Soy, Soy Foods and Their Role in Vegetarian Diets
Abstract
:1. Introduction
2. Soy Consumption
3. Soy Nutrients and Bioactive Compounds
3.1. Protein Content and Protein Quality
3.2. Isoflavones
3.3. Lipids and Phytosterols
3.4. Other Bioactive Compounds
4. Health Effects
4.1. Possible Beneficial Effects
4.1.1. Cardiovascular and Metabolic Protection
4.1.2. Anticancer Properties
4.1.3. Menopause and Osteoporosis
4.1.4. Other Beneficial Effects
4.2. Possible Detrimental Effects
4.2.1. Thyroid Gland Disturbance
4.2.2. Sex Hormones Perturbation
4.2.3. Procancer Activity
5. Soy Foods
New Generation Foods
6. Food Safety
6.1. The Early Stage of Infant Nutrition
6.2. Soy Allergy
7. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Dietetic Association |
| APP | alternate protein product |
| BV | biological value |
| CHD | coronary heart disease |
| CVD | cardiovascular disease |
| DIAAS | digestible indispensable amino acid score |
| EFSA | European Food Safety Authority |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| ER | oestrogen receptor |
| FAO | Food and Agriculture Organization |
| FDA | Food and Drug Administration |
| GMO | genetically modified organism |
| HDL | high density lipoprotein |
| HHL | health-conscious lifestyle |
| LC-MS/MS | liquid chromatography tandem-mass spectrometry |
| LDL | low density lipoprotein |
| MRI | magnetic resonance imaging |
| NAHANES | National Health and Nutrition Examination Survey |
| NPU | net protein utilization |
| OR | odds ratio |
| PDCAAS | protein digestibility corrected for amino acid score |
| PER | protein efficiency ratio |
| PSA | prostate specific antigen |
| PUFA | polyunsaturated fatty acids |
| SERM | selective oestrogen receptor modulator |
| SPI | soy protein isolate |
| STEAR | selective tissue estrogenic activity |
| TSH | thyroid-stimulating hormone |
| TVP | textured vegetable protein |
| USDA | United States Department of Agriculture |
| WHO | World Health Organization |
| w/w | weight per weight |
References
- Key, T.J.; Davey, G.K.; Appleby, P.N. Health benefits of a vegetarian diet. Proc. Nutr. Soc. 1999, 58, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Health and ecological implications of fish consumption: A deeper insight. Mediterr. J. Nutr. Metab. 2016, 9, 7–22. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [PubMed]
- Mangels, A.R.; Messina, V. Considerations in planning vegan diets: Infants. J. Am. Diet. Assoc. 2001, 101, 670–677. [Google Scholar] [CrossRef]
- Messina, V.; Mangels, A.R. Considerations in planning vegan diets: Children. J. Am. Diet. Assoc. 2001, 101, 661–669. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; United Nations University. Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organ: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Imura, K.; Okada, A. Amino acid metabolism in pediatric patients. Nutrition 1998, 14, 143–148. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant Protein and Animal Proteins: Do They Differentially Affect Cardiovascular Disease Risk? Adv. Nutr. Int. Rev. J. 2015, 6, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Izquierdo Pulido, M.; Sánchez-González, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Morr, C.V.; Ha, E.Y.W. Off-flavors of whey protein concentrates: A literature review. Int. Dairy J. 1991, 1, 1–11. [Google Scholar] [CrossRef]
- US Department of Agricolture (USDA). Food Composition Databases Show Foods List. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 7 September 2017).
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Gerber, P.J., Food and Agriculture Organization of the United Nations, Eds.; Food and Agriculture Organization of The United Nations: Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Ko, K.-P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.A. Soy isoflavones: Hope or hype? Maturitas 2008, 61, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy isoflavones: A safety review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Seidlová-Wuttke, D. Isoflavones—Safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol. Nutr. Food Res. 2009, 53, S266–S309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, W.; Custer, L.J.; Dai, Q.; Shu, X.O.; Jin, F.; Franke, A.A. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr. Cancer 1999, 33, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Egami, I.; Kato, K.; Kawamura, T.; Tamakoshi, A.; Lin, Y.; Nakayama, T.; Wada, M.; Ohno, Y. Dietary intake and sources of isoflavones among Japanese. Nutr. Cancer 1999, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kwon, C.S. Estimated dietary isoflavone intake of Korean population based on National Nutrition Survey. Nutr. Res. 2001, 21, 947–953. [Google Scholar] [CrossRef]
- Shu, X.O.; Jin, F.; Dai, Q.; Wen, W.; Potter, J.D.; Kushi, L.H.; Ruan, Z.; Gao, Y.T.; Zheng, W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 483–488. [Google Scholar]
- Ho, S.C.; Woo, J.L.; Leung, S.S.; Sham, A.L.; Lam, T.H.; Janus, E.D. Intake of soy products is associated with better plasma lipid profiles in the Hong Kong Chinese population. J. Nutr. 2000, 130, 2590–2593. [Google Scholar] [PubMed]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chan, S.G.; Yip, Y.B.; Chan, C.S.Y.; Woo, J.L.F.; Sham, A. Change in bone mineral density and its determinants in pre- and perimenopausal Chinese women: The Hong Kong Perimenopausal Women Osteoporosis Study. Osteoporos. Int. 2008, 19, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dai, Q.; Tseng, M.; Shu, X.-O.; Gao, Y.-T.; Zheng, W. Dietary patterns and breast cancer risk in the shanghai breast cancer study. Cancer Epidemiol. Epidemiol. Prev. 2007, 16, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. Int. J. Epidemiol. 2000, 29, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Barua, K.; Hauck, C.C. Solvent extraction selection in the determination of isoflavones in soy foods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 129–138. [Google Scholar] [CrossRef]
- Morton, M.S.; Wilcox, G.; Wahlqvist, M.L.; Griffiths, K. Determination of lignans and isoflavonoids in human female plasma following dietary supplementation. J. Endocrinol. 1994, 142, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.S.; Arisaka, O.; Miyake, N.; Morgan, L.D.; Evans, B.A.J. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J. Nutr. 2002, 132, 3168–3171. [Google Scholar] [PubMed]
- Van Erp-Baart, M.A.J.; Brants, H.A.M.; Kiely, M.; Mulligan, A.; Turrini, A.; Sermoneta, C.; Kilkkinen, A.; Valsta, L.M. Isoflavone intake in four different European countries: The VENUS approach. Br. J. Nutr. 2003, 89, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, J.H.; Nam, S.J.; Ryu, S.; Kong, G. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr. Cancer 2008, 60, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wang, C.; Ren, C. Intakes of total and individual flavonoids by US adults. Int. J. Food Sci. Nutr. 2014, 65, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Kuhnle, G.G.C.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-h dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ross, P.L.; Lee, M.; John, E.M.; Koo, J. Sources of phytoestrogen exposure among non-Asian women in California, USA. Cancer Causes Control 2000, 11, 299–302. [Google Scholar] [CrossRef] [PubMed]
- De Kleijn, M.J.; van der Schouw, Y.T.; Wilson, P.W.; Adlercreutz, H.; Mazur, W.; Grobbee, D.E.; Jacques, P.F. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: The Framingham study (1–4). J. Nutr. 2001, 131, 1826–1832. [Google Scholar] [PubMed]
- Boker, L.K.; Van der Schouw, Y.T.; De Kleijn, M.J.J.; Jacques, P.F.; Grobbee, D.E.; Peeters, P.H.M. Intake of dietary phytoestrogens by Dutch women. J. Nutr. 2002, 132, 1319–1328. [Google Scholar] [PubMed]
- Keinan-Boker, L.; Peeters, P.H.M.; Mulligan, A.A.; Navarro, C.; Slimani, N.; Mattisson, I.; Lundin, E.; McTaggart, A.; Allen, N.E.; Overvad, K.; et al. Soy product consumption in 10 European countries: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2002, 5, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.S.; Appleby, P.N.; Spencer, E.A.; Key, T.J. Soy intake and blood cholesterol concentrations: A cross-sectional study of 1033 pre- and postmenopausal women in the Oxford arm of the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2004, 80, 1391–1396. [Google Scholar] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Rapisarda, G.; Marventano, S.; Galvano, F.; Mistretta, A.; Grosso, G. Association between polyphenol intake and adherence to the Mediterranean diet in Sicily, southern Italy. NFS J. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.L.; Fraser, G.E.; Beeson, W.L.; Knutsen, S.F.; Herring, R.P.; Chan, J.; Sabaté, J.; Montgomery, S.; Haddad, E.; Preston-Martin, S.; et al. Cohort profile: The Adventist Health Study-2 (AHS-2). Int. J. Epidemiol. 2008, 37, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegl, K.; Fraser, G.E.; Chan, J.; Franke, A.; Sabaté, J. Validation of soy protein estimates from a food-frequency questionnaire with repeated 24-h recalls and isoflavonoid excretion in overnight urine in a Western population with a wide range of soy intakes. Am. J. Clin. Nutr. 2008, 87, 1422–1427. [Google Scholar] [PubMed]
- Frankenfeld, C.L.; Patterson, R.E.; Kalhorn, T.F.; Skor, H.E.; Howald, W.N.; Lampe, J.W. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. J. Am. Diet. Assoc. 2002, 102, 1407–1413. [Google Scholar] [CrossRef]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 6 September 2017).
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006; ISBN 978-92-5-105571-7. [Google Scholar]
- Ministero delle Politiche Agricole, Alimentari e Forestali (Mipaaf—Italia). Notifica a Ue Richiesta per Divieto Coltivazione Ogm in tutto il Territorio. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/9284 (accessed on 11 September 2017).
- Directive (EU) 2015/412 of the European Parliament and of the Council of 11 March 2015. Available online: http://eur-lex.europa.eu/legal-content/IT/TXT/?uri=OJ%3AJOL_2015_068_R_0001 (accessed on 11 September 2017).
- Genetically Modified Organisms—European Commission. Available online: http://ec.europa.eu/food/dyna/gm_register/index_en.cfm (accessed on 29 November 2017).
- De Vendômois, J.S.; Cellier, D.; Vélot, C.; Clair, E.; Mesnage, R.; Séralini, G.-E. Debate on GMOs health risks after statistical findings in regulatory tests. Int. J. Biol. Sci. 2010, 6, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences, Engineering and Medicine. Genetically Engineered Crops: Experiences and Prospects; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-43738-7. [Google Scholar]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Daydé, J.; Widholm, J.M. Effect of Temperature and Soil Moisture Status during Seed Development on Soybean Seed Isoflavone Concentration and Composition. Crop Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Wasswa-Kintu, S. USDA Special Interest Databases on Flavonoids. Available online: https://data.nal.usda.gov/dataset/usda-special-interest-databases-flavonoids (accessed on 7 September 2017).
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.S. Soy protein. J. Perinat. Educ. 2003, 12, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Boersma, B.; Patel, R.; Kirk, M.; Darley-Usmar, V.M.; Kim, H.; Xu, J. Isoflavonoids and chronic disease: Mechanisms of action. BioFactors 2000, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mayr, U.; Butsch, A.; Schneider, S. Validation of two in vitro test systems for estrogenic activities with zearalenone, phytoestrogens and cereal extracts. Toxicology 1992, 74, 135–149. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Ota, Y.; Hatanaka, C. Extraction and purification of pectic polysaccharides from soybean okara and enzymatic analysis of their structures. Carbohydr. Polym. 1996, 30, 265–273. [Google Scholar] [CrossRef]
- Armstrong, W.B.; Wan, X.S.; Kennedy, A.R.; Taylor, T.H.; Meyskens, F.L. Development of the Bowman-Birk inhibitor for oral cancer chemoprevention and analysis of Neu immunohistochemical staining intensity with Bowman-Birk inhibitor concentrate treatment. Laryngoscope 2003, 113, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.; Chen, N.; Macasieb, J.; de Lumen, B.O. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001, 61, 7473–7478. [Google Scholar] [PubMed]
- Lam, Y.; Galvez, A.; de Lumen, B.O. Lunasin suppresses E1A-mediated transformation of mammalian cells but does not inhibit growth of immortalized and established cancer cell lines. Nutr. Cancer 2003, 47, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Yamamoto, M.; Nakajima, K. Effects of Soybean Oligosaccharides on Human Digestive Organs. J. Clin. Biochem. Nutr. 1991, 10, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Prasad, K.N.; Xie, H.; Lin, S.; Jiang, Y. Structural characteristics of oligosaccharides from soy sauce lees and their potential prebiotic effect on lactic acid bacteria. Food Chem. 2011, 126, 590–594. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Rupérez, P. Soybean oligosaccharides: Potential as new ingredients in functional food. Nutr. Hosp. 2006, 21, 92–96. [Google Scholar] [PubMed]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lee, S.-O.; Hendrich, S.; Murphy, P.A. Quantification of the group B soyasaponins by high-performance liquid chromatography. J. Agric. Food Chem. 2002, 50, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Sung, M.K. Saponins as anticarcinogens. J. Nutr. 1995, 125, 717S–724S. [Google Scholar] [PubMed]
- Nakashima, H.; Okubo, K.; Honda, Y.; Tamura, T.; Matsuda, S.; Yamamoto, N. Inhibitory effect of glycosides like saponin from soybean on the infectivity of HIV in vitro. AIDS 1989, 3, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.C.; Berhow, M.A.; Badger, T.M. Estrogenic and antiproliferative properties of soy sapogenols in human breast cancer cells in vitro. Food Chem. Toxicol. 2002, 40, 1767–1774. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Badger, T.M. Comprehensive phytochemical profile of soy protein isolate. J. Agric. Food Chem. 2004, 52, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Istfan, N.; Murray, E.; Janghorbani, M.; Young, V.R. An evaluation of the nutritional value of a soy protein concentrate in young adult men using the short-term N-balance method. J. Nutr. 1983, 113, 2516–2523. [Google Scholar] [PubMed]
- Scrimshaw, N.S.; Wayler, A.H.; Murray, E.; Steinke, F.H.; Rand, W.M.; Young, V.R. Nitrogen balance response in young men given one of two isolated soy proteins or milk proteins. J. Nutr. 1983, 113, 2492–2497. [Google Scholar] [PubMed]
- Wayler, A.; Queiroz, E.; Scrimshaw, N.S.; Steinke, F.H.; Rand, W.M.; Young, V.R. Nitrogen balance studies in young men to assess the protein quality of an isolated soy protein in relation to meat proteins. J. Nutr. 1983, 113, 2485–2491. [Google Scholar] [PubMed]
- Young, V.R.; Puig, M.; Queiroz, E.; Scrimshaw, N.S.; Rand, W.M. Evaluation of the protein quality of an isolated soy protein in young men: Relative nitrogen requirements and effect of methionine supplementation. Am. J. Clin. Nutr. 1984, 39, 16–24. [Google Scholar] [PubMed]
- Young, V.R.; Wayler, A.; Garza, C.; Steinke, F.H.; Murray, E.; Rand, W.M.; Scrimshaw, N.S. A long-term metabolic balance study in young men to assess the nutritional quality of an isolated soy protein and beef proteins. Am. J. Clin. Nutr. 1984, 39, 8–15. [Google Scholar] [PubMed]
- Beer, W.H.; Murray, E.; Oh, S.H.; Pedersen, H.E.; Wolfe, R.R.; Young, V.R. A long-term metabolic study to assess the nutritional value of and immunological tolerance to two soy-protein concentrates in adult humans. Am. J. Clin. Nutr. 1989, 50, 997–1007. [Google Scholar] [PubMed]
- García, M.C.; Torre, M.; Marina, M.L.; Laborda, F. Composition and characterization of soyabean and related products. Crit. Rev. Food Sci. Nutr. 1997, 37, 361–391. [Google Scholar] [CrossRef] [PubMed]
- Grieshop, C.M.; Fahey, G.C. Comparison of quality characteristics of soybeans from Brazil, China, and the United States. J. Agric. Food Chem. 2001, 49, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Grieshop, C.M.; Kadzere, C.T.; Clapper, G.M.; Flickinger, E.A.; Bauer, L.L.; Frazier, R.L.; Fahey, G.C. Chemical and nutritional characteristics of United States soybeans and soybean meals. J. Agric. Food Chem. 2003, 51, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R.; Viteri, F.; Elías, L.G.; de Zaghi, S.; Alvarado, J.; Odell, A.D. Protein quality of a soybean protein textured food in experimental animals and children. J. Nutr. 1967, 93, 349–360. [Google Scholar] [PubMed]
- Joint FAO/WHO Expert Consultation; World Health Organization; Food and Agriculture Organization of the United Nations. Protein Quality Evaluation: Report of the Joint FAO/WHO; FAO Food Nutrition Paper; FAO: Rome, Italy, 1991. [Google Scholar]
- Protein—Elements within the Nutrition Facts Table—Food—Canadian Food Inspection Agency. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrition-labelling/elements-within-the-nutrition-facts-table/eng/1389206763218/1389206811747?chap=7#s10c7 (accessed on 7 September 2017).
- World Health Organization. Report of a Joint FAO/WHO/UNU Expert Consultation. Energy and Protein Requirements; World Health Organization: Geneva, Switzerland, 1985; Volume 724, pp. 1–206. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition; FAO: Rome, Italy, 2013; Volume 92, pp. 1–66. [Google Scholar]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. The protein digestibility-corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [PubMed]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [PubMed]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [PubMed]
- Lee, W.T.; Weisell, R.; Albert, J.; Tomé, D.; Kurpad, A.V.; Uauy, R. Research Approaches and Methods for Evaluating the Protein Quality of Human Foods Proposed by an FAO Expert Working Group in 2014. J. Nutr. 2016, 146, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Istfan, N.; Murray, E.; Janghorbani, M.; Evans, W.J.; Young, V.R. The nutritional value of a soy protein concentrate (STAPRO-3200) for long-term protein nutritional maintenance in young men. J. Nutr. 1983, 113, 2524–2534. [Google Scholar] [PubMed]
- Amigo-Benavent, M.; Silván, J.M.; Moreno, F.J.; Villamiel, M.; Del Castillo, M.D. Protein quality, antigenicity, and antioxidant activity of soy-based foodstuffs. J. Agric. Food Chem. 2008, 56, 6498–6505. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kiel, D.P.; Tucker, K.L.; Dufour, A.B.; Hannan, M.T. Dietary protein is associated with musculoskeletal health independently of dietary pattern: The Framingham Third Generation Study. Am. J. Clin. Nutr. 2017, 105, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.C.; DiSilvestro, R.A.; Babaknia, A.; Devor, S.T. Soy versus whey protein bars: Effects on exercise training impact on lean body mass and antioxidant status. Nutr. J. 2004, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Società Italiana di Nutrizione Umana (SINU). LARN: Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana; IV Revisione; SICS Editore; Società Italiana di Nutrizione Umana: Firenze, Italy, 2014; ISBN 978-88-906852-2-4. [Google Scholar]
- U.S. Department of Agriculture (USDA). Questions and Answers on Alternate Protein Products (APP)—Food and Nutrition Service. Available online: https://www.fns.usda.gov/questions-and-answers-alternate-protein-products-app (accessed on 11 September 2017).
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [PubMed]
- Cabot, W. Phytoestrogens. J. Am. Acad. Orthop. Surg. 2003, 11, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Lozovaya, V.V.; Lygin, A.V.; Zernova, O.V.; Ulanov, A.V.; Li, S.; Hartman, G.L.; Widholm, J.M. Modification of phenolic metabolism in soybean hairy roots through down regulation of chalcone synthase or isoflavone synthase. Planta 2007, 225, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Zabala, G.; Zou, J.; Tuteja, J.; Gonzalez, D.O.; Clough, S.J.; Vodkin, L.O. Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol. 2006, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Stacey, G.; Yu, O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006, 48, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.M.; Duke, J.A.; Wahala, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef]
- USDA ARS. Nutrient Data Laboratory. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/ (accessed on 7 September 2017).
- Thuzar, M.; Puteh, A.B.; Abdullah, N.A.P.; Lassim, M.B.M.; Jusoff, K. The Effects of Temperature Stress on the Quality and Yield of Soya Bean [(Glycine max L.) Merrill.]. J. Agric. Sci. 2010, 2, 172. [Google Scholar] [CrossRef]
- Šertović, E.; Mujić, I.; Jokić, S.; Alibabić, V.; Sarić, Z. Effect of soybean cultivars on the content of isoflavones in soymilk. Rom. Biotechnol. Lett. 2012, 17, 7151–7159. [Google Scholar]
- Wang, H.; Murphy, P.A. Isoflavone Composition of American and Japanese Soybeans in Iowa: Effects of Variety, Crop Year, and Location. J. Agric. Food Chem. 1994, 42, 1674–1677. [Google Scholar] [CrossRef]
- Devi, M.K.A.; Gondi, M.; Sakthivelu, G.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Functional attributes of soybean seeds and products, with reference to isoflavone content and antioxidant activity. Food Chem. 2009, 114, 771–776. [Google Scholar] [CrossRef]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Larsen, J.C. Detection of weak estrogenic flavonoids using a recombinant yeast strain and a modified MCF7 cell proliferation assay. Chem. Res. Toxicol. 1998, 11, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Markkanen, H.; Watanabe, S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 1993, 342, 1209–1210. [Google Scholar] [CrossRef]
- Kapiotis, S.; Hermann, M.; Held, I.; Seelos, C.; Ehringer, H.; Gmeiner, B.M. Genistein, the dietary-derived angiogenesis inhibitor, prevents LDL oxidation and protects endothelial cells from damage by atherogenic LDL. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2868–2874. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.-A. Biological role of estrogen and estrogen receptors. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Meegan, M.J.; Lloyd, D.G. Advances in the science of estrogen receptor modulation. Curr. Med. Chem. 2003, 10, 181–210. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Hartmann, L.C. Selective estrogen-receptor modulators—Mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003, 348, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; O’Malley, B.W. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004, 25, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Ryder, J.J.; Kurzer, M.S.; Lampe, J.W.; Messina, M.J.; Phipps, W.R.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Update 2009, 15, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafsson, J.-A. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Boersma, B.J.; Crawford, J.H.; Hogg, N.; Kirk, M.; Kalyanaraman, B.; Parks, D.A.; Barnes, S.; Darley-Usmar, V. Antioxidant mechanisms of isoflavones in lipid systems: Paradoxical effects of peroxyl radical scavenging. Free Radic. Biol. Med. 2001, 31, 1570–1581. [Google Scholar] [CrossRef]
- Nakajima, N.; Nozaki, N.; Ishihara, K.; Ishikawa, A.; Tsuji, H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of a new isoflavone-enriched tempeh. J. Biosci. Bioeng. 2005, 100, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Song, T.; Buseman, G.; Barua, K.; Beecher, G.R.; Trainer, D.; Holden, J. Isoflavones in retail and institutional soy foods. J. Agric. Food Chem. 1999, 47, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Kobayashi-Hattori, K.; Kaneko, K.; Endo, H.; Takita, T. Effect of soybean varieties on the content and composition of isoflavone in rice-koji miso. Food Chem. 2007, 100, 369–374. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.P. Isoflavone phytoestrogen degradation in fermented soymilk with selected beta-glucosidase producing L. acidophilus strains during storage at different temperatures. Int. J. Food Microbiol. 2007, 115, 79–88. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Bik, E.M.; Costello, E.K.; Dethlefsen, L.; Haque, R.; Relman, D.A.; Singh, U. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Gordon, J.I. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Collison, M.W. Determination of total soy isoflavones in dietary supplements, supplement ingredients, and soy foods by high-performance liquid chromatography with ultraviolet detection: Collaborative study. J. AOAC Int. 2008, 91, 489–500. [Google Scholar] [PubMed]
- Mathias, K.; Ismail, B.; Corvalan, C.M.; Hayes, K.D. Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. J. Agric. Food Chem. 2006, 54, 7495–7502. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, M.D.; Le Guerroué, J.L.; Chagas, C.M.S.; Franco, O.L.; Bianchini, E.; João, M.J. Influence of Heat Treatment and Grain Germination on the Isoflavone Profile of Soy Milk. J. Food Biochem. 2006, 30, 234–247. [Google Scholar] [CrossRef]
- King, R.A.; Bursill, D.B. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998, 67, 867–872. [Google Scholar] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [PubMed]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [PubMed]
- Axelson, M.; Kirk, D.N.; Farrant, R.D.; Cooley, G.; Lawson, A.M.; Setchell, K.D. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982, 201, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [PubMed]
- Bolca, S.; Possemiers, S.; Herregat, A.; Huybrechts, I.; Heyerick, A.; De Vriese, S.; Verbruggen, M.; Depypere, H.; De Keukeleire, D.; Bracke, M.; et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J. Nutr. 2007, 137, 2242–2246. [Google Scholar] [PubMed]
- Setchell, K.D.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lydeking-Olsen, E.; Beck-Jensen, J.E.; Setchell, K.D.R.; Holm-Jensen, T. Soymilk or progesterone for prevention of bone loss—A 2 years randomized, placebo-controlled trial. Eur. J. Nutr. 2004, 43, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Berman, S.; Humbert, O.; Lampe, J.W. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J. Nutr. 2004, 134, 596–599. [Google Scholar] [PubMed]
- Karr, S.C.; Lampe, J.W.; Hutchins, A.M.; Slavin, J.L. Urinary isoflavonoid excretion in humans is dose dependent at low to moderate levels of soy-protein consumption. Am. J. Clin. Nutr. 1997, 66, 46–51. [Google Scholar] [PubMed]
- Kulling, S.E.; Honig, D.M.; Simat, T.J.; Metzler, M. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J. Agric. Food Chem. 2000, 48, 4963–4972. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Honig, D.M.; Metzler, M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, J.; Coward, L.; Kirk, M.; Barnes, S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J. Nutr. 1997, 127, 1260–1268. [Google Scholar] [PubMed]
- Doerge, D.R.; Chang, H.C.; Churchwell, M.I.; Holder, C.L. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab. Dispos. Biol. Fate Chem. 2000, 28, 298–307. [Google Scholar] [PubMed]
- Hargreaves, D.F.; Potten, C.S.; Harding, C.; Shaw, L.E.; Morton, M.S.; Roberts, S.A.; Howell, A.; Bundred, N.J. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J. Clin. Endocrinol. Metab. 1999, 84, 4017–4024. [Google Scholar] [CrossRef] [PubMed]
- Maubach, J.; Bracke, M.E.; Heyerick, A.; Depypere, H.T.; Serreyn, R.F.; Mareel, M.M.; De Keukeleire, D. Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 784, 137–144. [Google Scholar] [CrossRef]
- Morton, M.S.; Chan, P.S.; Cheng, C.; Blacklock, N.; Matos-Ferreira, A.; Abranches-Monteiro, L.; Correia, R.; Lloyd, S.; Griffiths, K. Lignans and isoflavonoids in plasma and prostatic fluid in men: Samples from Portugal, Hong Kong, and the United Kingdom. Prostate 1997, 32, 122–128. [Google Scholar] [CrossRef]
- Hedlund, T.E.; Maroni, P.D.; Ferucci, P.G.; Dayton, R.; Barnes, S.; Jones, K.; Moore, R.; Ogden, L.G.; Wähälä, K.; Sackett, H.M.; et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J. Nutr. 2005, 135, 1400–1406. [Google Scholar] [PubMed]
- Mounts, T.L.; Warner, K.; List, G.R.; Kleiman, R.; Fehr, W.R.; Hammond, E.G.; Wilcox, J.R. Effect of altered fatty acid composition on soybean oil stability. J. Am. Oil Chem. Soc. 1988, 65, 624–628. [Google Scholar] [CrossRef]
- Slavin, M.; Kenworthy, W.; Yu, L.L. Antioxidant properties, phytochemical composition, and antiproliferative activity of Maryland-grown soybeans with colored seed coats. J. Agric. Food Chem. 2009, 57, 11174–11185. [Google Scholar] [CrossRef] [PubMed]
- Whent, M.; Hao, J.; Slavin, M.; Zhou, M.; Song, J.; Kenworthy, W.; Yu, L.L. Effect of genotype, environment, and their interaction on chemical composition and antioxidant properties of low-linolenic soybeans grown in Maryland. J. Agric. Food Chem. 2009, 57, 10163–10174. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, I.A.; Katan, M.B.; Zock, P.L. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: A meta-analysis. J. Nutr. 2004, 134, 919–922. [Google Scholar] [PubMed]
- Dai, J.; Ziegler, T.R.; Bostick, R.M.; Manatunga, A.K.; Jones, D.P.; Goldberg, J.; Miller, A.; Vogt, G.; Wilson, P.W.; Jones, L.; et al. High habitual dietary alpha-linolenic acid intake is associated with decreased plasma soluble interleukin-6 receptor concentrations in male twins. Am. J. Clin. Nutr. 2010, 92, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kiribuchi, M.; Miura, K.; Tokuda, S.; Kaneda, T. Hypocholesterolemic effect of triterpene alcohols with soysterol on plasma cholesterol in rats. J. Nutr. Sci. Vitaminol. 1983, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Dennis, A.L.; Kumazawa, T.; Haynes, A.M.; Nes, W.D. Soybean sterol composition and utilization by Phytophthora sojae. Phytochemistry 2001, 58, 423–428. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Klingberg, S.; Ellegård, L.; Johansson, I.; Hallmans, G.; Weinehall, L.; Andersson, H.; Winkvist, A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am. J. Clin. Nutr. 2008, 87, 993–1001. [Google Scholar] [PubMed]
- Van Ee, J.H. Soy constituents: Modes of action in low-density lipoprotein management. Nutr. Rev. 2009, 67, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.W. Plant sterols and tissue cholesterol levels. Am. J. Clin. Nutr. 1958, 6, 644–649. [Google Scholar] [PubMed]
- Lin, X.; Ma, L.; Racette, S.B.; Anderson Spearie, C.L.; Ostlund, R.E. Phytosterol glycosides reduce cholesterol absorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G931–G935. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E. Phytosterols, cholesterol absorption and healthy diets. Lipids 2007, 42, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H.; Frohlich, J.J. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am. J. Med. 1999, 107, 588–594. [Google Scholar] [CrossRef]
- Andersson, S.W.; Skinner, J.; Ellegård, L.; Welch, A.A.; Bingham, S.; Mulligan, A.; Andersson, H.; Khaw, K.T. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. Eur. J. Clin. Nutr. 2004, 58, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Escurriol, V.; Cofán, M.; Moreno-Iribas, C.; Larrañaga, N.; Martínez, C.; Navarro, C.; Rodríguez, L.; González, C.A.; Corella, D.; Ros, E. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J. Lipid Res. 2010, 51, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Genser, B.; Silbernagel, G.; De Backer, G.; Bruckert, E.; Carmena, R.; Chapman, M.J.; Deanfield, J.; Descamps, O.S.; Rietzschel, E.R.; Dias, K.C.; et al. Plant sterols and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2012, 33, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Jones, P.J.H.; Abumweis, S.S. Plant sterols: Factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandberg, T.E.; Gylling, H.; Tilvis, R.S.; Miettinen, T.A. Serum plant and other noncholesterol sterols, cholesterol metabolism and 22-year mortality among middle-aged men. Atherosclerosis 2010, 210, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegl, K.; Lütjohann, D.; Sirirat, R.; Mashchak, A.; Fraser, G.E.; Haddad, E. Variations in dietary intake and plasma concentrations of plant sterols across plant-based diets among North American adults. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439S–450S. [Google Scholar] [PubMed]
- Peñalvo, J.L.; Heinonen, S.-M.; Nurmi, T.; Deyama, T.; Nishibe, S.; Adlercreutz, H. Plant lignans in soy-based health supplements. J. Agric. Food Chem. 2004, 52, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, C. Soybean saponins: Chemistry, analysis, and potential of health effects. In Soybeans as Functional Foods and Ingredients; AOCS Press: Columbia, MO, USA, 2004; pp. 73–100. ISBN 1-893997-33-2. [Google Scholar]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J. Nutr. 1995, 125, 606S–611S. [Google Scholar] [PubMed]
- Oakenfull, D. Saponins in food—A review. Food Chem. 1981, 7, 19–40. [Google Scholar] [CrossRef]
- Decroos, K.; Vincken, J.P.; Heng, L.; Bakker, R.; Gruppen, H.; Verstraete, W. Simultaneous quantification of differently glycosylated, acetylated, and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one-conjugated soyasaponins using reversed-phase high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. A 2005, 1072, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gestetner, B.; Birk, Y.; Tencer, Y. Soybean saponins. Fate of ingested soybean saponins and the physiological aspect of their hemolytic activity. J. Agric. Food Chem. 1968, 16, 1031–1035. [Google Scholar] [CrossRef]
- Hu, J.; Reddy, M.B.; Hendrich, S.; Murphy, P.A. Soyasaponin I and sapongenol B have limited absorption by Caco-2 intestinal cells and limited bioavailability in women. J. Nutr. 2004, 134, 1867–1873. [Google Scholar] [PubMed]
- Hu, J.; Zheng, Y.L.; Hyde, W.; Hendrich, S.; Murphy, P.A. Human fecal metabolism of soyasaponin I. J. Agric. Food Chem. 2004, 52, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Ustündağ, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, D.; Sidhu, G.S. Could saponins be a useful treatment for hypercholesterolaemia? Eur. J. Clin. Nutr. 1990, 44, 79–88. [Google Scholar] [PubMed]
- Oakenfull, D.G.; Topping, D.L. Saponins and plasma cholesterol. Atherosclerosis 1983, 48, 301–303. [Google Scholar] [CrossRef]
- Potter, J.; Topping, D.; Oakenfull, D. Soya, saponins, and plasma-cholesterol. Lancet 1979, 313, 223. [Google Scholar] [CrossRef]
- Oakenfull, D. Soy protein, saponins and plasma cholesterol. J. Nutr. 2001, 131, 2971–2972. [Google Scholar] [PubMed]
- Fonseca, N.D.; Villar, M.P.M.; Donangelo, C.M.; Perrone, D. Isoflavones and soyasaponins in soy infant formulas in Brazil: Profile and estimated consumption. Food Chem. 2014, 143, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Hu, J.; Barua, K.; Hauck, C.C. Group B Saponins in Soy Products in the U.S. Department of Agriculture—Iowa State University Isoflavone Database and Their Comparison with Isoflavone Contents. J. Agric. Food Chem. 2008, 56, 8534–8540. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Doré, J.; Blaut, M. Bioavailability of lignans in human subjects. Nutr. Res. Rev. 2006, 19, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Meselhy, M.R.; Li, Y.; Qin, G.W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef] [PubMed]
- HeYuan, J.; FeiJie, L.; JianXiang, T. Bioactive components of soyabeans and their functions. Soybean Sci. 2000, 19, 160–164. [Google Scholar]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Prieto, R.M.; Costa-Bauzá, A.; March, J.G.; Shamsuddin, A.M. Absorption and excretion of orally administered inositol hexaphosphate (IP(6) or phytate) in humans. BioFactors 2001, 15, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E.; Welch, R.; Theil, E.C.; Beard, J.L. Women with low iron stores absorb iron from soybeans. Am. J. Clin. Nutr. 2003, 77, 180–184. [Google Scholar] [PubMed]
- Zielińska-Dawidziak, M. Plant ferritin—A source of iron to prevent its deficiency. Nutrients 2015, 7, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Burton, J.W.; Theil, E.C. Purified ferritin and soybean meal can be sources of iron for treating iron deficiency in rats. J. Nutr. 1996, 126, 154–160. [Google Scholar] [PubMed]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Weaver, C.M.; Fitzsimmons, M.L. Soybean phytate content: Effect on calcium absorption. Am. J. Clin. Nutr. 1991, 53, 745–747. [Google Scholar] [PubMed]
- Omoni, A.O.; Aluko, R.E. Soybean foods and their benefits: Potential mechanisms of action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244S–1299S. [Google Scholar] [PubMed]
- Food and Drug Administration. Food labeling: Health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed. Regist. 1999, 64, 57700–57733. [Google Scholar]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Benkhedda, K.; Boudrault, C.; Sinclair, S.E.; Marles, R.J.; Xiao, C.W.; Underhill, L. Food Risk Analysis Communication. Issued by health canada’s food directorate. Health Canada’s proposal to accept a health claim about soy products and cholesterol lowering. Int. Food Risk Anal. J. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Harland, J.I.; Haffner, T.A. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis 2008, 200, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [PubMed]
- Nagata, C.; Takatsuka, N.; Kurisu, Y.; Shimizu, H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J. Nutr. 1998, 128, 209–213. [Google Scholar] [PubMed]
- Erdman, J.W. AHA Science Advisory: Soy protein and cardiovascular disease: A statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation 2000, 102, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to isolated soy protein and reduction of blood LDL-cholesterol concentrations pursuant to Article 14 of Regulation (EC) No 1924/2006: Isolated soy protein and reduction of blood LDL-cholesterol concentrations. EFSA J. 2012, 10, 2555. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Albertazzi, P.; Lise Nielsen, I.; Hall, W.; Williamson, G.; Tetens, I.; Atkins, S.; Cross, H.; Manios, Y.; Wolk, A.; et al. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proc. Nutr. Soc. 2006, 65, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef]
- Badger, T.M.; Ronis, M.J.; Hakkak, R. Developmental effects and health aspects of soy protein isolate, casein, and whey in male and female rats. Int. J. Toxicol. 2001, 20, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Eason, R.R.; Till, S.R.; Velarde, M.C.; Geng, Y.; Chatman, L.; Gu, L.; Badger, T.M.; Simmen, F.A.; Simmen, R.C.M. Uterine phenotype of young adult rats exposed to dietary soy or genistein during development. J. Nutr. Biochem. 2005, 16, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Martin, B.; Morris, K.; Greig, I.; McKinnell, C.; McNeilly, A.S.; Walker, M. Infant feeding with soy formula milk: Effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum. Reprod. 2002, 17, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Safford, B.; Dickens, A.; Halleron, N.; Briggs, D.; Carthew, P.; Baker, V. A model to estimate the oestrogen receptor mediated effects from exposure to soy isoflavones in food. Regul. Toxicol. Pharmacol. 2003, 38, 196–209. [Google Scholar] [CrossRef]
- Merritt, R.J.; Jenks, B.H. Safety of soy-based infant formulas containing isoflavones: The clinical evidence. J. Nutr. 2004, 134, 1220S–1224S. [Google Scholar] [PubMed]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [PubMed]
- Wisniewski, A.B.; Klein, S.L.; Lakshmanan, Y.; Gearhart, J.P. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J. Urol. 2003, 169, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Fielden, M.R.; Samy, S.M.; Chou, K.C.; Zacharewski, T.R. Effect of human dietary exposure levels of genistein during gestation and lactation on long-term reproductive development and sperm quality in mice. Food Chem. Toxicol. 2003, 41, 447–454. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Andrews, W.W.; Advis, J.P.; White, S.S. Recent advances in the endocrinology of puberty. Endocr. Rev. 1980, 1, 228–257. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.D.; Judd, H.L.; Young, P.E.; Jones, O.W.; Yen, S.S. Amniotic fluid androgens and estrogens in midgestation. J. Clin. Endocrinol. Metab. 1977, 45, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Zhao, X.; Lindley, S.L.; Heubi, J.E.; King, E.C.; Messina, M.J. Soy isoflavone phase II metabolism differs between rodents and humans: Implications for the effect on breast cancer risk. Am. J. Clin. Nutr. 2011, 94, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Helppi, J.; Müller, D.R.; Zierau, O.; Watzl, B.; Vollmer, G.; Diel, P.; Bub, A.; Kulling, S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Arch. Toxicol. 2016, 90, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Van der Schouw, Y.T.; de Kleijn, M.J.; Peeters, P.H.; Grobbee, D.E. Phyto-oestrogens and cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 154–167. [Google Scholar] [PubMed]
- Merz-Demlow, B.E.; Duncan, A.M.; Wangen, K.E.; Xu, X.; Carr, T.P.; Phipps, W.R.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am. J. Clin. Nutr. 2000, 71, 1462–1469. [Google Scholar] [PubMed]
- Zhang, X.; Gao, Y.T.; Yang, G.; Li, H.; Cai, Q.; Xiang, Y.B.; Ji, B.T.; Franke, A.A.; Zheng, W.; Shu, X.O. Urinary isoflavonoids and risk of coronary heart disease. Int. J. Epidemiol. 2012, 41, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Ganry, O. Phytoestrogens and prostate cancer risk. Prev. Med. 2005, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Sonoda, T.; Mori, M.; Miyanaga, N.; Okumura, K.; Goto, K.; Naito, S.; Fujimoto, K.; Hirao, Y.; Takahashi, A.; et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J. Nutr. 2007, 137, 1974–1979. [Google Scholar] [PubMed]
- Zuniga, K.E.; Clinton, S.K.; Erdman, J.W. The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev. Res. 2013, 6, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Lintermans, A.; Dieudonné, A.S.; Neven, P. Management of menopausal symptoms in breast cancer patients. Maturitas 2011, 68, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; McGlynn, H.; Rowland, I.R. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004, 208, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.H.M.; Keinan-Boker, L.; van der Schouw, Y.T.; Grobbee, D.E. Phytoestrogens and breast cancer risk: Review of the epidemiological evidence. IARC Sci. Publ. 2002, 156, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Western diet and Western diseases: Some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. Suppl. 1990, 201, 3–23. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Kronenberg, F.; Kurzer, M.S.; Messina, M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: Systematic review and meta-analysis of randomized controlled trials. Menopause 2012, 19, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B. Soy phytoestrogens: What will be their role in postmenopausal hormone replacement therapy? Menopause 2000, 7, 71–75. [Google Scholar] [PubMed]
- Alekel, D.L.; Germain, A.S.; Peterson, C.T.; Hanson, K.B.; Stewart, J.W.; Toda, T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am. J. Clin. Nutr. 2000, 72, 844–852. [Google Scholar] [PubMed]
- Horiuchi, T.; Onouchi, T.; Takahashi, M.; Ito, H.; Orimo, H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos. Int. 2000, 11, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Wangen, K.E.; Duncan, A.M.; Merz-Demlow, B.E.; Xu, X.; Marcus, R.; Phipps, W.R.; Kurzer, M.S. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: Meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2008, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Henderson, V.W.; Paganini-Hill, A.; Miller, B.L.; Elble, R.J.; Reyes, P.F.; Shoupe, D.; McCleary, C.A.; Klein, R.A.; Hake, A.M.; Farlow, M.R. Estrogen for Alzheimer’s disease in women: Randomized, double-blind, placebo-controlled trial. Neurology 2000, 54, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Atkinson, C.; Liggins, J.; Bluck, L.; Coward, A. Phyto-oestrogens: Where are we now? Br. J. Nutr. 1998, 79, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.J.; Ng, K.M.; Luo, K.Q. Extraction and purification of isoflavones from soybeans and characterization of their estrogenic activities. J. Agric. Food Chem. 2007, 55, 6940–6950. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Fotsis, T.; Lampe, J.; Wähälä, K.; Mäkelä, T.; Brunow, G.; Hase, T. Quantitative determination of lignans and isoflavonoids in plasma of omnivorous and vegetarian women by isotope dilution gas chromatography-mass spectrometry. Scand. J. Clin. Lab. Investig. Suppl. 1993, 215, 5–18. [Google Scholar] [CrossRef]
- Irvine, C.; Fitzpatrick, M.; Robertson, I.; Woodhams, D. The potential adverse effects of soybean phytoestrogens in infant feeding. N. Z. Med. J. 1995, 108, 208–209. [Google Scholar] [PubMed]
- Sheehan, D.M. Herbal medicines, phytoestrogens and toxicity: Risk:benefit considerations. Proc. Soc. Exp. Biol. Med. 1998, 217, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Divi, R.L.; Chang, H.C.; Doerge, D.R. Anti-thyroid isoflavones from soybean: Isolation, characterization, and mechanisms of action. Biochem. Pharmacol. 1997, 54, 1087–1096. [Google Scholar] [CrossRef]
- Sun, C.L.; Yuan, J.M.; Arakawa, K.; Low, S.H.; Lee, H.P.; Yu, M.C. Dietary soy and increased risk of bladder cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1674–1677. [Google Scholar]
- White, L.; Petrovitch, H.; Ross, G.W.; Masaki, K.H.; Abbott, R.D.; Teng, E.L.; Rodriguez, B.L.; Blanchette, P.L.; Havlik, R.J.; Wergowske, G.; et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA 1996, 276, 955–960. [Google Scholar] [CrossRef] [PubMed]
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; Roberts, S.A.; Howell, A.; Potten, C.S.; Bundred, N.J. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 1998, 68, 1431S–1435S. [Google Scholar] [PubMed]
- Petrakis, N.L.; Barnes, S.; King, E.B.; Lowenstein, J.; Wiencke, J.; Lee, M.M.; Miike, R.; Kirk, M.; Coward, L. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol. Biomark. Prev. 1996, 5, 785–794. [Google Scholar]
- Li, Y.; Saldanha, S.N.; Tollefsbol, T.O. Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. AAPS J. 2014, 16, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [PubMed]
- Adlercreutz, H.; Honjo, H.; Higashi, A.; Fotsis, T.; Hämäläinen, E.; Hasegawa, T.; Okada, H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991, 54, 1093–1100. [Google Scholar] [PubMed]
- Price, K.R.; Fenwick, G.R. Naturally occurring oestrogens in foods—A review. Food Addit. Contam. 1985, 2, 73–106. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Eden, J.A. Phytoestrogens—A short review. Maturitas 1995, 22, 167–175. [Google Scholar] [CrossRef]
- Mazur, W. Phytoestrogen content in foods. Baillieres Clin. Endocrinol. Metab. 1998, 12, 729–742. [Google Scholar] [CrossRef]
- Mazur, W.; Fotsis, T.; Wähälä, K.; Ojala, S.; Salakka, A.; Adlercreutz, H. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal. Biochem. 1996, 233, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.M.; Wähälä, K.; Rasku, S.; Salakka, A.; Hase, T.; Adlercreutz, H. Lignan and isoflavonoid concentrations in tea and coffee. Br. J. Nutr. 1998, 79, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. Beta-conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro. J. Agric. Food Chem. 2008, 56, 10533–10543. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; Gonzalez de Mejia, E. Protein hydrolysates from beta-conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation in vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; Gonzalez de Mejia, E. Peptides from purified soybean beta-conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. beta-Conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Krehl, W.A.; Stone, D.B.; Lopez, A. Dietary carbohydrates and low cholesterol diets: Effects on serum lipids on man. Am. J. Clin. Nutr. 1967, 20, 198–208. [Google Scholar] [PubMed]
- Jenkins, D.J.A.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.C.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bowen, R.; Cai, Q.; Barnes, S.; Wang, Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc. Soc. Exp. Biol. Med. 1995, 208, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Chen, G.; Doerge, D.R.; Heilbrun, L.K.; Kucuk, O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001, 172, 1–6. [Google Scholar] [CrossRef]
- Rimbach, G.; Weinberg, P.D.; de Pascual-Teresa, S.; Alonso, M.G.; Ewins, B.A.; Turner, R.; Minihane, A.M.; Botting, N.; Fairley, B.; Matsugo, S.; et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta 2004, 1670, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; O’Reilly, J.D.; Adlercreutz, H.; Mallet, A.I.; Bowey, E.A.; Rowland, I.R.; Sanders, T.A. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000, 72, 395–400. [Google Scholar] [PubMed]
- Sirtori, C.R.; Even, R.; Lovati, M.R. Soybean protein diet and plasma cholesterol: From therapy to molecular mechanisms. Ann. N. Y. Acad. Sci. 1993, 676, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M.; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [PubMed]
- Dong, J.Y.; Tong, X.; Wu, Z.W.; Xun, P.C.; He, K.; Qin, L.Q. Effect of soya protein on blood pressure: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2011, 106, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Pan, A.; Manson, J.E.; Willett, W.C.; Malik, V.; Rosner, B.; Giovannucci, E.; Hu, F.B.; Sun, Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: A pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016, 70, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; González, C.A.; Buijsse, B.; Guevara, M.; van der Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: The EPIC-InterAct study. Diabetes Care 2013, 36, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Takahashi, Y.; Kirii, K.; Inoue, M.; Noda, M.; Tsugane, S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J. Nutr. 2010, 140, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, X.; Li, C.; Jiao, S.; Dong, W. Association between consumption of soy and risk of cardiovascular disease: A meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2017, 24, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- González Cañete, N.; Durán Agüero, S. Soya isoflavones and evidences on cardiovascular protection. Nutr. Hosp. 2014, 29, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Natarajan, L.; Parker, B.; Gold, E.B.; Thomson, C.; Newman, V.; Rock, C.L.; Pu, M.; Al-Delaimy, W.; Pierce, J.P. Soy food consumption and breast cancer prognosis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Shu, X.O.; Li, H.; Yang, G.; Cai, H.; Wen, W.; Ji, B.T.; Gao, J.; Gao, Y.T.; Zheng, W. Adolescent and adult soy food intake and breast cancer risk: Results from the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009, 89, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Kucuk, O.; Lampe, J.W. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J. AOAC Int. 2006, 89, 1121–1134. [Google Scholar] [PubMed]
- Zhou, J.R.; Gugger, E.T.; Tanaka, T.; Guo, Y.; Blackburn, G.L.; Clinton, S.K. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999, 129, 1628–1635. [Google Scholar] [PubMed]
- Vitale, S.G.; Laganà, A.S.; Nigro, A.; La Rosa, V.L.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Corrado, F.; Buscema, M.; et al. Peroxisome Proliferator-Activated Receptor Modulation during Metabolic Diseases and Cancers: Master and Minions. PPAR Res. 2016, 2016, 6517313. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S.; et al. Soy Intake and Breast Cancer Risk: An Evaluation Based on a Systematic Review of Epidemiologic Evidence Among the Japanese Population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H. Prostate cancer chemoprevention by soy isoflavones: Role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012, 103, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-term soy isoflavone intervention in patients with localized prostate cancer: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef] [PubMed]
- Dalais, F.S.; Meliala, A.; Wattanapenpaiboon, N.; Frydenberg, M.; Suter, D.A.I.; Thomson, W.K.; Wahlqvist, M.L. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology 2004, 64, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Spitznagel, E.L. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, I.; Takahashi, Y.; Yoshida, H.; Mizushina, Y. Inhibitory Effect of Isoflavones from Processed Soybeans on Human DNA Topoisomerase II Activity. J. Plant Biochem. Physiol. 2013. [Google Scholar] [CrossRef]
- Dagdemir, A.; Durif, J.; Ngollo, M.; Bignon, Y.J.; Bernard-Gallon, D. Histone lysine trimethylation or acetylation can be modulated by phytoestrogen, estrogen or anti-HDAC in breast cancer cell lines. Epigenomics 2013, 5, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Dagdemir, A.; Durif, J.; Ngollo, M.; Bignon, Y.J.; Bernard-Gallon, D. Breast cancer: Mechanisms involved in action of phytoestrogens and epigenetic changes. In Vivo 2013, 27, 1–9. [Google Scholar] [PubMed]
- Hilakivi-Clarke, L.; Andrade, J.E.; Helferich, W. Is soy consumption good or bad for the breast? J. Nutr. 2010, 140, 2326S–2334S. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jing, X.; Li, H.; Zhao, X.; Wang, D. Soy isoflavone consumption and colorectal cancer risk: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 25939. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.S.; Ge, J.; Chen, S.W.; Xiong, Y.Q.; Ma, S.J.; Chen, Q. Association between Dietary Isoflavones in Soy and Legumes and Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Wu, A.H.; Fears, T.; Nomura, A.M.Y.; West, D.W.; Kolonel, L.N.; Pike, M.C.; Hoover, R.N.; Ziegler, R.G. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Wan, P.; Hankin, J.; Tseng, C.C.; Yu, M.C.; Pike, M.C. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 2002, 23, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Colonese, F.; La Rosa, V.L.; Laganà, A.S.; Vitale, S.G.; Cortinovis, D.; Bidoli, P. Comment on: “Is there a role for vitamin D in human reproduction?”. Horm. Mol. Biol. Clin. Investig. 2017, 29, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Sapia, F.; La Rosa, V.L.; Vitale, S.G. Comment on “Inositols: From physiology to rational therapy in gynecological clinical practice”. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [PubMed]
- Cianci, A.; Colacurci, N.; Paoletti, A.M.; Perino, A.; Cicinelli, E.; Maffei, S.; Di Martino, M.; Daguati, R.; Stomati, M.; Pilloni, M.; et al. Soy isoflavones, inulin, calcium, and vitamin D3 in post-menopausal hot flushes: An observational study. Clin. Exp. Obstet. Gynecol. 2015, 42, 743–745. [Google Scholar] [PubMed]
- Husain, D.; Khanna, K.; Puri, S.; Haghighizadeh, M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J. Am. Coll. Nutr. 2015, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Arjomand-Wölkart, K.; Birkhäuser, M.H.; Genazzani, A.R.; Gruber, D.M.; Huber, J.; Kölbl, H.; Kreft, S.; Leodolter, S.; Linsberger, D.; et al. Consensus: Soy isoflavones as a first-line approach to the treatment of menopausal vasomotor complaints. Gynecol. Endocrinol. 2016, 32, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Nishi, N.; Omori, T.; Kurzer, M.S. Soy isoflavones for osteoporosis: An evidence-based approach. Maturitas 2011, 70, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F. PHYTOS Investigators Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: A randomized, double-blind, placebo controlled study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [PubMed]
- Vupadhyayula, P.M.; Gallagher, J.C.; Templin, T.; Logsdon, S.M.; Smith, L.M. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women—A 2-year randomized, double-blind, placebo-controlled trial. Menopause 2009, 16, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [PubMed]
- Chen, Y.M.; Ho, S.C.; Lam, S.S.H.; Ho, S.S.S.; Woo, J.L.F. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: A double-blind, randomized, controlled trial. J. Clin. Endocrinol. Metab. 2003, 88, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Roughead, Z.K.; Hunt, J.R.; Johnson, L.K.; Badger, T.M.; Lykken, G.I. Controlled substitution of soy protein for meat protein: Effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.A.; Lipscomb, E.R.; Cadogan, J.; Martin, B.; Wastney, M.E.; Peacock, M.; Weaver, C.M. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: A randomized crossover study. Am. J. Clin. Nutr. 2005, 81, 916–922. [Google Scholar] [PubMed]
- Matthews, V.L.; Knutsen, S.F.; Beeson, W.L.; Fraser, G.E. Soy milk and dairy consumption is independently associated with ultrasound attenuation of the heel bone among postmenopausal women: The Adventist Health Study-2. Nutr. Res. 2011, 31, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric J. Int. Menopause Soc. 2015, 18, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B.; Utian, W.H.; Barnes, S.; Gold, E.B.; Basaria, S.S.; Aso, T.; Kronenberg, F.; Frankenfeld, C.L.; Cline, J.M.; Landgren, B.M.; et al. The role of soy isoflavones in menopausal health: Report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause 2011, 18, 732–753. [Google Scholar] [CrossRef]
- Li, L.; Lv, Y.; Xu, L.; Zheng, Q. Quantitative efficacy of soy isoflavones on menopausal hot flashes. Br. J. Clin. Pharmacol. 2015, 79, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Jenks, B.H.; Iwashita, S.; Nakagawa, Y.; Ragland, K.; Lee, J.; Carson, W.H.; Ueno, T.; Uchiyama, S. A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency. J. Womens Health 2012, 21, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Colonese, F.; Laganà, A.S.; Colonese, E.; Sofo, V.; Salmeri, F.M.; Granese, R.; Triolo, O. The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: An overview on a hot topic. BioMed Res. Int. 2015, 2015, 986281. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; Otten, J.J., Hellwig, J.P., Meyers, L.D., Eds.; The National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-15742-1. [Google Scholar]
- Fredlund, K.; Isaksson, M.; Rossander-Hulthén, L.; Almgren, A.; Sandberg, A.S. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J. Trace Elem. Med. Biol. 2006, 20, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ørgaard, A.; Jensen, L. The effects of soy isoflavones on obesity. Exp. Biol. Med. 2008, 233, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Palomba, S.; Laganà, A.S.; Orio, F. Inositols in the Treatment of Insulin-Mediated Diseases. Int. J. Endocrinol. 2016, 2016, 3058393. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [PubMed]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Chalvon-Demersay, T.; Azzout-Marniche, D.; Arfsten, J.; Egli, L.; Gaudichon, C.; Karagounis, L.G.; Tomé, D. A Systematic Review of the Effects of Plant Compared with Animal Protein Sources on Features of Metabolic Syndrome. J. Nutr. 2017, 147, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. Glycemic index and disease. Am. J. Clin. Nutr. 2002, 76, 290S–298S. [Google Scholar] [PubMed]
- Neacsu, M.; Fyfe, C.; Horgan, G.; Johnstone, A.M. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: A randomized crossover trial. Am. J. Clin. Nutr. 2014, 100, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W. Beneficial effects of soy protein consumption for renal function. Asia Pac. J. Clin. Nutr. 2008, 17, 324–328. [Google Scholar] [PubMed]
- Lee, Y.B.; Lee, H.J.; Sohn, H.S. Soy isoflavones and cognitive function. J. Nutr. Biochem. 2005, 16, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Borchers, T.A.; Park, J.S.; Chew, B.P.; McGuire, M.K.; Fournier, L.R.; Beerman, K.A. Soy isoflavones modulate immune function in healthy postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 1118–1125. [Google Scholar] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Raudales, D.; Hoeflinger, J.L.; Bringe, N.A.; Cox, S.B.; Dowd, S.E.; Miller, M.J.; Gonzalez de Mejia, E. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes 2012, 3, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, S.; Ohashi, Y.; Narai-Kanayama, A.; Aso, K.; Nakagaki, T.; Fujisawa, T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int. J. Food Sci. Nutr. 2012, 63, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.E.; Lopetcharat, K.; Drake, M.A. Preference Mapping of Soymilk with Different U.S. Consumers. J. Food Sci. 2016, 81, S463–S476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Yun, T.T.; Liu, S.; Qi, W.T.; Zhao, L.Q.; Liu, J.R.; Li, A.K. Analysis of water-soluble bioactive compounds in commonly consumed soymilk in China. J. Food Compos. Anal. 2016, 46, 29–35. [Google Scholar] [CrossRef]
- Nakasato, K.; Ono, T.; Ishiguro, T.; Takamatsu, M.; Tsukamoto, C.; Mikami, M. Rapid quantitative analysis of the major components in soymilk using Fourier-transform infrared spectroscopy (FT-IR). Food Sci. Technol. Res. 2004, 10, 137–142. [Google Scholar] [CrossRef]
- Hou, J.W.; Yu, R.C.; Chou, C.C. Changes in some components of soymilk during fermentation with bifidobacteria. Food Res. Int. 2000, 33, 393–397. [Google Scholar] [CrossRef]
- Piacentini, G.; Peroni, D.; Bessi, E.; Morelli, L. Molecular characterization of intestinal microbiota in infants fed with soymilk. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, H.; Xie, M. Antibacterial mechanism of soybean isoflavone on Staphylococcus aureus. Arch. Microbiol. 2010, 192, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [PubMed]
- Cheng, I.C.; Shang, H.F.; Lin, T.F.; Wang, T.H.; Lin, H.S.; Lin, S.H. Effect of fermented soy milk on the intestinal bacterial ecosystem. World J. Gastroenterol. 2005, 11, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Park, Y.; Lee, M.; Chae, S.W.; Park, K.; Kim, Y.; Lee, H.S. Doenjang, a Korean fermented soy food, exerts antiobesity and antioxidative activities in overweight subjects with the PPAR-γ2 C1431T polymorphism: 12-week, double-blind randomized clinical trial. J. Med. Food 2014, 17, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Bedani, R.; Pauly-Silveira, N.D.; Roselino, M.N.; de Valdez, G.F.; Rossi, E.A. Effect of fermented soy product on the fecal microbiota of rats fed on a beef-based animal diet. J. Sci. Food Agric. 2010, 90, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Han, H.W.; Yim, S.Y. Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food Funct. 2015, 6, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, G.; Penas, E.; Pedrazuela, A.; Prestamo, G. Intestinal microbiota in rats fed with tofu (soy curd) treated under high pressure. Eur. Food Res. Technol. 2005, 220, 395–400. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Peisker, M. The impact of soy oligosaccharides on digestion and intestinal health in weaning piglets. Livest. Sci. 2010, 134, 187–189. [Google Scholar] [CrossRef]
- Li, T.; Zhong, J.Z.; Wan, J.; Liu, C.M.; Le, B.Y.; Liu, W.; Fu, G.M. Effects of micronized okara dietary fiber on cecal microbiota, serum cholesterol and lipid levels in BALB/c mice. Int. J. Food Sci. Nutr. 2013, 64, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Kim, K.A.; Han, M.J.; Kim, D.H. Doenjang, a fermented Korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.C.U.; Suzuki, J.Y.; Abdalla, D.S.P.; Vendramini, R.C.; Pauly-Silveira, N.D.; Roselino, M.N.; Pinto, R.A.; Rossi, E.A. Influence of a probiotic soy product on fecal microbiota and its association with cardiovascular risk factors in an animal model. Lipids Health Dis. 2011, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- McCARRISON, R. The goitrogenic action of soya-bean and ground-nut. Indian J. Med. Res. 1933, 21, 179–181. [Google Scholar]
- Doerge, D.R.; Chang, H.C. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 269–279. [Google Scholar] [CrossRef]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Atteritano, M.; Altavilla, D.; Mazzaferro, S.; Marini, H.; Adamo, E.B.; D’Anna, R.; Granese, R.; Corrado, F.; et al. Genistein aglycone does not affect thyroid function: Results from a three-year, randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2010, 95, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Sosvorová, L.; Mikšátková, P.; Bičíková, M.; Kaňová, N.; Lapčík, O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem. Toxicol. 2012, 50, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- De Souza Dos Santos, M.C.; Gonçalves, C.F.L.; Vaisman, M.; Ferreira, A.C.F.; de Carvalho, D.P. Impact of flavonoids on thyroid function. Food Chem. Toxicol. 2011, 49, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Jaceldo-Siegl, K.; Messina, M.; Haddad, E.; Fraser, G.E. The association between soya consumption and serum thyroid-stimulating hormone concentrations in the Adventist Health Study-2. Public Health Nutr. 2016, 19, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Shepard, T.H.; Pyne, G.E.; Kirschvink, J.F.; McLean, M.C. Soybean Goiter. N. Engl. J. Med. 1960, 262, 1099–1103. [Google Scholar] [CrossRef]
- Van Wyk, J.J.; Arnold, M.B.; Wynn, J.; Pepper, F. The effects of a soybean product on thyroid function in humans. Pediatrics 1959, 24, 752–760. [Google Scholar] [PubMed]
- Hydovitz, J.D. Occurrence of goiter in an infant on a soy diet. N. Engl. J. Med. 1960, 262, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Polito, F.; Adamo, E.B.; Bitto, A.; Squadrito, F.; Benvenga, S. Update on genistein and thyroid: An overall message of safety. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch. Dis. Child. 2004, 89, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Liwanpo, L.; Hershman, J.M. Conditions and drugs interfering with thyroxine absorption. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, H.W.; Underwood, E.J.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946, 22, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Shutt, D.A. The effects of plant oestrogens on animal reproduction. Endeavour 1976, 35, 110–113. [Google Scholar] [CrossRef]
- Siepmann, T.; Roofeh, J.; Kiefer, F.W.; Edelson, D.G. Hypogonadism and erectile dysfunction associated with soy product consumption. Nutrition 2011, 27, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Lewi, J.E. An unusual case of gynecomastia associated with soy product consumption. Endocr. Pract. 2008, 14, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soybean isoflavone exposure does not have feminizing effects on men: A critical examination of the clinical evidence. Fertil. Steril. 2010, 93, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum. Reprod. 2008, 23, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Kochman, L.; Andolina, E.; Herko, R.C.; Brewer, K.J.; Lewis, V. Beneficial effects of dietary intake of plant phytoestrogens on semen parameters and sperm DNA integrity in infertile men. Fertil. Steril. 2006, 86, S49. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Cawood, E.; Kinniburgh, D.; Provan, A.; Collins, A.R.; Irvine, D.S. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin. Sci. 2001, 100, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 539, 1–266. [Google Scholar]
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Womens Health 2014, 6, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, J.; Greer, F.; American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP-CERHR monograph on Soy Infant Formula. NTP CERHR Monogr. 2010, 23, i-661. [Google Scholar]
- Cao, Y.; Calafat, A.M.; Doerge, D.R.; Umbach, D.M.; Bernbaum, J.C.; Twaddle, N.C.; Ye, X.; Rogan, W.J. Isoflavones in urine, saliva, and blood of infants: Data from a pilot study on the estrogenic activity of soy formula. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Adgent, M.A.; Daniels, J.L.; Rogan, W.J.; Adair, L.; Edwards, L.J.; Westreich, D.; Maisonet, M.; Marcus, M. Early-life soy exposure and age at menarche. Paediatr. Perinat. Epidemiol. 2012, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A.; Drulis, J.M.; Nelson, S.E.; Hanson, S.A. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001, 286, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Pribis, P.; Messina, M.; Oda, K.; Sabaté, J. Is soy intake related to age at onset of menarche? A cross-sectional study among adolescents with a wide range of soy food consumption. Nutr. J. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 545, 1–240. [Google Scholar]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Respective contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor alpha induced transcriptional activity by isoflavones and equol: Consequence on breast cancer cell proliferation. Mol. Nutr. Food Res. 2009, 53, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Virant, S.M.; Helferich, W.G. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001, 61, 5045–5050. [Google Scholar] [PubMed]
- Du, M.; Yang, X.; Hartman, J.A.; Cooke, P.S.; Doerge, D.R.; Ju, Y.H.; Helferich, W.G. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis 2012, 33, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Ferrari, P.; González, C.A.; Tjønneland, A.; Olsen, A.; Bredsdorff, L.; Overvad, K.; Touillaud, M.; Perquier, F.; Fagherazzi, G.; et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res. Treat. 2013, 139, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Speirs, V.; Carder, P.J.; Lane, S.; Dodwell, D.; Lansdown, M.R.J.; Hanby, A.M. Oestrogen receptor beta: What it means for patients with breast cancer. Lancet Oncol. 2004, 5, 174–181. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Garcia, A.; Tseng, C.C.; Hovanessian-Larsen, L.; Sheth, P.; Martin, S.E.; Hawes, D.; Russell, C.; MacDonald, H.; et al. Double-Blind Randomized 12-Month Soy Intervention Had No Effects on Breast MRI Fibroglandular Tissue Density or Mammographic Density. Cancer Prev. Res. 2015, 8, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Allen, N.E.; Appleby, P.N.; Spencer, E.A.; Roddam, A.W.; Key, T.J. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int. J. Cancer 2008, 122, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Lamartiniere, C.A.; Moore, J.; Holland, M.; Barnes, S. Neonatal genistein chemoprevents mammary cancer. Proc. Soc. Exp. Biol. Med. 1995, 208, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Cheftel, J.C. Nutritional effects of extrusion-cooking. Food Chem. 1986, 20, 263–283. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581S–588S. [Google Scholar] [PubMed]
- Friedman, M. Nutritional consequences of food processing. Forum Nutr. 2003, 56, 350–352. [Google Scholar] [PubMed]
- Csáky, I.; Fekete, S. Soybean: Feed quality and safety. Part 1: Biologically active components. A review. Acta Vet. Hung. 2004, 52, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Molnár-Perl, I.; Pintér-Szakács, M. Monitoring of Maillard reactions in soy products. Z. Lebensm. Unters. Forsch. 1986, 183, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Biological effects of Maillard browning products that may affect acrylamide safety in food: Biological effects of Maillard products. Adv. Exp. Med. Biol. 2005, 561, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Saiga, S.; Kobayashi, A. Relationships between Aroma Components and Sensory Evaluation of Miso. J. Food Sci. Technol. 1992, 39, 1098–1104. [Google Scholar] [CrossRef]
- Wang, H.; Jenner, A.M.; Lee, C.Y.J.; Shui, G.; Tang, S.Y.; Whiteman, M.; Wenk, M.R.; Halliwell, B. The identification of antioxidants in dark soy sauce. Free Radic. Res. 2007, 41, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ginting, E.; Arcot, J. High-performance liquid chromatographic determination of naturally occurring folates during tempe preparation. J. Agric. Food Chem. 2004, 52, 7752–7758. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P. Fermentation of non-dairy foods. Food Biotechnol. 1991, 5, 293–303. [Google Scholar] [CrossRef]
- Setchell, K.D.; Welsh, M.B.; Lim, C.K. High-performance liquid chromatographic analysis of phytoestrogens in soy protein preparations with ultraviolet, electrochemical and thermospray mass spectrometric detection. J. Chromatogr. 1987, 386, 315–323. [Google Scholar] [CrossRef]
- Heller, K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001, 73, 374S–379S. [Google Scholar] [PubMed]
- Council Regulation (EEC) No. 1898/87 of 2 July 1987 on the Protection of Designations Used in Marketing of Milk and Milk Products. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31987R1898 (accessed on 9 September 2017).
- Klemola, T.; Vanto, T.; Juntunen-Backman, K.; Kalimo, K.; Korpela, R.; Varjonen, E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: A prospective, randomized study with a follow-up to the age of 2 years. J. Pediatr. 2002, 140, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, R.S.; Sampson, H.A.; Bock, S.A.; Burks, A.W.; Harden, K.; Noone, S.; Martin, D.; Leung, S.; Wilson, G. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J. Pediatr. 1999, 134, 614–622. [Google Scholar] [CrossRef]
- Allen, K.J.; Davidson, G.P.; Day, A.S.; Hill, D.J.; Kemp, A.S.; Peake, J.E.; Prescott, S.L.; Shugg, A.; Sinn, J.K.H.; Heine, R.G. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J. Paediatr. Child Health 2009, 45, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.M.; Chen, R.Y.; Leung, L.K.; Chan, F.L.; Huang, Y.; Chen, Z.-Y. Difference in flavonoid and isoflavone profile between soybean and soy leaf. Biomed. Pharmacother. 2002, 56, 289–295. [Google Scholar] [CrossRef]
- Reinli, K.; Block, G. Phytoestrogen content of foods—A compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [PubMed]
- Setchell, K.D.R.; Cole, S.J. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J. Agric. Food Chem. 2003, 51, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Hankin, J.H.; Yu, M.C.; Maskarinec, G.; Low, S.H.; Custer, L.J. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J. Agric. Food Chem. 1999, 47, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Haron, H.; Ismail, A.; Azlan, A.; Shahar, S.; Peng, L.S. Daidzein and genestein contents in tempeh and selected soy products. Food Chem. 2009, 115, 1350–1356. [Google Scholar] [CrossRef]
- Simonne, A.H.; Smith, M.; Weaver, D.B.; Vail, T.; Barnes, S.; Wei, C.I. Retention and changes of soy isoflavones and carotenoids in immature soybean seeds (Edamame) during processing. J. Agric. Food Chem. 2000, 48, 6061–6069. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Factors affecting the bioavailability of soy isoflavones in humans. J. AOAC Int. 2006, 89, 1182–1188. [Google Scholar] [PubMed]
- Chen, T.R.; Wei, Q.K. Analysis of bioactive aglycone isoflavones in soybean and soybean products. Nutr. Food Sci. 2008, 38, 540–547. [Google Scholar] [CrossRef]
- Astuti, M.; Meliala, A.; Dalais, F.S.; Wahlqvist, M.L. Tempe, a nutritious and healthy food from Indonesia. Asia Pac. J. Clin. Nutr. 2000, 9, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.; de Oliveira, M.C.N.; Mandarino, J.M.G.; da Silva, J.B.; Ida, E.I.; Carrão-Panizzi, M.C. Changes in the isoflavone profile and in the chemical composition of tempeh during processing and refrigeration. Pesq. Agropecu. Bras. 2011, 46, 1555–1561. [Google Scholar] [CrossRef] [Green Version]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Goeppinger, T.S.; Doerge, D.R.; Helferich, W.G. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis 2004, 25, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huff, H.E.; Hsieh, F. Texture and Chemical Characteristics of Soy Protein Meat Analog Extruded at High Moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, F.H. Protein–Protein Interactions in High Moisture-Extruded Meat Analogs and Heat-Induced Soy Protein Gels. J. Am. Oil Chem. Soc. 2007, 84, 741–748. [Google Scholar] [CrossRef]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Marsmana, G.J.P.; Gruppen, H.; van Zuilichem, D.J.; Resink, J.W.; Voragen, A.G.J. The influence of screw configuration on the in vitro digestibility and protein solubility of soybean and rapeseed meals. J. Food Eng. 1995, 26, 13–28. [Google Scholar] [CrossRef]
- MacDonald, R.S.; Pryzbyszewski, J.; Hsieh, F.H. Soy protein isolate extruded with high moisture retains high nutritional quality. J. Agric. Food Chem. 2009, 57, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, P.V.J.; Ahn, P.C. Nutritional comparisons between a soy-based meat analog and beef in the unheated and heated states. J. Food Sci. 1976, 41, 1133–1136. [Google Scholar] [CrossRef]
- Burton, J.W. Recent Developments in Breeding Soybeans for Improved Oil Quality. Lipid Fett 1991, 93, 121–128. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.C.; Liu, Z.; Xu, B. Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J. Agric. Food Chem. 2008, 56, 7957–7963. [Google Scholar] [CrossRef] [PubMed]
- De Carneiro, J.D.S.; Minim, V.P.R.; Deliza, R.; Silva, C.H.O.; Carneiro, J.C.S.; Leão, F.P. Labelling effects on consumer intention to purchase for soybean oil. Food Qual. Prefer. 2005, 16, 275–282. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Murphy, P.A. Mass Balance Study of Isoflavones during Soybean Processing. J. Agric. Food Chem. 1996, 44, 2377–2383. [Google Scholar] [CrossRef]
- Pandjaitan, N.; Hettiarachchy, N.; Ju, Z.Y.; Crandall, P.; Sneller, C.; Dombek, D. Evaluation of Genistin and Genistein Contents in Soybean Varieties and Soy Protein Concentrate Prepared with 3 Basic Methods. J. Food Sci. 2000, 65, 399–402. [Google Scholar] [CrossRef]
- Wiseman, H.; Casey, K.; Clarke, D.B.; Barnes, K.A.; Bowey, E. Isoflavone aglycon and glucoconjugate content of high- and low-soy U.K. foods used in nutritional studies. J. Agric. Food Chem. 2002, 50, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.R.; Cummings, J.H.; Morton, M.S.; Michael Steel, C.; Bolton-Smith, C.; Riches, A.C. A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br. J. Nutr. 2006, 95, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Hutabarat, L.S.; Greenfield, H.; Mulholland, M. Isoflavones and Coumestrol in Soybeans and Soybean Products from Australia and Indonesia. J. Food Compos. Anal. 2001, 14, 43–58. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Barnes, S.; Lee, M.; Coward, L.; Mandel, J.E.; Koo, J.; John, E.M.; Smith, M. Assessing phytoestrogen exposure in epidemiologic studies: Development of a database (United States). Cancer Causes Control 2000, 11, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Pillow, P.C.; Duphorne, C.M.; Chang, S.; Contois, J.H.; Strom, S.S.; Spitz, M.R.; Hursting, S.D. Development of a database for assessing dietary phytoestrogen intake. Nutr. Cancer 1999, 33, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Alezandro, M.R.; Granato, D.; Lajolo, F.M.; Genovese, M.I. Nutritional aspects of second generation soy foods. J. Agric. Food Chem. 2011, 59, 5490–5497. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.I.; Barbosa, A.C.L.; Pinto, M.D.S.; Lajolo, F.M. Commercial soy protein ingredients as isoflavone sources for functional foods. Plant Foods Hum. Nutr. 2007, 62, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Faughnan, M.; Wähälä, K.; Brants, H.; Mulligan, A. Phyto-oestrogen levels in foods: The design and construction of the VENUS database. Br. J. Nutr. 2003, 89, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Song, Y.; Joung, H.; Li, S.J.; Paik, H.Y. Establishment of an isoflavone database for usual Korean foods and evaluation of isoflavone intake among Korean children. Asia Pac. J. Clin. Nutr. 2007, 16, 129–139. [Google Scholar] [PubMed]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.C.; Dini, J.P.; Lavandier, C.; Rupasinghe, H.P.V.; Faulkner, H.; Poysa, V.; Buzzell, D.; DeGrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Uzzan, M.; Nechrebeki, J.; Labuza, T.P. Thermal and storage stability of nutraceuticals in a milk beverage dietary supplement. J. Food Sci. 2007, 72, E109–E114. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Petrovitch, H.; Ross, G.W.; Masaki, K. 487 Association of mid-life consumption of tofu with late life cognitive impairment and dementia: The Honolulu-Asia Aging Study. Neurobiol. Aging 1996, 17, S121. [Google Scholar] [CrossRef]
- White, L.R.; Petrovitch, H.; Ross, G.W.; Masaki, K.; Hardman, J.; Nelson, J.; Davis, D.; Markesbery, W. Brain aging and midlife tofu consumption. J. Am. Coll. Nutr. 2000, 19, 242–255. [Google Scholar] [CrossRef] [PubMed]
- File, S.E.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating soya improves human memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones: Safety of isoflavones from food supplements in menopausal women. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef]
- Messina, M. Post-Diagnosis Soy Isoflavone Intake Is Not Harmful to Women with Breast Cancer. Breast Dis. 2015, 26, 193–197. [Google Scholar] [CrossRef]
- Chi, F.; Wu, R.; Zeng, Y.C.; Xing, R.; Liu, Y.; Xu, Z.G. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.M.; Shu, X.; Ruan, Z.X.; Dai, Q.; Cai, Q.; Gao, Y.; Zheng, W. Soyfood intake and breast cancer survival: A followup of the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2005, 92, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Gaudet, M.M.; Abrahamson, P.E.; Bell, P.; Schroeder, J.C.; Teitelbaum, S.L.; et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Kwan, M.L.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The Life After Cancer Epidemiology study. Breast Cancer Res. Treat. 2009, 118, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P.; et al. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Q.; Wang, S.; Huang, X.; Jin, S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ 2010, 182, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Ruhräh, J. The Soy bean in Infant Feeding: Preliminary Report. Arch. Pediatr. 1909, 26, 496–501. [Google Scholar]
- Zoppi, G.; Gasparini, R.; Mantovanelli, F.; Gobio-Casali, L.; Astolfi, R.; Crovari, P. Diet and antibody response to vaccinations in healthy infants. Lancet 1983, 2, 11–14. [Google Scholar] [CrossRef]
- Zoppi, G.; Gerosa, F.; Pezzini, A.; Bassani, N.; Rizzotti, P.; Bellini, P.; Todeschini, G.; Zamboni, G.; Vazzoler, G.; Tridente, G. Immunocompetence and dietary protein intake in early infancy. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, G.; Zamboni, G.; Bassani, N.; Vazzoler, G. Gammaglobulin level and soy-protein intake in early infancy. Eur. J. Pediatr. 1979, 131, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Businco, L.; Bruno, G.; Grandolfo, M.E.; Novello, F.; Fiore, L.; Amato, C. Response to poliovirus immunization and type of feeding in babies of atopic families. Pediatr. Allergy Immunol. 1990, 1, 60–63. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Nutrition. Soy protein-based formulas: Recommendations for use in infant feeding. Pediatrics 1998, 101, 148–153. [Google Scholar]
- Rossen, L.M.; Simon, A.E.; Herrick, K.A. Types of Infant Formulas Consumed in the United States. Clin. Pediatr. 2016, 55, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gartne, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A. IBreastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the essential composition of infant and follow-on formulae: Essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef] [Green Version]
- The Australian College of Pediatrics. Position statement: soy protein formula. J. Paediatr. Child Health 1998, 34, 318–319. [Google Scholar]
- Andres, A.; Cleves, M.A.; Bellando, J.B.; Pivik, R.T.; Casey, P.H.; Badger, T.M. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics 2012, 129, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, J.B.; Ostrom, K.M.; Jacobs, J.R.; Blatter, M.M.; Ndife, L.I.; Gooch, W.M.; Cho, S. Growth of newborn, term infants fed soy formulas for 1 year. Clin. Pediatr. 1999, 38, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Churella, H.R.; Borschel, M.W.; Thomas, M.R.; Breen, M.; Jacobs, J. Growth and protein status of term infants fed soy protein formulas differing in protein content. J. Am. Coll. Nutr. 1994, 13, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Hillman, L.S.; Chow, W.; Salmons, S.S.; Weaver, E.; Erickson, M.; Hansen, J. Vitamin D metabolism, mineral homeostasis, and bone mineralization in term infants fed human milk, cow milk-based formula, or soy-based formula. J. Pediatr. 1988, 112, 864–874. [Google Scholar] [CrossRef]
- Mimouni, F.; Campaigne, B.; Neylan, M.; Tsang, R.C. Bone mineralization in the first year of life in infants fed human milk, cow-milk formula, or soy-based formula. J. Pediatr. 1993, 122, 348–354. [Google Scholar] [CrossRef]
- Venkataraman, P.S.; Luhar, H.; Neylan, M.J. Bone mineral metabolism in full-term infants fed human milk, cow milk-based, and soy-based formulas. Am. J. Dis. Child. 1992, 146, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O. Isoflavones, soy-based infant formulas, and relevance to endocrine function. Nutr. Rev. 1998, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Little, J.M.; Barone, G.W.; Chen, G.; Radominska-Pandya, A.; Badger, T.M. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J. Med. Food 2006, 9, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Businco, L.; Bruno, G.; Giampietro, P.G.; Furcolo, G.; Antoniazzi, F. Absence of Oestrogen Hormonal Effects in Long-Term Soy Formula Fed Children. Pediatr. Res. 1999, 45, 8A. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Castrellon, P.G.; Rivas, R.; Gutiérrez, C.J.; Garcia, L.D.; Jimenez, J.E.; Anzo, A.; Hegar, B.; Alarcon, P. Safety of soya-based infant formulas in children. Br. J. Nutr. 2014, 111, 1340–1360. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Pivik, R.T.; Gilchrist, J.M.; Badger, T.M. No difference indicated in electroencephalographic power spectral analysis in 3- and 6-month-old infants fed soy- or milk-based formula. Matern. Child. Nutr. 2008, 4, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, J.M.; Wiggins, P.; Brackenbury, J.; Worthen, A.; Crook, T.; Brodie, M.; Badger, T.M. Body composition among infants fed breast milk, milk-based formula, or soy-based formula Preliminary data from the Beginnings study. FASEB J. 2004, 18, 729–730. [Google Scholar]
- Andres, A.; Smith, S.A.; Badger, T.M.; Gilchrist, J.M. Bone mineralization and vitamin D/calcium intake of infants fed breast-milk, milk-based formula or soy-based formula. FASEB J. 2008, 22. [Google Scholar] [CrossRef]
- Badger, T.M.; Ronis, M.J.J.; Simmen, R.C.M.; Simmen, F.A. Soy protein isolate and protection against cancer. J. Am. Coll. Nutr. 2005, 24, 146S–149S. [Google Scholar] [CrossRef] [PubMed]
- Irvine, C.H.; Shand, N.; Fitzpatrick, M.G.; Alexander, S.L. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. Am. J. Clin. Nutr. 1998, 68, 1462S–1465S. [Google Scholar] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [PubMed]
- Franke, A.A.; Custer, L.J.; Tanaka, Y. Isoflavones in human breast milk and other biological fluids. Am. J. Clin. Nutr. 1998, 68, 1466S–1473S. [Google Scholar] [PubMed]
- Jochum, F.; Alteheld, B.; Meinardus, P.; Dahlinger, N.; Nomayo, A.; Stehle, P. Mothers’ Consumption of Soy Drink But Not Black Tea Increases the Flavonoid Content of Term Breast Milk: A Pilot Randomized, Controlled Intervention Study. Ann. Nutr. Metab. 2017, 70, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Halm, B.M.; Custer, L.J.; Tatsumura, Y.; Hebshi, S. Isoflavones in breastfed infants after mothers consume soy. Am. J. Clin. Nutr. 2006, 84, 406–413. [Google Scholar] [PubMed]
- Sampson, H.A. Food sensitivity and the pathogenesis of atopic dermatitis. J. R. Soc. Med. 1997, 90, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, P.; Docena, G.H.; Añón, M.C.; Fossati, C.A. Detection and identification of a soy protein component that cross-reacts with caseins from cow’s milk. Clin. Exp. Immunol. 2002, 130, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H. Food protein-induced enterocolitis syndrome: Case presentations and management lessons. J. Allergy Clin. Immunol. 2005, 115, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics 1987, 79, 683–688. [Google Scholar] [PubMed]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.J.; Singer, A.M.; Baptist, A.P. Food allergy and food allergy attitudes among college students. J. Allergy Clin. Immunol. 2009, 124, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Ostblom, E.; Lilja, G.; Pershagen, G.; van Hage, M.; Wickman, M. Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2008, 38, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Ostblom, E.; Wickman, M.; van Hage, M.; Lilja, G. Reported symptoms of food hypersensitivity and sensitization to common foods in 4-year-old children. Acta Paediatr. 2008, 97, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Stoneham, M.D.; Petruckevitch, A.; Barton, J.; Rona, R. A population study of food intolerance. Lancet 1994, 343, 1127–1130. [Google Scholar] [CrossRef]
- Brugman, E.; Meulmeester, J.F.; Spee-van der Wekke, A.; Beuker, R.J.; Radder, J.J.; Verloove-Vanhorick, S.P. Prevalence of self-reported food hypersensitivity among school children in The Netherlands. Eur. J. Clin. Nutr. 1998, 52, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Emmett, S.E.; Angus, F.J.; Fry, J.S.; Lee, P.N. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy 1999, 54, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, I.; Ardal, B.; Jonsson, J.S.; Sigurdsson, J.A.; Foldevi, M.; Björkstén, B. Adverse reactions to food and food allergy in young children in Iceland and Sweden. Scand. J. Prim. Health Care 1999, 17, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, T.; Böhler, E.; Ruhdorfer, S.; Weigl, L.; Wessner, D.; Heinrich, J.; Filipiak, B.; Wichmann, H.E.; Ring, J. Epidemiology of food allergy/food intolerance in adults: Associations with other manifestations of atopy. Allergy 2001, 56, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-S.; Frias, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; de Mejia, E.G. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hefle, S.L.; Lambrecht, D.M.; Nordlee, J.A. Soy sauce retains allergenicity through the fermentation/production process. J. Allergy Clin. Immunol. 2005, 115, S32. [Google Scholar] [CrossRef]
- Kilgallen, I.; Gibney, M.J. Parental perception of food allergy or intolerance in children under 4 years of age. J. Hum. Nutr. Diet. 1996, 9, 473–478. [Google Scholar] [CrossRef]
- Chin, B.; Chan, E.S.; Goldman, R.D. Early exposure to food and food allergy in children. Can. Fam. Phys. 2014, 60, 338–339. [Google Scholar]
- Fleischer, D.M.; Sicherer, S.; Greenhawt, M.; Campbell, D.; Chan, E.; Muraro, A.; Halken, S.; Katz, Y.; Ebisawa, M.; Eichenfield, L.; et al. Consensus Communication on Early Peanut Introduction and Prevention of Peanut Allergy in High-Risk Infants. Pediatr. Dermatol. 2016, 33, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Schyver, T.; Smith, C. Reported attitudes and beliefs toward soy food consumption of soy consumers versus nonconsumers in natural foods or mainstream grocery stores. J. Nutr. Educ. Behav. 2005, 37, 292–299. [Google Scholar] [CrossRef]
- Russin, T.A.; Boye, J.I.; Arcand, Y.; Rajamohamed, S.H. Alternative Techniques for Defatting Soy: A Practical Review. Food Bioprocess Technol. 2011, 4, 200–223. [Google Scholar] [CrossRef]
- Lazor, K.; Chapman, N.; Levine, E. Soy goes to school: Acceptance of healthful, vegetarian options in Maryland middle school lunches. J. Sch. Health 2010, 80, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.K.; Lanou, A.J.; Barnard, N.D.; Seidl, K.; Green, A.A. Acceptability of soymilk as a calcium-rich beverage in elementary school children. J. Am. Diet. Assoc. 2006, 106, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Endres, J.; Barter, S.; Theodora, P.; Welch, P. Soy-enhanced lunch acceptance by preschoolers. J. Am. Diet. Assoc. 2003, 103, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.I.; Lajolo, F.M. Isoflavones in soy-based foods consumed in Brazil: Levels, distribution, and estimated intake. J. Agric. Food Chem. 2002, 50, 5987–5993. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.d.S.; Lajolo, F.M.; Genovese, M.I. Effect of storage temperature and water activity on the content and profile of isoflavones, antioxidant activity, and in vitro protein digestibility of soy protein isolates and defatted soy flours. J. Agric. Food Chem. 2005, 53, 6340–6346. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Gass, M.L.; Maki, P.M.; Newton, K.M.; Pinkerton, J.V.; Taylor, M.; Utian, W.H.; Schnatz, P.F.; Kaunitz, A.M.; Shapiro, M.; et al. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015, 22, 1155–1174. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- American Institute for Cancer Research (AICR). Soy is Safe for Breast Cancer Survivors. Available online: http://www.aicr.org/press/press-releases/soy-safe-breast-cancer-survivors.html (accessed on 10 September 2017).
- World Cancer Research Fund International (WCRF). Breast cancer survivors. Available online: http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/breast-cancer-survivors (accessed on 10 September 2017).
- Erdman, J.W.; Badger, T.M.; Lampe, J.W.; Setchell, K.D.R.; Messina, M. Not all soy products are created equal: Caution needed in interpretation of research results. J. Nutr. 2004, 134, 1229S–1233S. [Google Scholar] [PubMed]
- D’Adamo, C.R.; Sahin, A. Soy foods and supplementation: A review of commonly perceived health benefits and risks. Altern. Ther. Health Med. 2014, 20, 39–51. [Google Scholar] [PubMed]
- Valtueña, S.; Cashman, K.; Robins, S.P.; Cassidy, A.; Kardinaal, A.; Branca, F. Investigating the role of natural phyto-oestrogens on bone health in postmenopausal women. Br. J. Nutr. 2003, 89, S87–S99. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Ridout, C.L.; Wharf, S.G.; Price, K.R.; Johnson, I.T.; Fenwick, G.R. UK mean daily intakes of saponins-intestine-permeabilizing factors in legumes. Food Sci. Nutr. 1988, 42, 111–116. [Google Scholar] [CrossRef]
- Demonty, I.; Lamarche, B.; Jones, P.J.H. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr. Rev. 2003, 61, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Rossetti, P.; Buscema, M.; La Vignera, S.; Condorelli, R.A.; Gullo, G.; Granese, R.; Triolo, O. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int. J. Endocrinol. 2016, 2016, 6306410. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Palomba, S.; Laganà, A.S.; Orio, F. Current Insights Into Inositol Isoforms, Mediterranean and Ketogenic Diets for Polycystic Ovary Syndrome: From Bench to Bedside. Curr. Pharm. Des. 2016, 22, 5554–5557. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Rossetti, P.; Sapia, F.; Chiofalo, B.; Buscema, M.; Valenti, G.; Rapisarda, A.M.C.; Vitale, S.G. Evidence-Based and Patient-Oriented Inositol Treatment in Polycystic Ovary Syndrome: Changing the Perspective of the Disease. Int. J. Endocrinol. Metab. 2017, 15, e43695. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Pizzo, A. Authors’ reply to: “Empiric” inositol supplementation in normal-weight non-insulin resistant women with polycystic ovarian disease: From the absence of benefit to the potential adverse effects. Arch. Gynecol. Obstet. 2015, 291, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Barbaro, L.; Pizzo, A. Evaluation of ovarian function and metabolic factors in women affected by polycystic ovary syndrome after treatment with D-Chiro-Inositol. Arch. Gynecol. Obstet. 2015, 291, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, A.; Laganà, A.S.; Barbaro, L. Comparison between effects of myo-inositol and D-chiro-inositol on ovarian function and metabolic factors in women with PCOS. Gynecol. Endocrinol. 2014, 30, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Gulino, F.A.; Leonardi, E.; Marilli, I.; Musmeci, G.; Vitale, S.G.; Leanza, V.; Palumbo, M.A. Effect of treatment with myo-inositol on semen parameters of patients undergoing an IVF cycle: In vivo study. Gynecol. Endocrinol. 2016, 32, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Rossetti, P.; Corrado, F.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Valenti, G.; Sapia, F.; Laganà, A.S.; Buscema, M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int. J. Endocrinol. 2016, 2016, 4987436. [Google Scholar] [CrossRef] [PubMed]
- Villar, H.C.C.E.; Saconato, H.; Valente, O.; Atallah, A.N. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Aoki, Y.; Belin, R.M.; Clickner, R.; Jeffries, R.; Phillips, L.; Mahaffey, K.R. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007, 17, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.L.; Miller, G.A.; Wang, R.Y.; Jain, R.B.; Jones, R.L. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid 2008, 18, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Delange, F.; de Benoist, B.; Burgi, H.; ICCIDD Working Group. International Council for Control of Iodine Deficiency Disorders Determining median urinary iodine concentration that indicates adequate iodine intake at population level. Bull. World Health Organ. 2002, 80, 633–636. [Google Scholar] [PubMed]
- Perrine, C.G.; Herrick, K.; Serdula, M.K.; Sullivan, K.M. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J. Nutr. 2010, 140, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicová-Kudlácková, M.; Bucková, K.; Klimes, I.; Seboková, E. Iodine deficiency in vegetarians and vegans. Ann. Nutr. Metab. 2003, 47, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, A.; Koschizke, J.W.; Leitzmann, C.; Hahn, A. Dietary intakes and lifestyle factors of a vegan population in Germany: Results from the German Vegan Study. Eur. J. Clin. Nutr. 2003, 57, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battino, M. VegPlate: A Mediterranean-Based Food Guide for Italian Adult, Pregnant, and Lactating Vegetarians (ARTICLE IN PRESS). J. Acad. Nutr. Diet. 2017. [Google Scholar] [CrossRef] [PubMed]
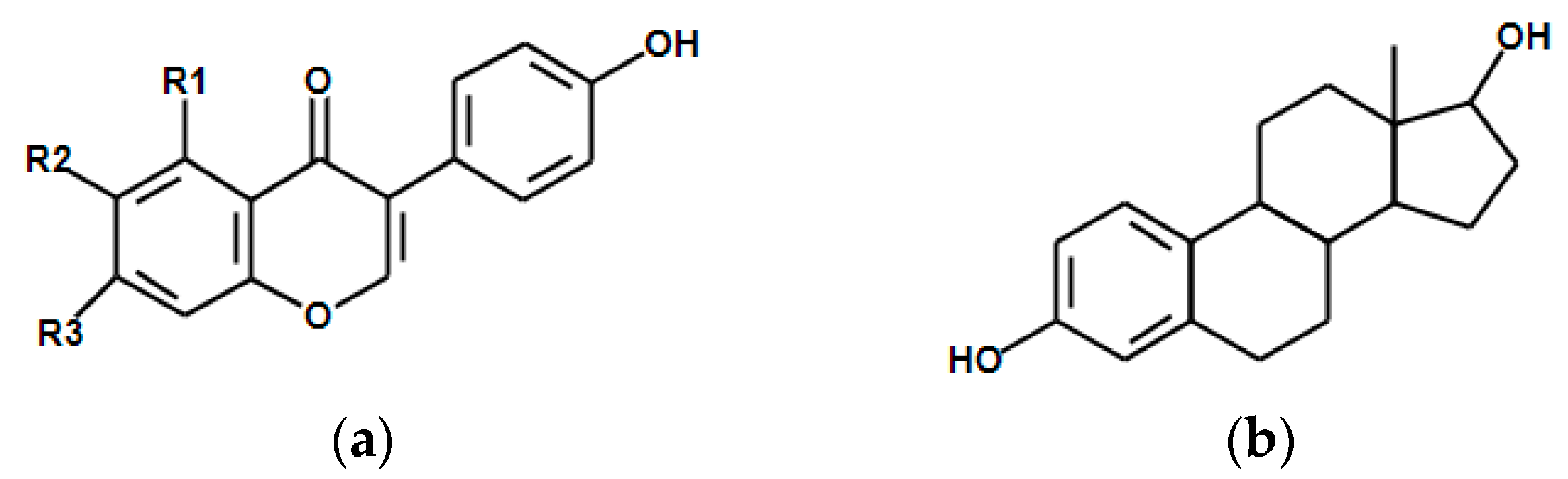

| Food | Energy 2 | Protein 3 | Carbohydrate 3 | Fats 3 | Fibre 3 | PUFA 3,4 | Iron 5 | Calcium 5 |
|---|---|---|---|---|---|---|---|---|
| Azuki beans | 329 | 19.87 | 62.90 | 0.53 | 12.7 | 0.113 | 4.98 | 66 |
| Fava beans | 341 | 26.12 | 58.29 | 1.53 | 25.0 | 0.627 | 6.70 | 103 |
| Chickpeas | 378 | 20.47 | 62.95 | 6.04 | 12.2 | 2.731 | 4.31 | 57 |
| Green peas | 352 | 23.82 | 63.74 | 1.16 | 25.5 | 0.495 | 4.82 | 37 |
| Kidney beans | 333 | 23.58 | 60.01 | 0.83 | 24.9 | 0.457 | 8.20 | 143 |
| Lentils | 352 | 24.63 | 63.35 | 1.06 | 10.7 | 0.526 | 6.51 | 35 |
| Lima beans | 338 | 21.46 | 36.38 | 0.69 | 19.0 | 0.309 | 7.51 | 81 |
| Lupins | 371 | 36.17 | 40.37 | 9.74 | 18.9 | 2.439 | 4.36 | 176 |
| Mug beans | 347 | 23.86 | 62.62 | 1.15 | 16.3 | 0.384 | 6.74 | 132 |
| Mungo beans | 341 | 25.21 | 58.99 | 1.64 | 18.3 | 1.071 | 7.51 | 138 |
| Navy beans | 337 | 22.33 | 60.75 | 1.50 | 15.3 | 0.873 | 5.49 | 147 |
| Peanuts | 567 | 25.8 | 16.13 | 49.24 | 8.5 | 15.558 | 4.58 | 92 |
| Pinto beans | 347 | 21.42 | 62.55 | 1.23 | 15.5 | 0.407 | 5.07 | 113 |
| Soy beans | 446 | 36.49 | 30.16 | 19.94 | 9.3 | 11.255 | 15.70 | 277 |
| Nationality | Soy and Soy Foods 2 | Soy Protein 2 | Isoflavones 3 |
|---|---|---|---|
| USA | NA | NA | 0.73–3.3 |
| Europe | NA | NA | 0.37–4.5 |
| Vegetarians and soy users | NA | 8.42–9.25 | 3.2–30 |
| China | 23.5–135.4 | 2.5–10.3 | 6.2–75.7 |
| Japan | 50.7–102.1 | 6–11.3 | 22.6–54.3 |
| Korea | 21.07 | 7.4–8.5 | 14.88 |
| Country | Isoflavone per 100 g |
|---|---|
| Australia | 120.84 |
| Brazil | 99.82 |
| China | 118.28 |
| Europe | 103.56 |
| Japan | 130.56 |
| Korea | 178.81 |
| Taiwan | 85.68 |
| USA | 159.98 |
| Method | Description |
|---|---|
| Net Protein Utilization (NPU) | Difference between nitrogen retention in carcass of animal group feed with test protein and nitrogen content in carcass of animal group with free protein diet normalized for dietary nitrogen intake. |
| Protein Efficiency Ratio (PER) | Gain in body mass of animal model divided for protein intake |
| Nitrogen Balance | Protein intake requirement to attain nitrogen equilibrium. The difference between nitrogen intake and nitrogen loss with urine and faeces (nitrogen absorption and retention). |
| Biological Value (BV) | Nitrogen absorbed form test protein divided for nitrogen incorporated into the body and standardized for a reference protein. |
| True Digestibility | Difference between nitrogen intake and nitrogen loss corrected for protein-free diet loss. |
| Amino Acid Score | mg of amino acid in 1 g of test protein divided for mg of amino acid in requirement pattern. |
| Protein Digestibility corrected for amino acid score (PDCAAS) | Amino acid score multiplied for true faecal protein digestibility. |
| Digestible Indispensable Amino Acid Score (DIAAS) | Amino acid score for selected pattern multiplied for true ileal amino acid digestibility. |
| Source | PDCAAS | Digestibility (%) | Amino Acid Score | PER | BV |
|---|---|---|---|---|---|
| Soy | 0.92–1.00 | 95–98 | 0.94 | 2.2 | 74 |
| Wheat | 0.25 | 96–99 | 0.26 | 0.8 | 64 |
| Beef | 0.92 | 94–98 | 0.94 | 2.9 | 80 |
| Egg | 1.00 | 97–98 | 1.21 | 3.8–3.9 | 100 |
| Milk | 1.00 | 95 | 1.27 | 2.5–3.1 | 91 |
| Food | mg per 100 g |
|---|---|
| Miso | 41.45 |
| Edamame | 17.92 |
| Natto | 82.29 |
| Soy cheese 2 | 6.02–25.72 |
| Soy four, textured | 172.55 |
| Soy four, defatted | 150.94 |
| Soy lecithin | 15.7 |
| Soy protein concentrate 3 | 94.65 |
| Soy protein concentrate 4 | 11.49 |
| Soy protein isolate | 91.05 |
| Shoyu (soy sauce) | 1.18 |
| Soy beans, roasted | 148.5 |
| Soy beans, raw | 154.53 |
| Yuba/foo jook | 44.67 |
| Soy milk 5 | 0.7–10.73 |
| Sufu | 13.75 |
| Tempeh | 3.82 |
| Tofu 6 | 13.1–34.78 |
| Okara | 9.39 |
| Fuyu | 45.51 |
| Soybean oil | 0 |
| Item | Description |
|---|---|
| Tofu | Soaking and heating procedures with addition of protein coagulants such as calcium sulphate to soymilk. Pressed soy curd can undergo smoking or marinating processes. |
| Soy milk | Water extraction of hulled and crushed soy beans. Boiling after filtration of raw milk removes beany flavour. |
| Tempeh | Dehulled soy beans fermented with Rhizopius oligosporus. |
| Natto | Soy beans fermented with Bacillus natto and Bacillus subtilis. |
| Sufu | Tofu fermented with Actinomucor elegans. |
| Edamame | Immature, green soy beans. |
| Miso | Fermented soy beans with Aspergillus orzae or Aspergillus soyae. |
| Yuba | Drying of skin (film) formed in soymilk production during boiling. |
| Soy Sauce | Soy beans or soy flakes fermented with different bacteria, yeast or treated with isolated enzymes. Pressing and filtration after fermentation is needed to extract aqueous phase. |
| Textured vegetable protein | Extrusion and cooking of soy flour, full fat or defatted, in moisture and temperature controlled conditions. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. https://doi.org/10.3390/nu10010043
Rizzo G, Baroni L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients. 2018; 10(1):43. https://doi.org/10.3390/nu10010043
Chicago/Turabian StyleRizzo, Gianluca, and Luciana Baroni. 2018. "Soy, Soy Foods and Their Role in Vegetarian Diets" Nutrients 10, no. 1: 43. https://doi.org/10.3390/nu10010043
APA StyleRizzo, G., & Baroni, L. (2018). Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients, 10(1), 43. https://doi.org/10.3390/nu10010043






