Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Study Design
2.3. Questionnaire
2.4. Laboratory Tests
2.5. Ethics
2.6. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study and Future Research
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gerasimoula, K.; Lefkothea, L.; Maria, L.; Victoria, A.; Paraskevi, T.; Maria, P. Quality of life in hemodialysis patients. Mater. Sociomed. 2015, 27, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.B.; Schmidt, D.B.; Amatneeks, T.M.; Santos, J.C.; Cavallet, L.H.; Michel, R.B. Quality of life in hemodialysis patients and the relationship with mortality, hospitalizations and poor treatment adherence. J. Bras. Nefrol. 2016, 38, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Mollaoğlu, M. Quality of life in patients undergoing hemodialysis. In Hemodialysis; Suzuki, H., Ed.; InTech: Rijeka, Croatia, 2013; pp. 823–841. [Google Scholar]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis of CKD: An update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.C.; Kalantar-Zadeh, K.; Mehrotra, R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. Suppl. 2005, 95, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pickering, W.P.; Price, S.R.; Bircher, G.; Marinovic, A.C.; Mitch, W.E.; Walls, J. Nutrition in CAPD: Serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002, 61, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Moio, M.R.; Fois, A.; Sofronie, A.; Gendrot, L.; Cabiddu, G.; D’Alessandro, C.; Cupisti, A. The diet and haemodialysis dyad: Three eras, four open questions and four paradoxes. A narrative review, towards a personalized, patient-centered approach. Nutrients 2017, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Khoueiry, G.; Waked, A.; Goldman, M.; El-Charabaty, E.; Dunne, E.; Smith, M.; Kleiner, M.; Lafferty, J.; Kalantar-Zadeh, K.; El-Sayegh, S. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J. Ren. Nutr. 2011, 21, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Deepak, S.; Block, D.; Block, G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J. Ren. Nutr. 2002, 12, 17–31. [Google Scholar] [CrossRef] [PubMed]
- KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Available online: http://www2.kidney.org/professionals/kdoqi/guidelines_nutrition/doqi_nut.html (accessed on 25 December 2017).
- Dąbrowski, P.; Olszanecka-Glinianowicz, M.; Chudek, J. Żywienie w przewlekłej chorobie nerek. Endokrynologia Otyłość i Zaburzenia Przemiany Materii 2011, 7, 229–237. [Google Scholar]
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am. J. Kidney Dis. 2003, 42, 1–202. [Google Scholar]
- Kalantar-Zadeh, K.; Brown, A.; Chen, J.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left. PMC J. 2016, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Tsuiji, T.; Sakao, Y.; Ohashi, N.; Yasuda, H.; Fujimoto, T.; Takita, T.; Furuhashi, M.; Kumagai, H. A comparison of systemic inflammation-based prognostic scores in patients on regular hemodialysis. Nephron Extra 2013, 3, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Borek, P.; Duszyńska, A.; Małgorzewicz, S.; Chmielewski, M.; Rutkowski, B. Ryzyko utraty masy ciała i stan odżywienia pacjentów hemodializowanych. Nefrol. Dial. Pol. 2014, 18, 123–127. [Google Scholar]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: A systematic review and meta-analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Radziszewski, A.; Sułowicz, W. Mocznica jako stan zapalny. Postępy w Nefrologii i Nadciśnieniu Tętniczym 2008, 7, 103–110. [Google Scholar]
- Wing, M.R.; Patel, S.S.; Ramezani, A.; Raj, S.D. Gut microbiome in chronic kidney disease. Exp. Physiol. 2016, 101, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G.; Kotanko, P.; Kamer, A.R.; Nathan, W.L. Periodontal diseases—A modifiable source of systemic inflammation for the end-stage renal disease patient on haemodialysis therapy? Nephrol. Dial. Transplant. 2007, 22, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, K.; Juneja, V.; Shanaman, E. Medical Nutrition Therapy for Renal Disorders. In Krause’s Food and Nutrition Care Process; Mahan, K., Raymond, J., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 700–727. [Google Scholar]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.; Bammens, B.; Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient non-equivalence: Does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kokot, F.; Ficek, R.; Bułanowski, M. Hiperkaliemia—Czy zawsze wymaga korekcji u chorych z przewlekłą niewydolnością nerek. Postępy w Nefrologii i Nadciśnieniu Tętniczym 2005, 4, 33–35. [Google Scholar]
- Chrysohoou, C.; Pnagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Zeimbekis, A.; Kastorini, C.M.; Stefanadis, C. Adherence to the Mediterranean diet is associated with renal function among healthy adults: The ATTICA study. J. Ren. Nutr. 2010, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hoe, E.; Natchanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-inflammatory effects of Vitamin D on human immune cells in the context of bacterial infection. Nutrients 2016, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.; Xun, P.; He, K. Corrigendum. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2015, 69, 410. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Biruete, A.; Jeong, J.H.; Barnes, J.; Wilund, K.R. Modified nutritional recommendations to improve dietary patterns and outcomes in hemodialysis patients. J. Ren. Nutr. 2017, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Whole Study Group (n = 98) | Men (n = 60) | Women (n = 38) | p-Value |
|---|---|---|---|---|
| Age, years | 62 ± 14 | 61 ± 16 | 65 ± 11 | 0.2 |
| Duration of dialysis, months | 63 (35–144) | 60 (36–108) | 84 (36–192) | 0.3 |
| Residence | ||||
| Country, n/% | 34/35 | 19/32 | 15/39 | 0.9 |
| Town <100,000 inhabitants, n/% | 10/10 | 6/10 | 4/11 | |
| City >100,000 inhabitants, n/% | 54/55 | 35/58 | 19/50 | |
| Body weight, kg | 71.6 ± 16.3 | 76.0 ± 14.4 | 64.8 ± 16.8 | <0.001 |
| BMI, kg/m2 | 25.2 ± 5.0 | 25.7 ± 4.4 | 24.5 ± 5.8 | 0.3 |
| Active smoking, n/% | 17/17 | 12/20 | 5/8 | 0.3 |
| Sleep | ||||
| ≤6 h/24 h, n/% | 32/33 | 17/28 | 15/39 | 0.6 |
| 7–8 h/24 h, n/% | 48/49 | 30/50 | 18/47 | |
| 9 h/24 h, n/% | 15/15 | 10/17 | 5/13 | |
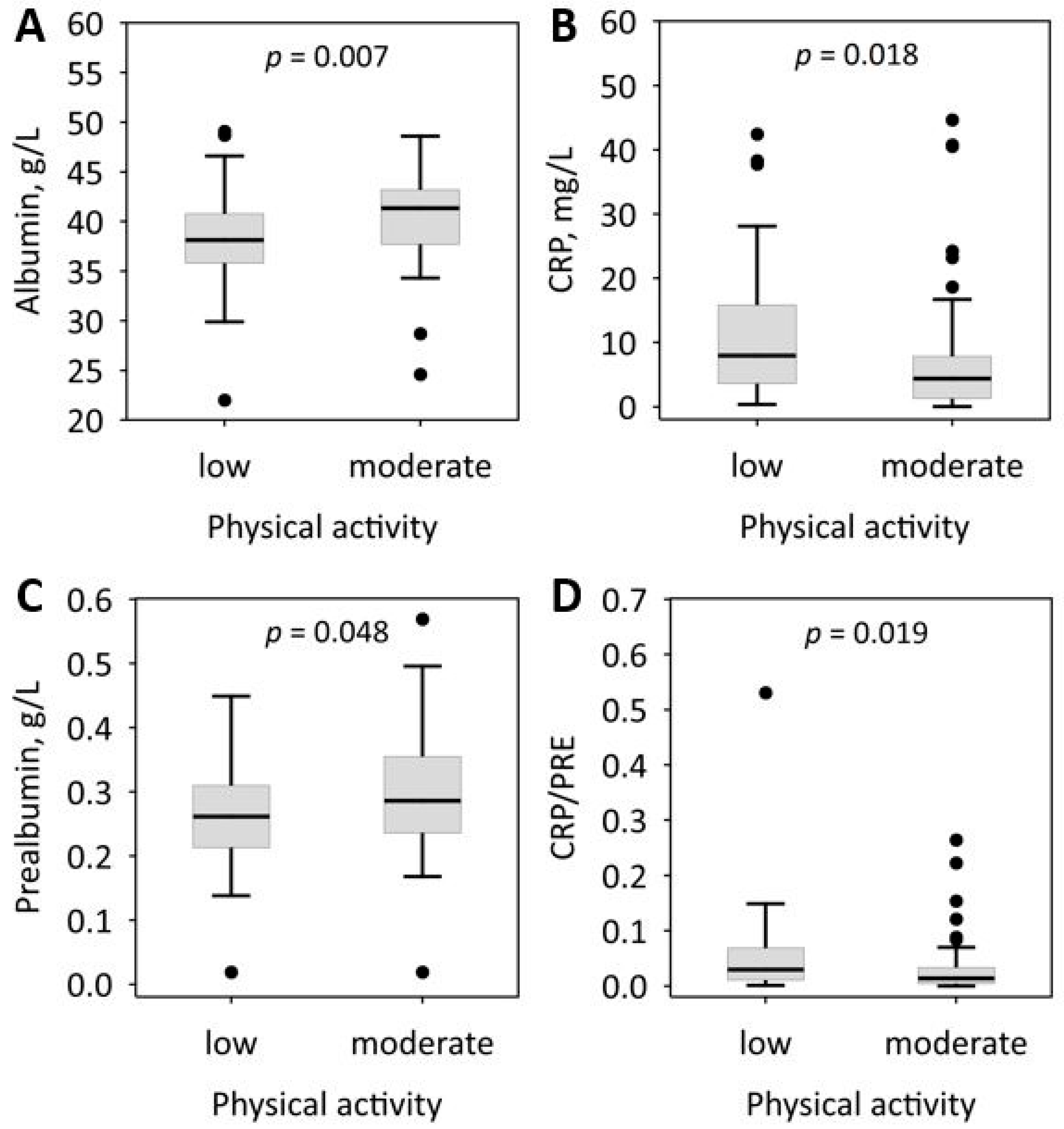
| Physical activity | ||||
| Low, n/% | 51/52 | 28/47 | 23/61 | 0.3 |
| Moderate, n/% | 44/45 | 29/48 | 15/39 | |
| Dietary supplements’ use, n/% | 31/32 | 18/30 | 13/34 | 0.7 |
| Vegetables frequency | ||||
| >2 portions/day, n/% | 3/3 | 2/3 | 1/3 | 0.8 |
| 1–2 portions/day, n/% | 47/48 | 30/50 | 17/45 | |
| A few portions/week, n/% | 36/37 | 20/33 | 16/42 | |
| <1 portion/week, n/% | 6/6 | 3/5 | 3/8 | |
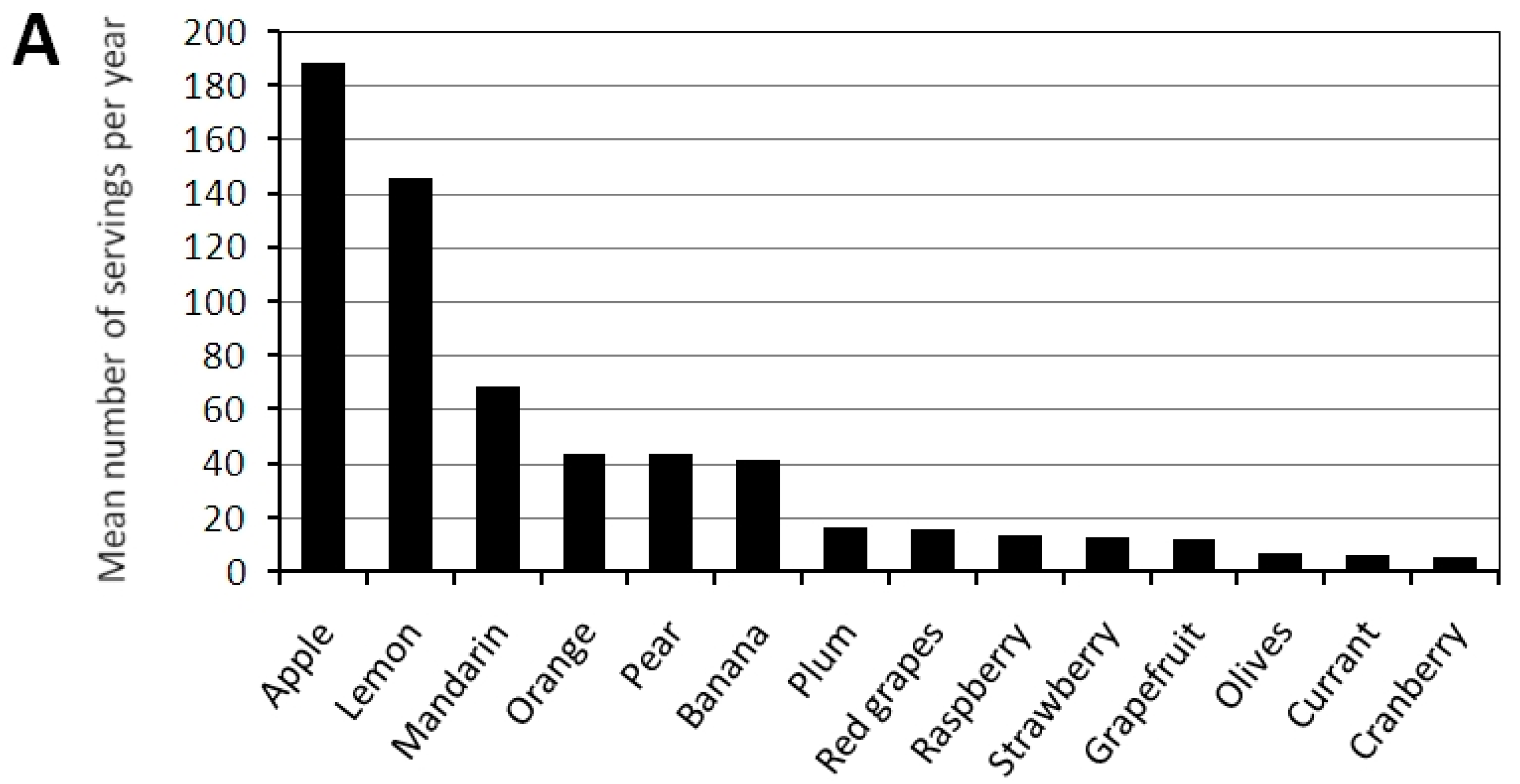
| Fruit frequency | ||||
| >2 portions/day, n/% | 5/5 | 0 | 5/13 | 0.005 |
| 1–2 portions/day, n/% | 52/53 | 34/57 | 18/47 | |
| A few portions/week, n/% | 20/20 | 9/15 | 11/29 | |
| <1 portion/week, n/% | 16/16 | 13/22 | 3/8 | |
| Fruit and vegetables processing | ||||
| Eating raw, n/% | 36/37 | 24/40 | 12/32 | 0.6 |
| Cooking, n/% | 42/43 | 24/40 | 18/47 | |
| Cooking with water change, n/% | 15/15 | 8/13 | 7/18 | |
| Study Parameters (Degree of Malnutrition) | Whole Study Group (n = 98) | Men (n = 60) | Women (n = 38) | p-Value |
|---|---|---|---|---|
| Albumin, g/L | 39.4 (36.4–42.8) | 40.7 (37.4–43.1) | 37.8 (36.1–40.9) | 0.022 |
| 30–34 g/L (mild), n/% | 10/10 | 6/10 | 4/11 | 0.5 |
| 21–29 g/L (moderate), n/% | 4/4 | 2/3 | 2/5 | |
| <21 g/L (severe), n/% | 0 | 0 | 0 | |
| Prealbumin, g/L | 0.27 (0.22–0.32) | 0.28 (0.24–0.33) | 0.25 (0.20–0.30) | 0.063 |
| 0.10–0.17 g/L (mild), n/% | 8/8 | 4/7 | 2/5 | 0.4 |
| 0.05–0.09 g/L (moderate), n/% | 0 | 0 | 2/5 | |
| <0.05 g/L (severe), n/% | 0 | 0 | 0 | |
| GPS 0, n/% | 56/57 | 36/60 | 20/53 | 0.5 |
| GPS 1, n/% | 25/25 | 16/27 | 9/24 | |
| GPS 2, n/% | 11/11 | 5/8 | 6/16 | |
| CRP/PRE | 0.019 (0.008–0.059) | 0.016 (0.006–0.052) | 0.024 (0.011–0.072) | 0.09 |
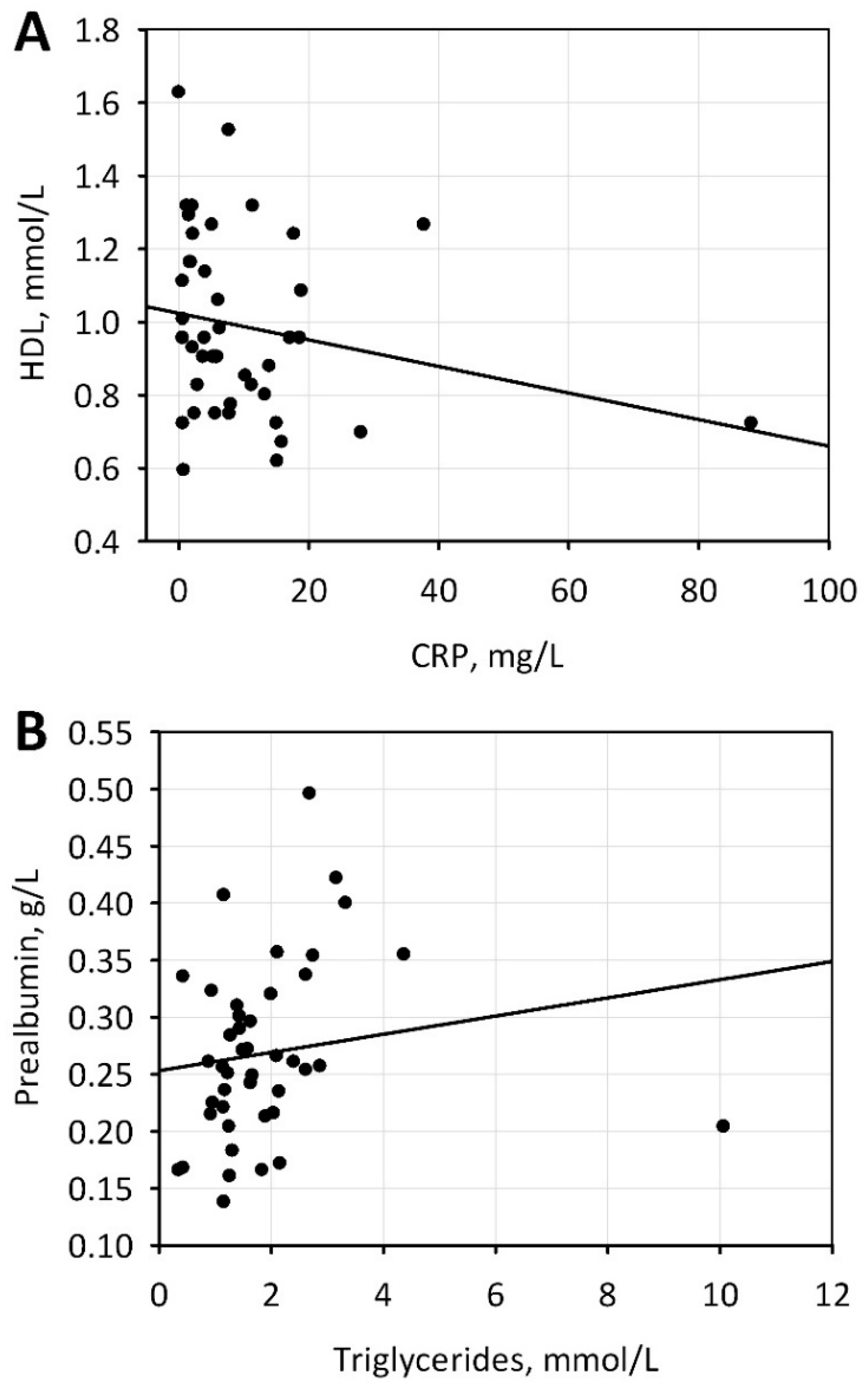
| CRP, mg/L | 5.7 (2.2–13.7) | 5.7 (1.7–12.1) | 5.9 (2.9–16.7) | 0.3 |
| CRP >5 mg/L, n/% | 52/53 | 30/50 | 22/58 | 0.3 |
| GPS | CRP/PRE | CRP | Prealbumin | Total Cholesterol | HGB | |
|---|---|---|---|---|---|---|
| Albumin | −0.52 * | −0.46 * | −0.42 * | 0.59 * | −0.04 NS | 0.36 * |
| Prealbumin | −0.61 * | −0.64 * | −0.50 * | - | 0.24 NS | 0.17 NS |
| CRP | 0.80 * | 0.97 * | - | - | 0.14 NS | −0.11 NS |
| CRP/PRE | 0.80 * | - | - | - | 0.11 NS | −0.14 NS |
| GPS | - | - | - | - | 0.06 NS | −0.10 NS |
| Albumin ≤ 34 g/L | Prealbumin ≤ 0.17 g/L | CRP > 5 mg/L | CRP/PRE > 0.019 | GPS > 0 | |
|---|---|---|---|---|---|
| Simple odds ratio (95% confidence interval) | |||||
| Age, per 1 year | 1.03 (0.99–1.08) NS | 1.01 (0.96–1.06) NS | 1.03 (0.99–1.06) NS | 1.04 (1.003–1.07) * | 1.05 (1.01–1.09) ** |
| Female sex | 1.27 (0.39–4.08) NS | 1.74 (0.40–7.60) NS | 1.52 (0.64–3.64) NS | 1.92 (0.81–4.57) NS | 1.29 (0.54–3.07) NS |
| Physical activity | 0.32 (0.08–1.32) NS | 0.63 (0.14–2.87) NS | 0.52 (0.22–1.22) NS | 0.34 (0.14–0.82) * | 0.37 (0.15–0.92) * |
| Fruit frequency ≥1 portion/day | 0.26 (0.07–0.96) * | 0.62 (0.14–2.72) NS | 1.57 (0.65–3.79) NS | 1.00 (0.42–2.39) NS | 1.19 (0.49–2.90) NS |
| Vegetables’ frequency ≥1 portion/day | 0.51 (0.15–1.81) NS | 0.80 (0.18–3.48) NS | 1.50 (0.62–3.60) NS | 1.09 (0.46–2.58) NS | 0.82 (0.34–1.99) NS |
| Age-adjusted odds ratio (95% confidence interval); p-value | |||||
| Physical activity | - | - | - | 0.40 (0.16–1.01) NS | 0.46 (0.18–1.20) NS |
| Laboratory Test | Results | Reference Range | Results < Reference Range, n/% | Results > Reference Range, n/% |
|---|---|---|---|---|
| Complete blood counts | ||||
| WBC, ×103/µL | 6.5 (5.1–7.8) | 4.0–10.0 | 8/8 | 8/8 |
| RBC, ×106/µL | ||||
| Men | 3.8 ± 0.6 | 4.5–6.5 | 55/92 | 0 |
| Women | 3.6 ± 0.5 | 3.5–5.0 | 19/50 | 0 |
| HGB, g/dL | ||||
| Men | 11.3 ± 1.5 | 12.0–17.0 | 43/72 | 0 |
| Women | 10.7 ± 1.2 | 11.0–15.0 | 21/55 | 0 |
| HCT, % | ||||
| Men | 35.0 ± 4.6 | 40.0–54.0 | 53/88 | 0 |
| Women | 33.1 ± 3.5 | 37.0–47.0 | 31/82 | 0 |
| MCV, fL | 92.9 ± 6.0 | 82.0–92.0 | 3/3 | 53/54 |
| MCH, pg | 30.0 ± 2.0 | 27.0–31.0 | 8/8 | 29/30 |
| MCHC, g/dL | 32.3 ± 1.1 | 32.0–36.0 | 31/32 | 0 |
| PLT, × 103/µL | 203.8 ± 67.4 | 125.0–340.0 | 11/11 | 3/3 |
| RDW-CV, % | 15.2 (14.0–16.2) | 11.0–15 | 0 | 52/53 |
| Biochemistry | ||||
| Sodium, mmol/L | 137 (136–139) | 136–145 | 24/24 | 0 |
| Potassium, mmol/L | 5.1 ± 0.9 | 3.5–5.1 | 1/1 | 48/49 |
| Calcium, mmol/L | 1.89 (1.18–2.27) | 2.15–2.55 | 63/64 | 5/5 |
| Phosphate, mmol/L | 2.50 (1.65–4.50) | 0.81–1.45 | 3/3 | 80/82 |
| Iron, µmol/L | 11.80 (9.12–15.0) | 5.83–34.50 | 3/5 | 0 |
| UIBC, µmol/L | ||||
| Men | 30.7 ± 10.8 | 22.3–61.7 | 10/25 | 0 |
| Women | 30.8 ± 10.7 | 24.2–70.1 | 7/26 | 0 |
| TIBC, µmol/L | 42.2 ± 10.3 | 40.8–76.6 | 38/53 | 0 |
| Urea, mmol/L | 20.85 ± 7.90 | 2.76–8.07 | 1/1 | 88/90 |
| Total cholesterol, mmol/L | 4.2 ± 1.4 | 3.2–5.2 | 11/25 | 11/25 |
| HDL-C, mmol/L | 1.0 ± 0.3 | 0.9–3.0 | 13/30 | 0 |
| LDL-C, mmol/L | 2.3 ± 1.1 | 0.2–3.4 | 0 | 8/18 |
| Triglycerides, mmol/L | 1.5 (1.2–2.2) | 0.2–2.3 | 0 | 9/20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraj, M.; Kuśnierz-Cabala, B.; Dumnicka, P.; Gala-Błądzińska, A.; Gawlik, K.; Pawlica-Gosiewska, D.; Ząbek-Adamska, A.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuźniewski, M. Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients 2018, 10, 69. https://doi.org/10.3390/nu10010069
Maraj M, Kuśnierz-Cabala B, Dumnicka P, Gala-Błądzińska A, Gawlik K, Pawlica-Gosiewska D, Ząbek-Adamska A, Mazur-Laskowska M, Ceranowicz P, Kuźniewski M. Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients. 2018; 10(1):69. https://doi.org/10.3390/nu10010069
Chicago/Turabian StyleMaraj, Małgorzata, Beata Kuśnierz-Cabala, Paulina Dumnicka, Agnieszka Gala-Błądzińska, Katarzyna Gawlik, Dorota Pawlica-Gosiewska, Anna Ząbek-Adamska, Małgorzata Mazur-Laskowska, Piotr Ceranowicz, and Marek Kuźniewski. 2018. "Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis" Nutrients 10, no. 1: 69. https://doi.org/10.3390/nu10010069
APA StyleMaraj, M., Kuśnierz-Cabala, B., Dumnicka, P., Gala-Błądzińska, A., Gawlik, K., Pawlica-Gosiewska, D., Ząbek-Adamska, A., Mazur-Laskowska, M., Ceranowicz, P., & Kuźniewski, M. (2018). Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients, 10(1), 69. https://doi.org/10.3390/nu10010069








