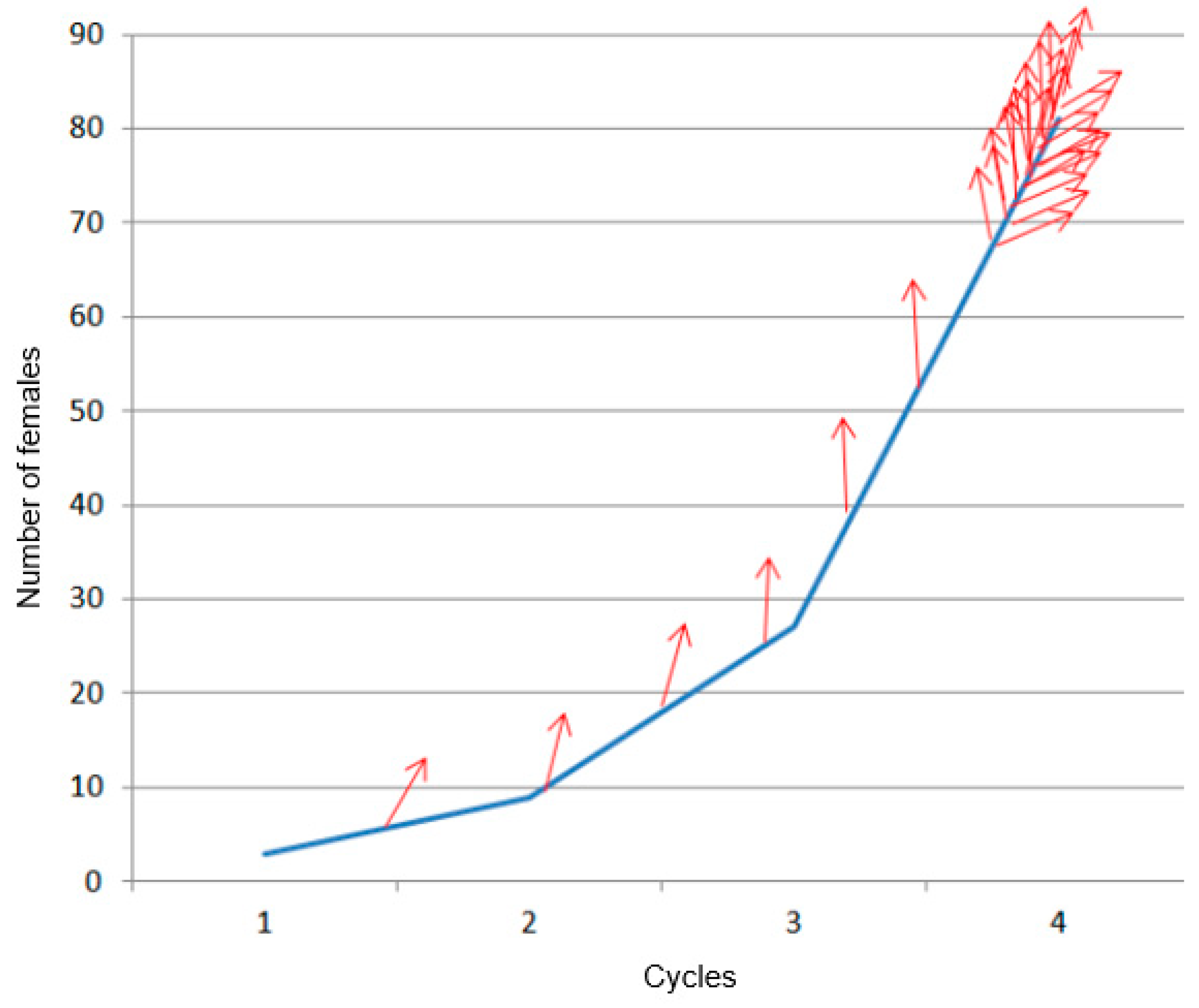
3.1. Visual Inspection
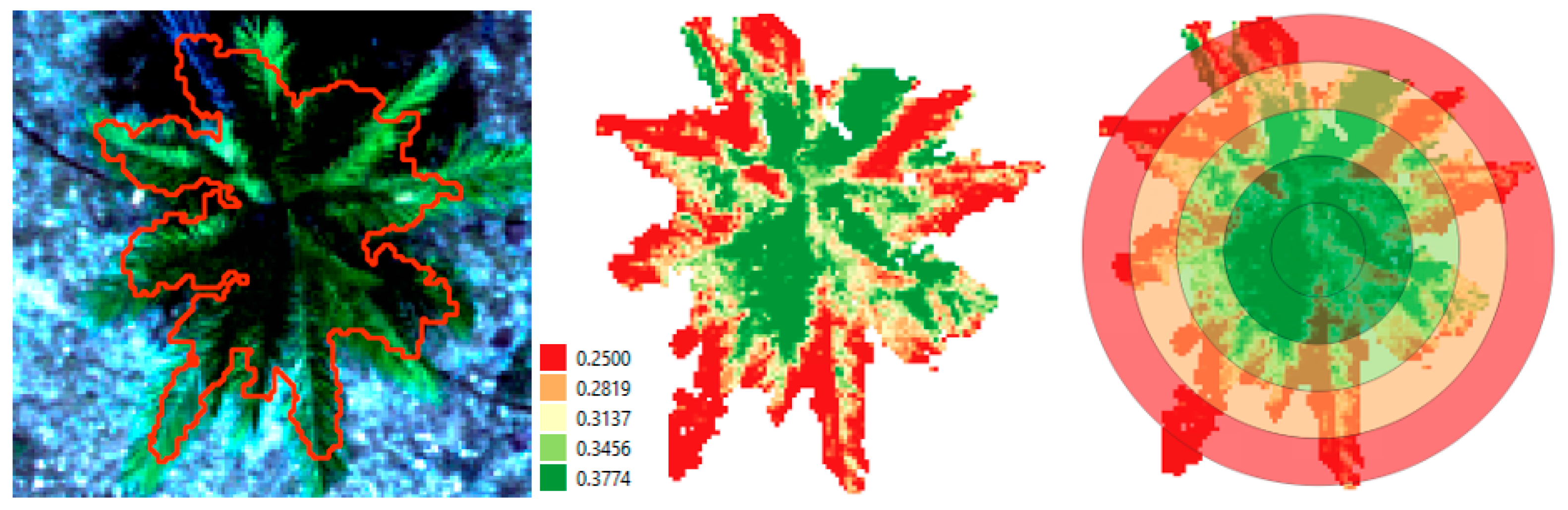
3.2. UAV Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferry, M.; Cousin, R.; Chabernaud, D.; Ferrero, F. An Effective Strategy to Obtain Very Rapidly the Red Palm Weevil Decline in an Area Planted with Ornamental Palms. Arab. J. Plant Prot. 2019, 37, 188–197. [Google Scholar] [CrossRef]
- Elshafie, H.; Faleiro, J.; Abdel Farag El-Shafie, H.; Romeno Faleiro, J. Red Palm Weevil, Rhynchophorus Ferrugineus (Coleoptera: Curculionidae): Global Invasion, Current Management Options, Challenges, and Future Prospects Characterization of Local Entomopathogenic Bacillus Strains View Project Red Palm Weevil Rhynchophorus Ferrugineus (Coleoptera: Curculionidae): Global Invasion, Current Management Options, Challenges and Future Prospects. Arab. J. Plant Prot. 2019, 37, 170–177. [Google Scholar]
- Mohammed, M.E.A.; El-Shafie, H.A.F.; Alhajhoj, M.R. Recent Trends in the Early Detection of the Invasive Red Palm Weevil, Rhynchophorus Ferrugineus (Olivier). In Invasive Species—Introduction Pathways, Economic Impact, and Possible Management Options; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Eldin, H.A.; Waleed, K.; Samir, M.; Tarek, M.; Sobeah, H.; Salam, M.A. A Survey on Detection of Red Palm Weevil Inside Palm Trees: Challenges and Applications. In Proceedings of the ACM International Conference Proceeding Series; Association for Computing Machinery, Cairo, Egypt, 11 November 2020; pp. 119–125. [Google Scholar]
- Koubaa, A.; Aldawood, A.; Saeed, B.; Hadid, A.; Ahmed, M.; Saad, A.; Alkhouja, H.; Alkanhal, M. Smart Palm: An IoT Framework for Red Palm Weevil Early Detection. Agronomy 2019, 10, 987. [Google Scholar] [CrossRef]
- Hetzroni, A.; Soroker, V.; Cohen, Y. Toward Practical Acoustic Red Palm Weevil Detection. Comput. Electron. Agric. 2016, 124, 100–106. [Google Scholar] [CrossRef]
- Mankin, R.W. Recent Developments in the Use of Acoustic Sensors and Signal Processing Tools to Target Early Infestations of Red Palm Weevil in Agricultural Environments. Fla. Entomol. 2011, 94, 761–765. [Google Scholar] [CrossRef]
- Rach, M.M.; Gomis, H.M.; Granado, O.L.; Malumbres, M.P.; Campoy, A.M.; Martín, J.J.S. On the Design of a Bioacoustic Sensor for the Early Detection of the Red Palm Weevil. Sensors 2013, 13, 1706–1729. [Google Scholar] [CrossRef]
- Mankin, R.W.; Al-Ayedh, H.Y.; Aldryhim, Y.; Rohde, B. Acoustic Detection of Rhynchophorus Ferrugineus (Coleoptera: Dryophthoridae) and Oryctes Elegans (Coleoptera: Scarabaeidae) in Phoenix Dactylifera (Arecales: Arecacae) Trees and Offshoots in Saudi Arabian Orchards. J. Econ. Entomol. 2016, 109, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Pinhas, J.; Soroker, V.; Hetzroni, A.; Mizrach, A.; Teicher, M.; Goldberger, J. Automatic Acoustic Detection of the Red Palm Weevil. Comput. Electron. Agric. 2008, 63, 131–139. [Google Scholar] [CrossRef]
- Siriwardena, K.A.P.; Fernando, L.C.P.; Nanayakkara, N.; Perera, K.F.G.; Kumara, A.D.N.T.; Nanayakkara, T. Portable Acoustic Device for Detection of Coconut Palms Infested by Rynchophorus Ferrugineus (Coleoptera: Curculionidae). Crop. Prot. 2010, 29, 25–29. [Google Scholar] [CrossRef]
- Rizzolo, A.; Bianchi, G.; Lucido, P.; Cangelosi, B.; Pozzi, L.; Villa, G.; Clematis, F.; Pasini, C.; Curir, P. Electronic Nose for the Early Detection of Red Palm Weevil (Rhynchophorus Ferrugineous Olivier) Infestation in Palms: Preliminary Results. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science, Angers, France, 25 September 2015; Volume 1099, pp. 347–356. [Google Scholar]
- Suma, P.; la Pergola, A.; Longo, S.; Soroker, V. The Use of Sniffing Dogs for the Detection of Rhynchophorus Ferrugineus. Phytoparasitica 2013, 42, 269–274. [Google Scholar] [CrossRef]
- Nakash, J.; Osem, Y.; Kehat, M. A Suggestion to Use Dogs for Detecting Red Palm Weevil (Rhynchophorus Ferrugineus) Infestation in Date Palms in Israel. Phytoparasitica 2000, 28, 153–155. [Google Scholar] [CrossRef]
- Ashry, I.; Mao, Y.; Al-Fehaid, Y.; Al-Shawaf, A.; Al-Bagshi, M.; Al-Brahim, S.; Ng, T.K.; Ooi, B.S. Early Detection of Red Palm Weevil Using Distributed Optical Sensor. Sci. Rep. 2020, 10, 3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soroker, V.; Suma, P.; la Pergola, A.; Cohen, Y.; Alchanatis, V.; Colomb, O. Early Detection and Monitoring of Red Palm Weevil: Approaches and Challenges. In Proceedings of the Palm Pest Mediterranean Conference, Nice, France, 16–18 January 2013. [Google Scholar]
- Culman, M.; Delalieux, S.; Tricht, K.V. Palm Tree Inventory from Aerial Images Using Retinanet. In Proceedings of the 2020 Mediterranean and Middle-East Geoscience and Remote Sensing Symposium, M2GARSS 2020, Tunis, Tunisia, 9–11 March 2020. [Google Scholar]
- Culman, M.; Delalieux, S.; van Tricht, K. Individual Palm Tree Detection Using Deep Learning on RGB Imagery to Support Tree Inventory. Remote Sens 2020, 12, 3476. [Google Scholar] [CrossRef]
- Culman, M.; Rodríguez, A.C.; Wegner, J.D.; Delalieux, S.; Somers, B. Deep Learning for Sub-Pixel Palm Tree Classification Using Spaceborne Sentinel-2 Imagery. In Proceedings of the SPIE—The International Society for Optical Engineering; (online) Spain, 13–18 September 2021, Volume 11856. [CrossRef]
- Zheng, J.; Fu, H.; Li, W.; Wu, W.; Zhao, Y.; Dong, R.; Yu, L. Cross-Regional Oil Palm Tree Counting and Detection via a Multi-Level Attention Domain Adaptation Network. ISPRS J. Photogramm. Remote Sens. 2020, 167, 154–177. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, W.; Yuan, S.; Fu, H.; Li, W.; Yu, L. Multisource-Domain Generalization-Based Oil Palm Tree Detection Using Very-High-Resolution (VHR) Satellite Images. IEEE Geosci. Remote Sens. Lett. 2022, 19, 1–5. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, S.; Wu, W.; Li, W.; Yu, L.; Fu, H.; Coomes, D. Surveying Coconut Trees Using High-Resolution Satellite Imagery in Remote Atolls of the Pacific Ocean. Remote Sens. Environ. 2023, 287, 113485. [Google Scholar] [CrossRef]
- Bannari, A.; Mohamed, A.M.A.; Peddle, D.R. Biophysiological Spectral Indices Retrieval and Statistical Analysis for Red Palm Weevil Stressattack Prediction Using Worldview-3 Data. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2016; Volume 2016, pp. 3512–3515. [Google Scholar]
- Bannari, A.; Mohamed, A.M.A.; El’battay, A. Water Stress Detection as an Indicator of Red Palm Weevil Attack Using WorldView 3 Data. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017. [Google Scholar]
- Poblete, T.; Navas-Cortes, J.A.; Camino, C.; Calderon, R.; Hornero, A.; Gonzalez-Dugo, V.; Landa, B.B.; Zarco-Tejada, P.J. Discriminating Xylella Fastidiosa from Verticillium Dahliae Infections in Olive Trees Using Thermal- and Hyperspectral-Based Plant Traits. ISPRS J. Photogramm. Remote Sens. 2021, 179, 133–144. [Google Scholar] [CrossRef]
- Golomb, O.; Alchanatis, V.; Cohen, Y.; Levin, N.; Cohen, Y.; Soroker, V. Detection of Red Palm Weevil Infected Trees Using Thermal Imaging. In Proceedings of the Precision Agriculture 2015—Papers Presented at the 10th European Conference on Precision Agriculture, ECPA, Tel Aviv, Israel, 12–16 July 2015; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 643–650. [Google Scholar]
- Zheng, J.; Fu, H.; Li, W.; Wu, W.; Yu, L.; Yuan, S.; Tao, W.Y.W.; Pang, T.K.; Kanniah, K.D. Growing Status Observation for Oil Palm Trees Using Unmanned Aerial Vehicle (UAV) Images. ISPRS J. Photogramm. Remote Sens. 2021, 173, 95–121. [Google Scholar] [CrossRef]
- Wood, D.J.A.; Preston, T.M.; Powell, S.; Stoy, P.C. Multiple UAV Flights across the Growing Season Can Characterize Fine Scale Phenological Heterogeneity within and among Vegetation Functional Groups. Remote Sens. 2022, 14, 1290. [Google Scholar] [CrossRef]
- Ecke, S.; Dempewolf, J.; Frey, J.; Schwaller, A.; Endres, E.; Klemmt, H.J.; Tiede, D.; Seifert, T. UAV-Based Forest Health Monitoring: A Systematic Review. Remote Sens. 2022, 14, 3205. [Google Scholar] [CrossRef]
- Vanbrabant, Y.; Tits, L.; Delalieux, S.; Pauly, K.; Verjans, W.; Somers, B. Multitemporal Chlorophyll Mapping in Pome Fruit Orchards from Remotely Piloted Aircraft Systems. Remote Sens. 2019, 11, 1468. [Google Scholar] [CrossRef] [Green Version]
- Vanbrabant, Y.; Delalieux, S.; Tits, L.; Pauly, K.; Vandermaesen, J.; Somers, B. Pear Flower Cluster Quantification Using RGB Drone Imagery. Agronomy 2020, 10, 407. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.M.; Milton, E.J. The Use of the Empirical Line Method to Calibrate Remotely Sensed Data to Reflectance. Int. J. Remote Sens. 1999, 20, 2653–2662. [Google Scholar] [CrossRef]
- Bivand, R.; Krug, R.; Neteler, M.; Jeworutzki, S.; Vanderhaeghe, F. Package “rgrass7” Title Interface Between GRASS Geographical Information System and R. 2022. Available online: https://cran.r-project.org/web/packages/rgrass/ (accessed on 24 February 2023).
- Woebbecke, D.M.; Sentinel, B.S.; Meyer, G.E.; von Bargen, K.; Mortensen, D.A. Color Indices for Weed /Identification under Various Soil, Residue, and Lighting Conditions; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1994. [Google Scholar]
- Meyer, G.E. Machine Vision Identification of Plants; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Hunt, E.R., Jr.; Cavigelli, M.; Daughtry, C.S.T.; McMurtrey, J.I.I.I.; Walthall, C.L. Evaluation of Digital Photography from Model Aircraft for Remote Sensing of Crop Biomass and Nitrogen Status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef] [Green Version]
- de Swaef, T.; Maes, W.H.; Aper, J.; Baert, J.; Cougnon, M.; Reheul, D.; Steppe, K.; Roldán-Ruiz, I.; Lootens, P. Applying Rgb-and Thermal-Based Vegetation Indices from UAVs for High-Throughput Field Phenotyping of Drought Tolerance in Forage Grasses. Remote Sens. 2021, 13, 147. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C.; Merzlyak, M.N. Nondestructive Estimation of Anthocyanin Content in Grapevine Nondestructive Estimation of Anthocyanin Content in Grapevine Leaves Leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar]
- Weng, Q. Proceedings of the EORSA 2018: The Fifth International Workshop on Earth Observation and Remote Sensing Applications (EORSA 2018), X’an, China, 18–20 June 2018; ISBN 9781538666425.
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-Based Plant Height from Crop Surface Models, Visible, and near Infrared Vegetation Indices for Biomass Monitoring in Barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Hague, T.; Tillett, N.D.; Wheeler, H. Automated Crop and Weed Monitoring in Widely Spaced Cereals. Precis. Agric. 2006, 7, 21–32. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Perry, C.R.; Lautenschlager, L.F. Functional Equivalence of Spectral Vegetation Indices. Remote Sens. Environ. 1984, 14, 169–182. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a Two-Band Enhanced Vegetation Index without a Blue Band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Pinty, B.; Verstraete, M.M. GEMI: A Non-Linear Index to Monitor Global Vegetation from Satellites. Vegetatio 1992, 101, 15–20. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Campos-Taberner, M.; Moreno-Martínez, Á.; Walther, S.; Duveiller, G.; Cescatti, A.; Mahecha, M.D.; Muñoz-Marí, J.; García-Haro, F.J.; Guanter, L.; et al. A Unified Vegetation Index for Quantifying the Terrestrial Biosphere. Sci. Adv. 2021, 7, eabc7447. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Great Plains Corridor: College Station, TX, USA, 1973. [Google Scholar]
- Barnes, E.; Clarke, T.; Richards, S.; Colaizzi, P.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident Detection OF Crop Water Stress, Nitrogen Status and Canopy Density Using Ground-Based Multispectral Data. In Proceedings of the 5th International Conference on Precision Agriculture and other resource management, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- McFEETERS, S.K. The Use of the Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and Limits of Vegetation Indices for LAI and APAR Assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Birth, G.S.; Mcvey, G.R. Measuring the Color of Growing Turf with a Reflectance Spectrophotometer1. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Thiam, A.K. Geographic Information Systems and Remote Sensing Methods for Assessing and Monitoring Land Degradation in the Sahel Region: The Case of Southern Mauritania; Clark University: Worcester, MA, USA, 1998. [Google Scholar]
- Deering, D.W. Measuring Forage Production of Grazing Units from Landsat MSS Data. In Proceedings of the 10th International Symposium on Remote Sensing of Environment, ERIM, Ann Arbor, MI, USA, 6 October 1975; pp. 1169–1178. [Google Scholar]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto. Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Kataoka, T.; Kaneko, T.; Okamoto, H.; Hata, S. Crop Growth Estimation System Using Machine Vision. In Proceedings of the 2003 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM 2003), Kobe, Japan, 20–24 July 2003; Volume 2, pp. b1079–b1083. [Google Scholar]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B.; Ripley, B. Package “Rpart”. Available online: https://cran.r-project.org/web/packages/rpart (accessed on 24 February 2023).
- Delalieux, S.; van Aardt, J.; Keulemans, W.; Schrevens, E.; Coppin, P. Detection of Biotic Stress (Venturia Inaequalis) in Apple Trees Using Hyperspectral Data: Non-Parametric Statistical Approaches and Physiological Implications. Eur. J. Agron. 2007, 27, 130–143. [Google Scholar] [CrossRef]
- Fan, Y.; Roupsard, O.; Bernoux, M.; le Maire, G.; Panferov, O.; Kotowska, M.M.; Knohl, A. A Sub-Canopy Structure for Simulating Oil Palm in the Community Land Model (CLM-Palm): Phenology, Allocation and Yield. Geosci. Model Dev. 2015, 8, 3785–3800. [Google Scholar] [CrossRef] [Green Version]
- Yones, M.; El-Shirbeny, M.A.; Khdery, G.; Ali, A.M. Hyperspectral Indices for Assessing Damage by the Red Palm Weevil Rhynchophorus Ferrugineus (Coleoptera: Curculionidae) in Date Palms Bt Project View Project Precision Farming View Project. Int. J. Geosci. Geomat. 2014, 2, 16–23. [Google Scholar]
- Kurdi, H.; Al-Aldawsari, A.; Al-Turaiki, I.; Aldawood, A.S. Early Detection of Red Palm Weevil, Rhynchophorus Ferrugineus (Olivier), Infestation Using Data Mining. Plants 2021, 10, 95. [Google Scholar] [CrossRef]
- El-Faki, M.S.; El-Shafie, H.A.F.; Al-Hajhoj, M.B.R. Potentials for Early Detection of Red Palm Weevil (Coleoptera: Curculionidae)-Infested Date Palm (Arecaceae) Using Temperature Differentials. Can. Entomol. 2015, 148, 239–245. [Google Scholar] [CrossRef]
- Mozib, E.-F. Effect of Red Palm Weevil, Rhynchophorus Ferrugineus (Olivier) Infestation on Temperature Profiles of Date Palm Tree. J. Entomol. Nematol. 2013, 5, 77–83. [Google Scholar] [CrossRef] [Green Version]






| Acquisition Date | RGB | Multispectral | Thermal |
|---|---|---|---|
| 29/9/2020 | ✓ | ✓ | No sensor available |
| 15/12/2020 | ✓ | ✓ | No sensor available |
| 11/05/2021 | Bad weather | Bad weather | Bad weather |
| 23/06/2021 | ✓ | ✓ | ✓ |
| 22/07/2021 | ✓ | ✓ | ✓ |
| 06/09/2021 | ✓ | ✓ | ✓ |
| Sequoia | |||
| Lens | Bandwidth | Central Wavelength | Resolution |
| Green | 40 nm | 550 nm | 1280 × 960 |
| Red | 40 nm | 660 nm | 1280 × 960 |
| Red-edge | 10 nm | 735 nm | 1280 × 960 |
| NIR | 40 nm | 790 nm | 1280 × 960 |
| RGB | 4608 × 3456 | ||
| Micasense RedEdge-MX | |||
| Lens | Bandwidth | Central Wavelength | Resolution |
| Blue | 20 nm | 475 nm | 1280 × 960 |
| Green | 20 nm | 560 nm | 1280 × 960 |
| Red | 10 nm | 668 nm | 1280 × 960 |
| Red-edge | 10 nm | 717 nm | 1280 × 960 |
| NIR | 40 nm | 840 nm | 1280 × 960 |
| Zenmuse FC350 | |||
| RGB | 1280 × 960 | ||
| FLIR Duo Pro R | |||
| Thermal | 7.5–13.5 μm | 640 × 512 | |
| Acronym | Indices | Definition | Author |
|---|---|---|---|
| RCC | Red Chromatic Coordinate index | R/(R + G + B) | [34] |
| GCC | Green Chromatic Coordinate index | G/(R + G + B) | [34] |
| BCC | Blue Chromatic Coordinate index | B/(R + G + B) | [34] |
| ExG | Excess green index | 2G − B − R | [34] |
| ExG2 | Excess Green Index v2 | (2G − B − R)/(R + G + B) | [34] |
| ExR | Excess Red Index | (1.4R − G)/(R + G + B) | [35] |
| ExGR | Excess Green minus Excess Red | ExG2 − ExR | [35] |
| GRVI | Green Red vegetation index | (G − R)/(G + R) | [36,37] |
| GBVI | Green Blue Vegetation Index | (G − B)/(G + B) | [38] |
| BRVI | Blue Red Vegetation Index | (B − R)/(B + R) | [38] |
| GR | Simple red–green ratio | G/R | [39] |
| G_R | Green-Red Difference | G − R | [38] |
| B_G | Blue-Green Difference | B − G | [38] |
| VDVI | Visible-band Difference Vegetation Index | (2G − R − B)/(2G + R + B) | [40] |
| VARI | Visible atmospherically resistant index | (G − R)/(G + R − B) | [37] |
| MGRVI | Modified green–red vegetation index | (G^2 − R^2)/(G^2 + R^2) | [41] |
| CIVE | Colour Index Of Vegetation | 0.441R − 0.881G + 0.385B + 18.787 | [40] |
| VEG | Vegetative Index | G/(R^(0.667)*B^(0.334)) | [42] |
| WI | Woebbecke Index | (G − B)/(R − G) | [34] |
| CLG | Green-band Chlorophyll Index | (RE/G) − 1 | [43] |
| CTVI | Corrected Transformed Vegetation Index | ((NDVI + 0.5)/abs(NDVI + 0.5))*sqrt(abs(NDVI + 0.5)) | [44] |
| EVI2 | Two-band Enhanced Vegetation Index | G * (NIR − R)/(NIR + 2.4R +1) | [45] |
| GEMI | Global Environmental Monitoring Index | (((NIR^2 − R^2) * 2 + (NIR * 1.5) + (R * 0.5) )/(NIR + R + 0.5)) * (1 − ((((NIR^2 − R^2) * 2 + (NIR * 1.5) + (R * 0.5) )/(NIR + R + 0.5)) * 0.25)) − ((R − 0.125)/(1 − R)) | [46] |
| GNDVI | Green Normalised Difference Vegetation Index | (NIR − G)/(NIR + G) | [47] |
| KNDVI | Kernel Normalised Difference Vegetation Index | tanh(((NIR − R)/(NIR + R)))^2 | [48] |
| MCARI | Modified Chlorophyll Absorption Ratio Index | ((RE − R) − (RE − G))*(RE/R) | [49] |
| MSAVI | Modified Soil Adjusted Vegetation Index | NIR + 0.5 − (0.5 * sqrt((2 * NIR + 1)^2 − 8 * (NIR − (2 * R)))) | [50] |
| MSAVI2 | Modified Soil Adjusted Vegetation Index 2 | (2 * (NIR + 1) − sqrt((2 * NIR + 1)^2 − 8 * (NIR − R)))/2 | [50] |
| NDVI | Normalised Difference Vegetation Index | (NIR − R)/(NIR + R) | [51] |
| NDRE | Normalised DifferenceRed Edge Index | (NIR − RE)/(NIR + RE) | [52] |
| NDWI | Normalised Difference Water Index | (G − NIR)/(G + NIR) | [53] |
| NRVI | Normalised Ratio Vegetation Index | (R/NIR − 1)/(R/NIR + 1) | [54] |
| RVI | Ratio Vegetation Index | R/NIR | |
| SR | Simple Ratio Vegetation Index | NIR/R | [55] |
| TTVI | Thiam’s Transformed Vegetation Index | sqrt(abs((NIR − R)/(NIR + R) + 0.5)) | [56] |
| TVI | Transformed Vegetation Index | sqrt((NIR − R)/(NIR + R) + 0.5) | [57] |
| NGRDI | Normalized Green-Red Difference Index | (G − R)/(G + R) | [58] |
| GLI | Green Leaf Index | (2G − R − B)/(2G + R + B) | [59] |
| CIVE | Color index of vegetation extraction | 0.441R − 0.81G + 0.385B + 18.7874 | [60] |
| CCCI | Canopy Chlorophyll Content INDE | ((NIR − RE)/(NIR + RE))/((NIR − R)/(NIR + R)) | [52] |
| WDVI | Weighted Difference Vegetation Index | NIR − 2R | |
| CIred | Chlorophyll index | (NIR/R)-1 |
| Palm n° | 04/09/20 | 17/11/20 | 30/03/21 | 25/05/21 | 07/07/21 | 03/09/21 | 13/09/21 |
|---|---|---|---|---|---|---|---|
| 14 | SoI– | L-M | M | M | VI | VI | VVI |
| 16 | SoI | M | M | M-VI | VI | VI | VVI |
| 26 | SoI | L-M | L-M | M | M_VI | VVI | VVI |
| 28 | SoI | M | M | M-VI | VI | VI | VVI |
| 29 | SoI | M | M | VI | VVI | VVI | VVI |
| 30 | SoI | L-M | L-M | L-M | VI | VI | VVI |
| 37 | SoI | L-M | L-M | M | M-VI | M-VI | M-VI |
| 38 | SoI-L | L | L | L | L | M | VI |
| 41 | SoI | M | M | M | M-VI | M | VI |
| Temperature Statistics | June 21 p Value | July 21 p Value | September 21 p Value |
|---|---|---|---|
| Mean | 0.377 | 0.055 | 0.017 * |
| Median | 0.999 | 0.348 | 0.022 * |
| Standard deviation | 0.042 * | 0.016 * | 0.010 * |
| Min | 0.791 | 0.487 | 0.447 |
| Max | 0.033 * | 0.002 * | 0.001 * |
| Range | 0.051 | 0.005 * | 0.010 * |
| Minority | 0.791 | 0.487 | 0.447 |
| Majority | 0.536 | 0.236 | 0.028 * |
| Variety | 0.133 | 0.030 * | 0.008 * |
| Variance | 0.042 * | 0.016 * | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delalieux, S.; Hardy, T.; Ferry, M.; Gomez, S.; Kooistra, L.; Culman, M.; Tits, L. Red Palm Weevil Detection in Date Palm Using Temporal UAV Imagery. Remote Sens. 2023, 15, 1380. https://doi.org/10.3390/rs15051380
Delalieux S, Hardy T, Ferry M, Gomez S, Kooistra L, Culman M, Tits L. Red Palm Weevil Detection in Date Palm Using Temporal UAV Imagery. Remote Sensing. 2023; 15(5):1380. https://doi.org/10.3390/rs15051380
Chicago/Turabian StyleDelalieux, Stephanie, Tom Hardy, Michel Ferry, Susi Gomez, Lammert Kooistra, Maria Culman, and Laurent Tits. 2023. "Red Palm Weevil Detection in Date Palm Using Temporal UAV Imagery" Remote Sensing 15, no. 5: 1380. https://doi.org/10.3390/rs15051380






