Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice
Abstract
:1. Introduction
2. Results
2.1. Quantitative Analysis of Characteristic Components in Large Yellow Tea and Its Water Extract by High Pressure Liquid Chromatography LYT and LWE by HPLC
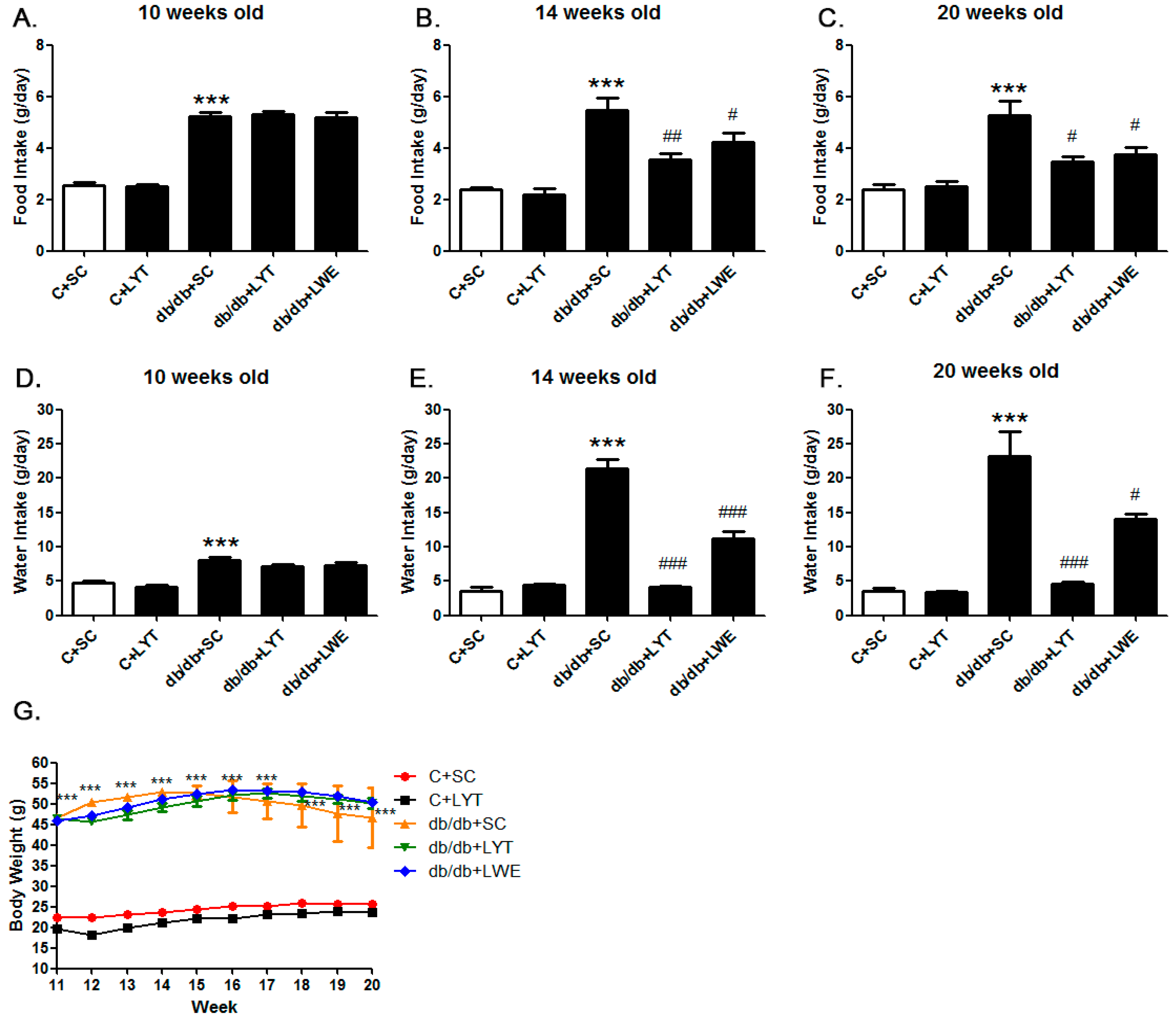
2.2. Effect of LYT and LWE on Diabetic Syndrome on db/db Mice
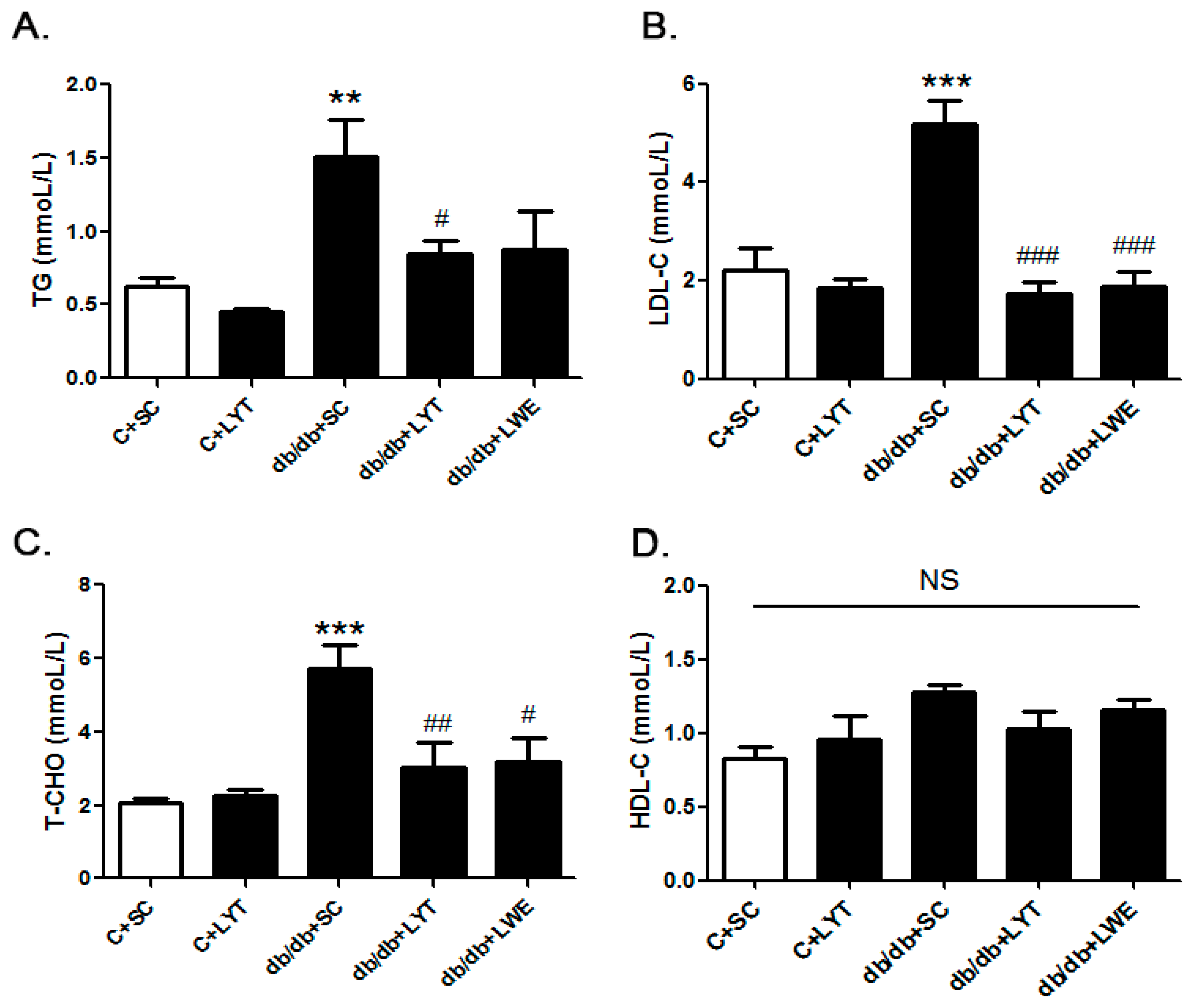
2.3. Protective Effect of LYT and LWE on Serum Lipids Profile of db/db Mice
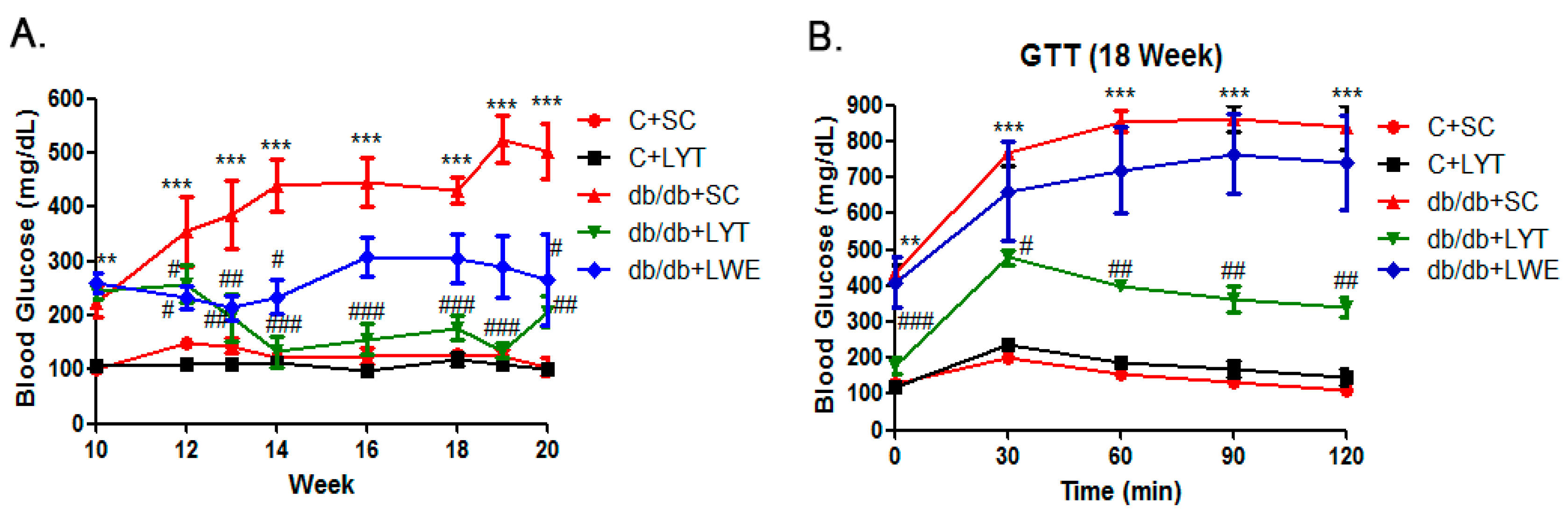
2.4. LYT Reduced the Blood Glucose and Improved Glucose Tolerance in db/db Mice
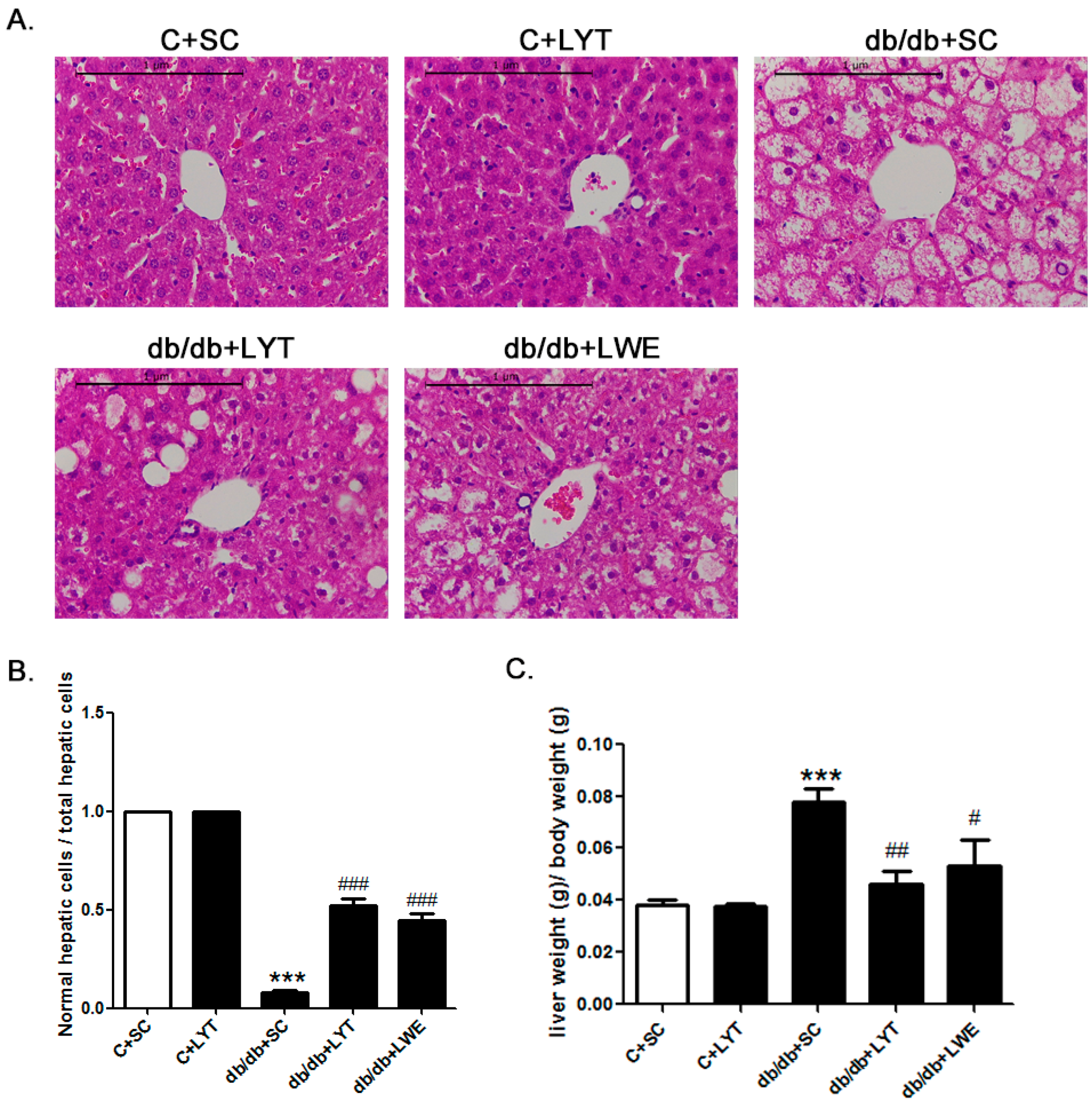
2.5. LYT and LWE Attenuate Hepatic Steatosis in db/db Mice
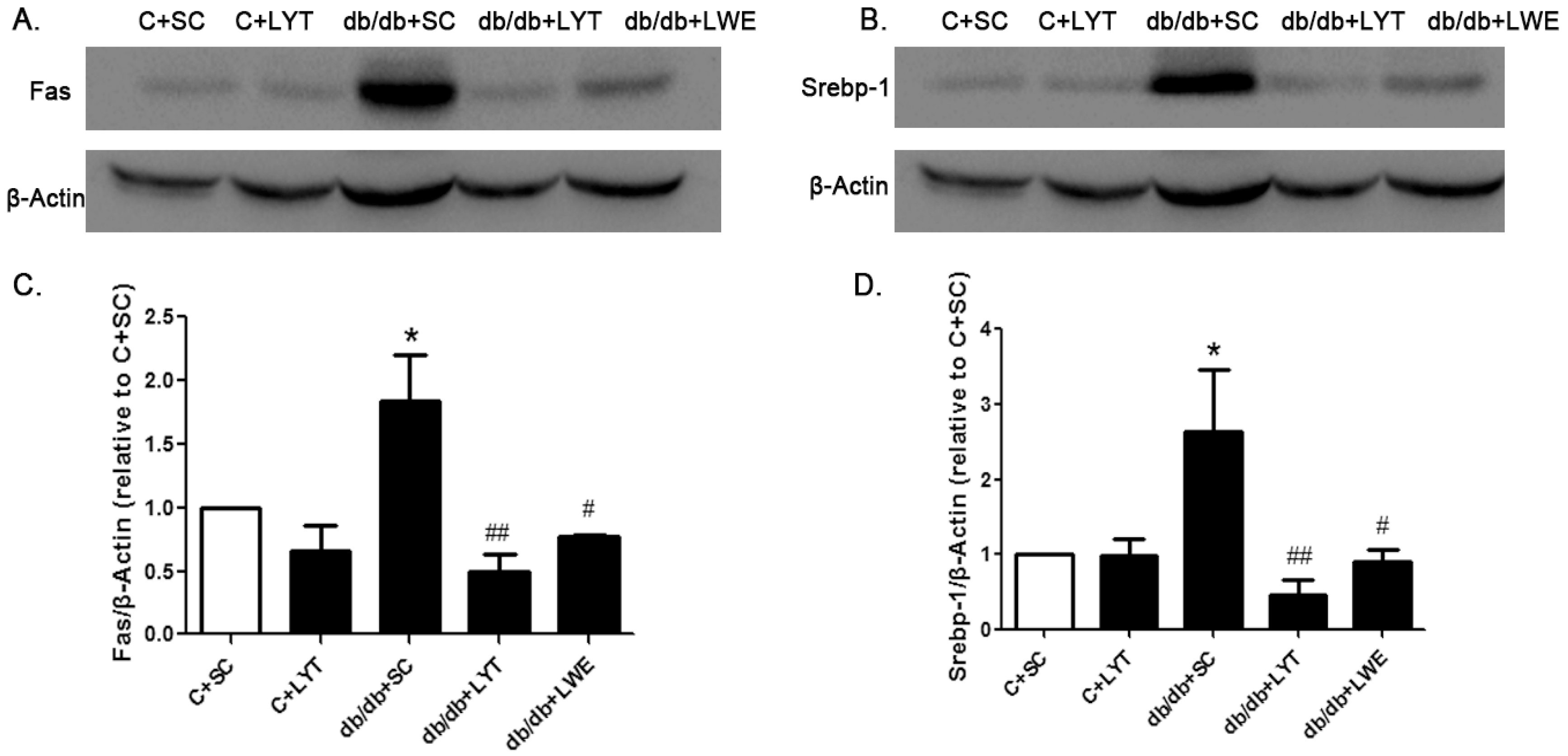
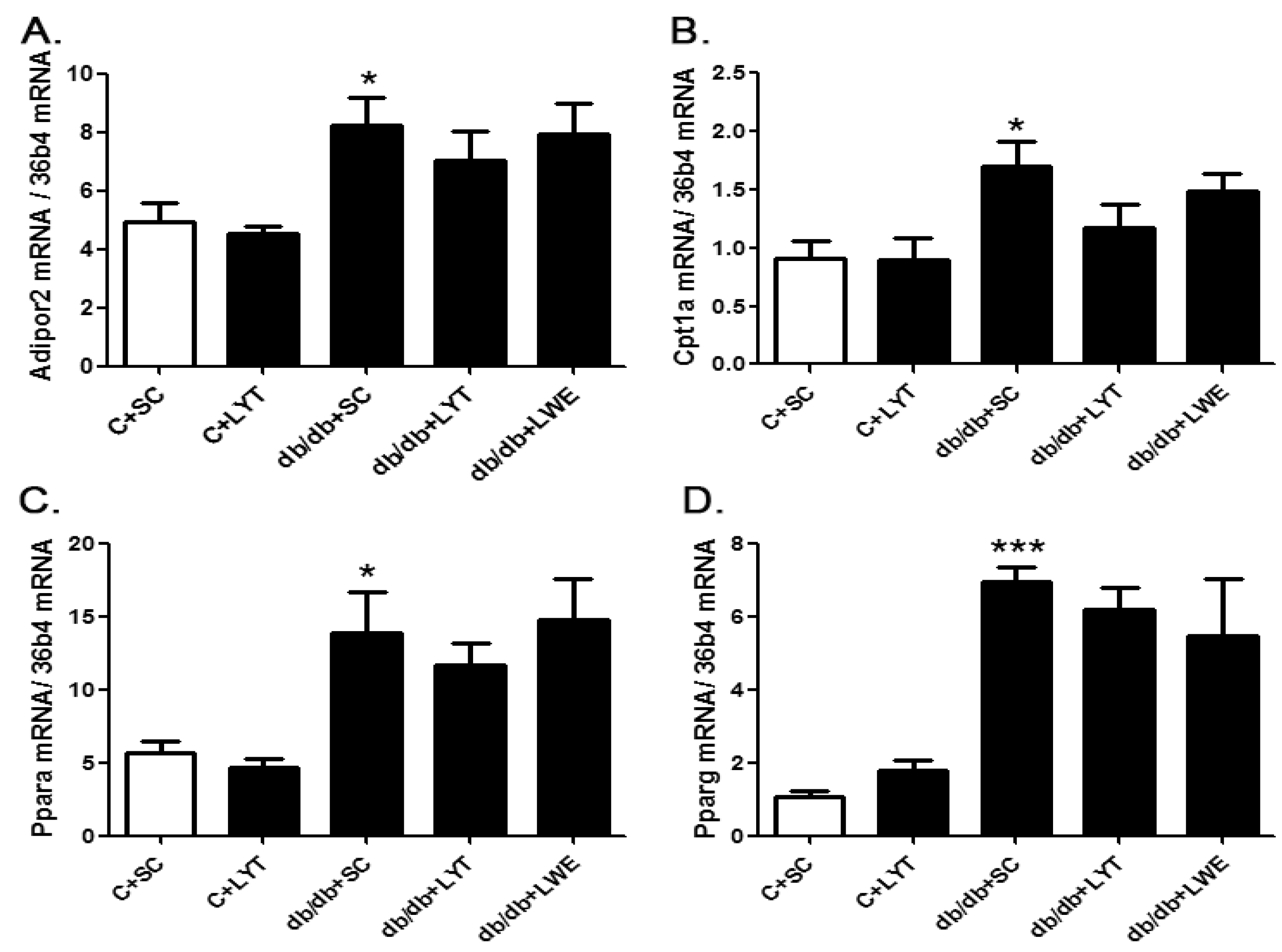
2.6. LYT and LWE Reduced Lipid Accumulation in Liver Tissues via Suppressing the Lipogenesis in db/db Mice
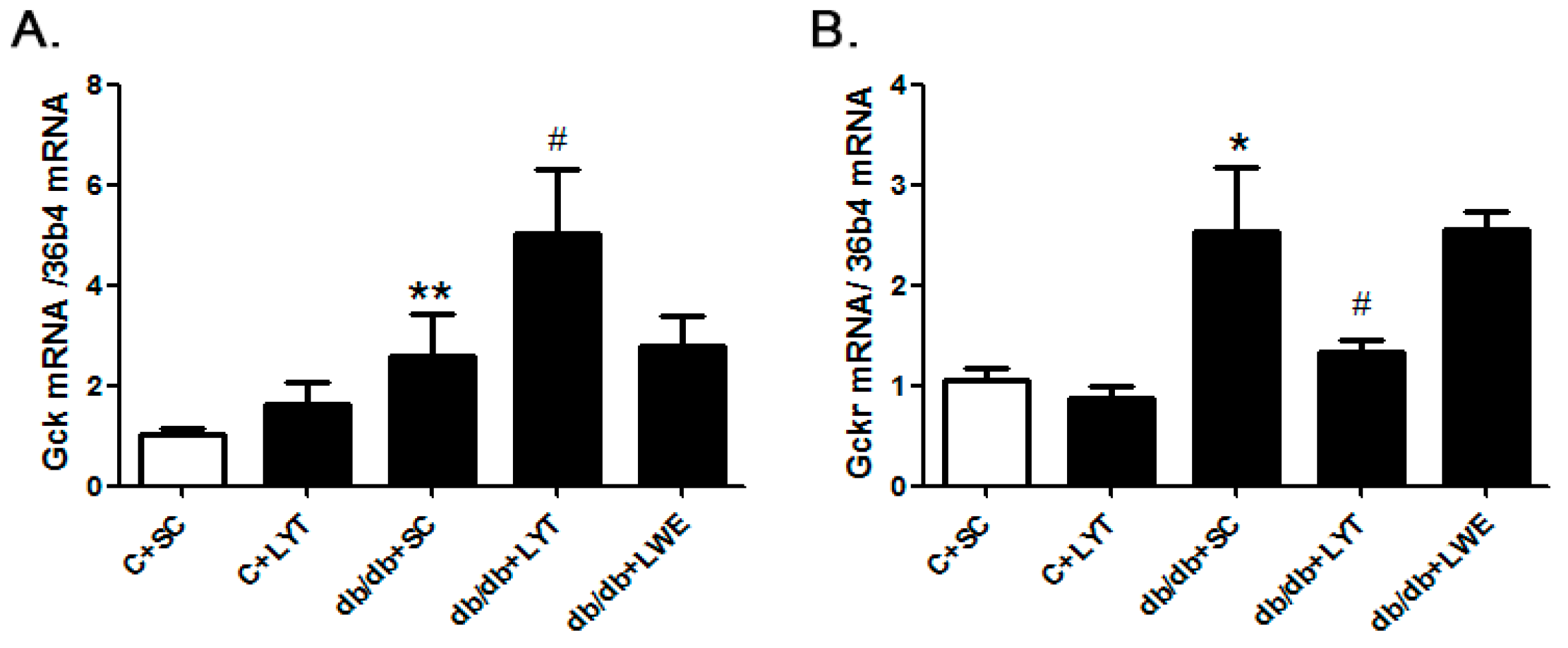
2.7. LYT Improved the Hepatic Glycometabolism in db/db Mice
3. Discussion
4. Materials and Methods
4.1. The Extract of Large Yellow Tea
4.2. Analysis of Major Chemical Components of Large Yellow Tea and Its Water Extract
4.3. Animal Experiments
4.4. Glucose Tolerance Test
4.5. Hematoxylin–Eosin Staining
4.6. Real-Time Polymerase Chain Reaction PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome, a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Fotheringham, A.K. Vascular complications in diabetes: Old messages, new thoughts. Diabetologia 2017. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome-a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Schulman, R.C.; Mechanick, J.L. Metabolic and nutrition support in the chronic cirtical illness syndrome. Respir. Care 2012, 57, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Oda, E. Metabolic syndrome: Its history, mechanisms, and limitations. Acta Diabetol. 2012, 49, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cryer, M.J.; Horani, T.; DiPette, D.J. Diabetes and Hypertension: A Comparative Review of Current Guidelines. J. Clin. Hypertens. 2016, 18, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Vague, J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am. J. Clin. Nutr. 1956, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Role of insulin resistance in human disease (syndrome X): An expanded definition. Annu. Rev. Med. 1993, 44, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sandouk, Z.; Lansang, M.C. Diabetes with obesity—Is there an ideal diet? Clevel. Clin. J. Med. 2017, 84, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Guo, Z.; Randall, D.C.; Cassis, L.; Brown, D.R.; Gong, M.C. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Berglund, E.D.; Yu, X.; Wang, M.Y.; Evans, M.R.; Scherer, P.E.; Holland, W.L.; Charron, M.J.; Roth, M.G.; Unger, R.H. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13217–13222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2003, 284, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, D.; Luo, X.; Ding, D.; Song, Y.; Zhang, Z.; Wan, X. Stepwise identification of six tea (Camellia sinensis (L.) categories based on catechins, caffeine, and theanine contents combined with fisher discriminant analysis. Food Anal. Method 2016, 9, 3242–3250. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Paradee, A.; Montira, P.; Oratai, T.; Bung-orn, S.; Narong, A.; Bandit, T.; Soontorn, K.; Srisuda, W.; Supat, S.; Pranithi, H. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trail. Physiol. Behav. 2008, 93, 486–491. [Google Scholar] [CrossRef]
- Kang, S.J.; Lee, J.E.; Lee, E.K.; Jung, D.H.; Song, C.H.; Park, S.J.; Choi, S.H.; Han, C.H.; Ku, S.K.; Lee, Y.J. Fermentation with Aquilariae Lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients 2014, 6, 3536–3571. [Google Scholar] [CrossRef] [PubMed]
- Ortsäter, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjöholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, G.; Wang, Y.; Wang, D.; Sun, F.; Ning, J.; Wan, X.; Zhang, J. Safety and anti-hyperglycemic efficacy of various tea types in mice. Sci. Rep. 2016, 6, 31703. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.C.; Jarvis, R.M.; Rosengren, R.J. In vitro mechanism of action for the cytotoxicity elicited by the combination of epigallocatechingallate and raloxifene in MDA-MB-231 cells. Oncol. Rep. 2010, 24, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechingallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In vitro toxicity of epigallocatechingallate in rat liver mitochondria and hepatocytes. Oxid. Med. Cell. Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Jung, Y.; Min, S.; Nam, M.; Heo, R.W.; Jeon, B.T.; Song, D.H.; Yi, C.O.; Jeong, E.A.; Kim, H.; et al. Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism. Sci. Rep. 2016, 6, 30111. [Google Scholar] [CrossRef] [PubMed]
- Porcellati, F.; Lucidi, P.; Bolli, G.B.; Fanelli, C.G. Thirty years of research on the dawn phenomenon: Lessons to optimize blood glucose control in diabetes. Diabetes Care 2013, 36, 3860–3862. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, A.T.; Tooke, J.E. The fetal insulin hypothesis: An alternative explanation of the association of low birth weight with diabetes and vascular disease. Lancet 1999, 353, 1789–1792. [Google Scholar] [CrossRef]
- Callejas, D.; Mann, C.J.; Ayuso, E.; Lage, R.; Grifoll, I.; Roca, C.; Andaluz, A.; Ruiz-de, G.R.; Montané, J.; Muñoz, S.; et al. Treatment of diabetes and long-term survival after insulin and glucokinase gene therapy. Diabetes 2013, 62, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Zelent, B.; Raimondo, A.; Barett, A.; Buettger, C.W.; Chen, P.; Gloyn, A.L.; Matschinsky, F.M. Analysis of the co-operative interaction between the allosterically regulated proteins GK and GKRP using tryptophan fluorescence. Biochem. J. 2014, 459, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.J.; St Jean, D.J., Jr.; Kurzeja, R.J.; Wahl, R.C.; Michelsen, K.; Cupples, R.; Chen, M.; Wu, J.; Sivits, G.; Helmering, J.; et al. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature 2013, 504, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Taniguchi, Y.; Saka, A.; Yoshida, A.; Yajima, H. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition 2011, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Dugan, C.E.; Smyth, J.A.; DiNatale, D.A.; Koo, S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008, 138, 323–331. [Google Scholar] [PubMed]
- Jurgens, T.M.; Whelan, A.M.; Killian, L.; Doucette, S.; Kirk, S.; Foy, E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst. Rev. 2012, 12, CD008650. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qiao, C.; Tang, R.H.; Jiang, J.; Li, J.; Martin, C.B.; Bulaklak, K.; Li, J.; Wang, D.W.; Xiao, X. Overcoming insulin insuffciency by forced follistatin expression in β-cells of db/db mice. Mol. Ther. 2015, 23, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Petta, S.; Dalle, G.R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Mardinoglu, A.; Zhang, C.; Lee, D.; Nielsen, J. Dysregulated signaling hubs of liver lipid metabolism reveal hepatocellular carcinoma pathogenesis. Nucleic Acids Res. 2016, 44, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Scandiuzzi, L.; Hasnian, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, S.; Trombetta, M.; Boselli, M.L.; Turrini, F.; Malerba, G.; Trabetti, E.; Pignatti, P.F.; Bonora, E.; Bonadonna, R.C. Variants of GCKR affect both β-cell and kidney function in patients with newly diagnosed type 2 diabetes: The Verona newly diagnosed type 2 diabetes study 2. Diabetes Care 2011, 34, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, R.; Wang, C.; Yu, W.; Lu, J.; Ma, X.; Wang, J.; Jiang, F.; Tang, S.; Bao, Y.; Xiang, K.; Jia, W. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS ONE 2010, 5, e11761. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; Camastra, S.; Astiarraga, B.; Nannipieri, M.; Anselmino, M.; Ferrannini, E. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol. Metab. 2014, 4, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Chen, Y.K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.J.; Lu, Y.P.; Yang, C.S. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [PubMed]
- Byu, J.K.; Yoon, B.Y.; Jhun, J.Y.; Oh, H.J.; Kim, E.K.; Min, J.K.; Cho, M.L. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol. Lett. 2014, 157, 51–59. [Google Scholar] [CrossRef]
- Okuda, M.H.; Zemdegs, J.C.; de Santana, A.A.; Santamarina, A.B.; Moreno, M.F.; Hachui, A.C.; dos Santos, B.; do Nascimento, C.M.; Ribero, E.B.; Oyama, L.M. Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. J. Nutr. Biochem. 2014, 25, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zeng, K.; Shao, W.; Yang, B.B.; Fantus, I.G.; Weng, J.; Jin, T. Short-Term Curcumin Gavage Sensitizes Insulin Signaling in Dexamethasone-Treated C57BL/6 Mice. J. Nutr. 2015, 145, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gong, M.C.; Su, W.; Turk, J.; Guo, Z. Group VIA phospholipase A2 (iPLA2β) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J. Biol. Chem. 2007, 282, 25278–25289. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, W.; Guo, Z.; Pang, H.; Post, S.R.; Gong, M.C. Up-regulation of CPI-17 phosphorylation in diabetic vasculature and high glucose cultured vascular smooth muscle cells. Cardiovasc. Res. 2006, 69, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, D.; Liu, S.; Calderon, L.; Zhao, G.; Turk, J.; Guo, Z. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key Cis-regulatory element for RGS2 transcriptional regulation by angiontensin II in cultured vascular smooth muscles. J. Biol. Chem. 2011, 286, 44646–44658. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef] [PubMed]

| Caffeine | EGC | C | EC | EGCG | GCG | ECG | Theanine | |
|---|---|---|---|---|---|---|---|---|
| LWE (mg/g) | 18.766 ± 0.394 | 1.133 ± 0.464 | 0.536 ± 0.096 | 1.231 ± 0.045 | 13.774 ± 0.543 | 2.970 ± 0.107 | 4.117 ± 0.145 | 7.625 ± 1.257 |
| LYT (mg/g) | 8.906 ± 0.282 ** | 0.731 ± 0.003 *** | 0.184 ± 0.002 * | 0.811 ± 0.002 *** | 4.250 ± 0.023 *** | 0.877 ± 0.002 *** | 1.237 ± 0.004 *** | 0.648 ± 0.166 *** |
| Primer Sequences | ||
|---|---|---|
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
| 36b4 | CCC TGA AGT GCT CGA CAT CA | TGC GGA CAC CCT CCA GAA |
| Fasn | CGT GTG ACC GCC ATC TAT ATC G | TGA GGT TGC TGT CGT CTG TAG TCT T |
| Srebf1 | AGT CCA GCC TTT GAG GAT AGC C | CCG TAG CAT CAG AGG GAG TGA G |
| Acaca | AGG AGG GAA AGG GAT CAG AAA AG | CAG AGC AGT CAC GAC CAA ACA AA |
| Adipor2 | CCT TTC GGG CCT GTT TTA AGA | GAG TGG CAG TAC ACC GTG TG |
| Cpt1a | CTT CAA AAA CAG CAA GAT AGG CAT A | TTA CAG TGT CCA TCC TCT GAG TAG C |
| Ppara | TAC GCT CCC GAC CCA TCT TTA | GAC TCC TTG GCA GTG TCC ATC T |
| Pparg | GAA AGA CAA CGG ACA AAT CAC CAT | CGG CTT CTA CGG ATC GAA ACT G |
| Gck | AGA CGA AAC ACC AGA TGT ATT CC | GAA GCC CTT GGT CCA GTT GAG |
| Gckr | GAC CCG GAA CTT GGA CAA AG | AAT GCC ATA CGA CCA GAG GTG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Li, D.; Guruvaiah, P.; Xu, N.; Xie, Z. Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice. Nutrients 2018, 10, 75. https://doi.org/10.3390/nu10010075
Teng Y, Li D, Guruvaiah P, Xu N, Xie Z. Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice. Nutrients. 2018; 10(1):75. https://doi.org/10.3390/nu10010075
Chicago/Turabian StyleTeng, Yun, Daxiang Li, Ponmari Guruvaiah, Na Xu, and Zhongwen Xie. 2018. "Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice" Nutrients 10, no. 1: 75. https://doi.org/10.3390/nu10010075





