Gender-Associated Impact of Early Leucine Supplementation on Adult Predisposition to Obesity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Ethics Statement
2.3. Follow up and Sample Collection
2.4. Challenge Tests
2.5. Gene Expression
2.6. Morphometric and Immunohistochemical Analysis
2.7. Statistical Analysis
3. Results
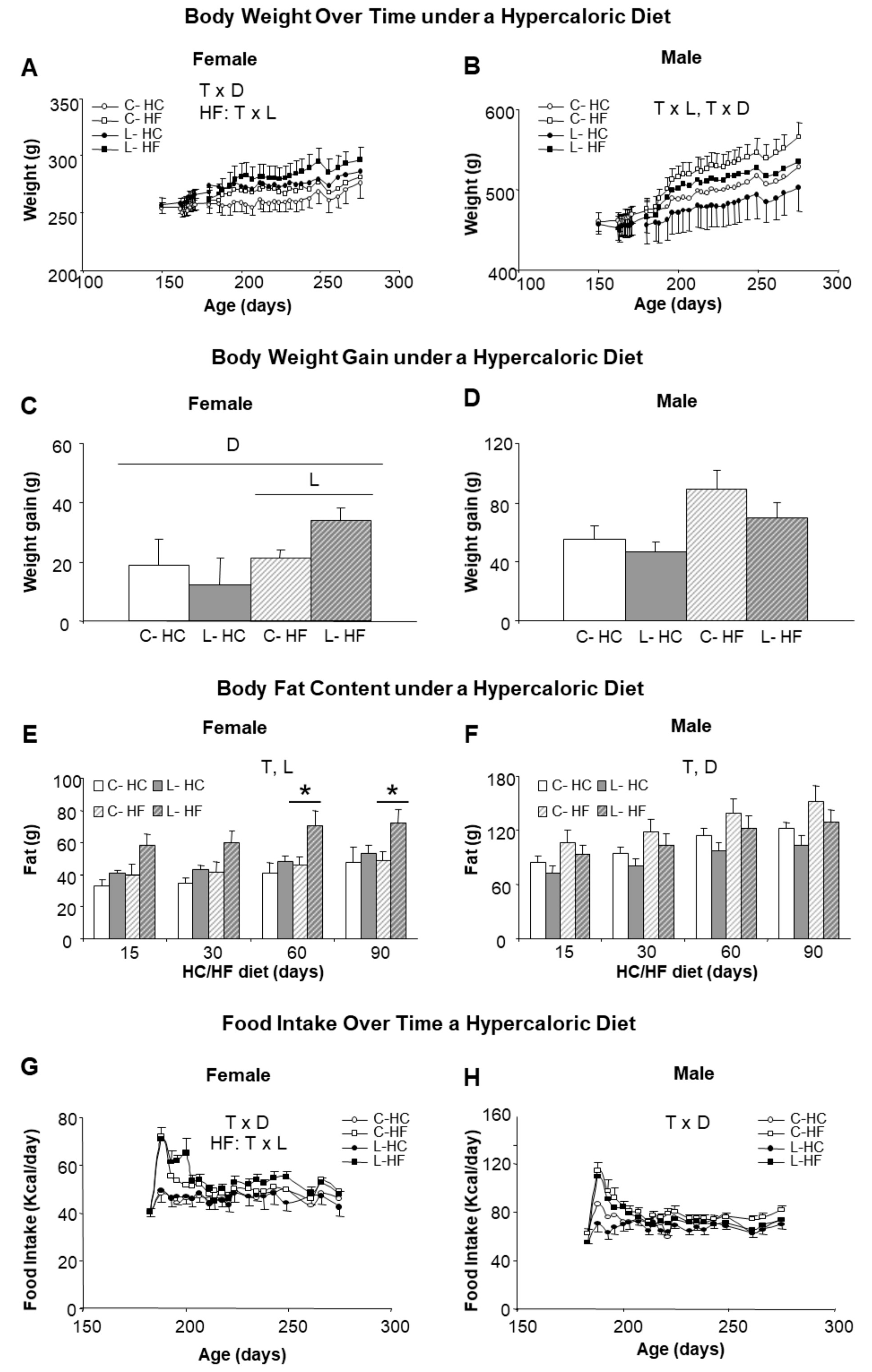
3.1. Maternal Leu Supplementation during Lactation Promoted Decreased Food Intake in Adult Offspring, Particularly for Carbohydrate Rich Diets, without Changes in Body Weight
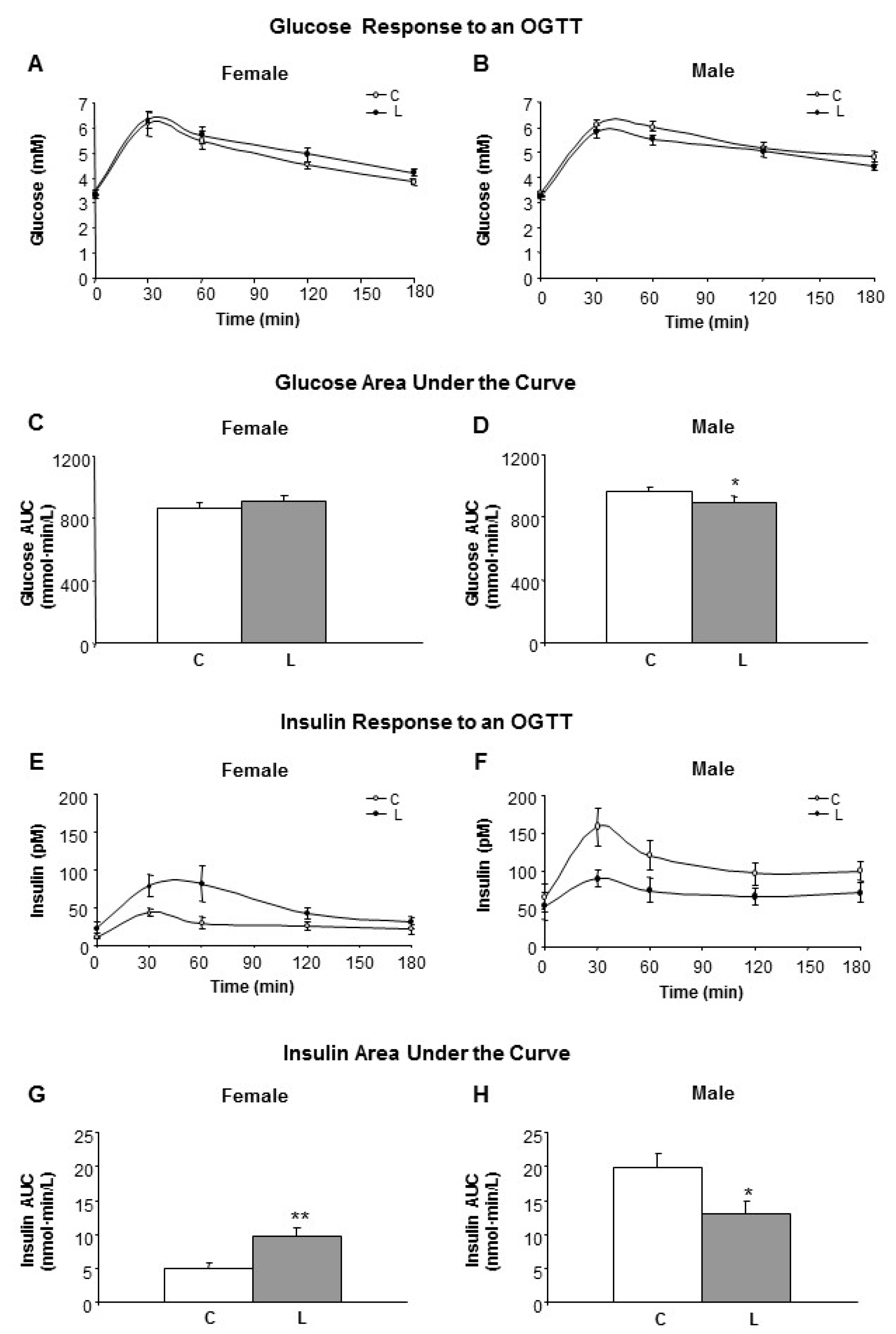
3.2. Glucose and Insulin Homeostasis Were Disturbed in Adult Offspring from Leu-Supplemented Dams in a Sex-Specific Manner
3.3. Higher Susceptibility to Adult Obesity Was Promoted by Early Maternal Leu-Supplementation in Female but Not in Male Offspring
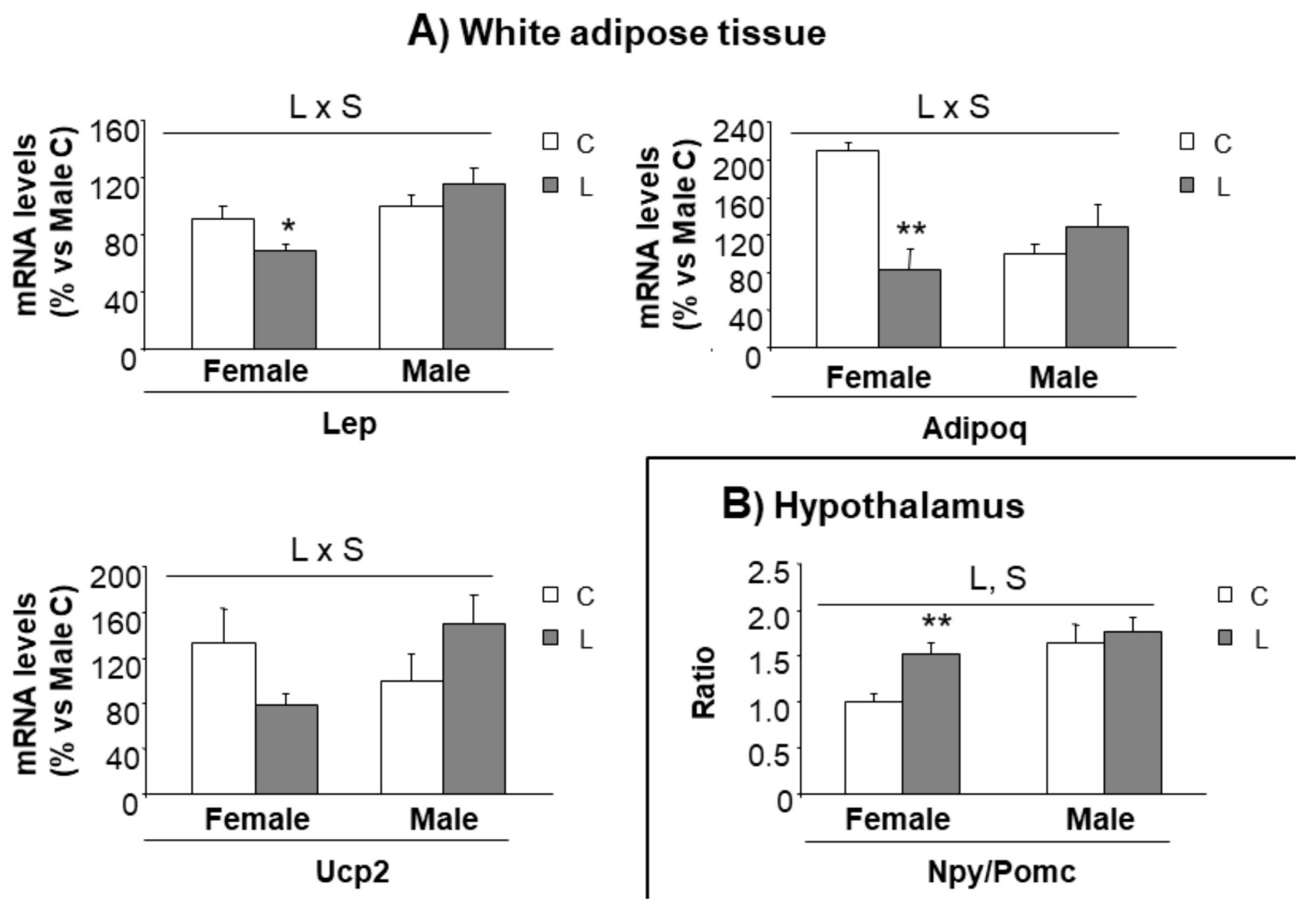
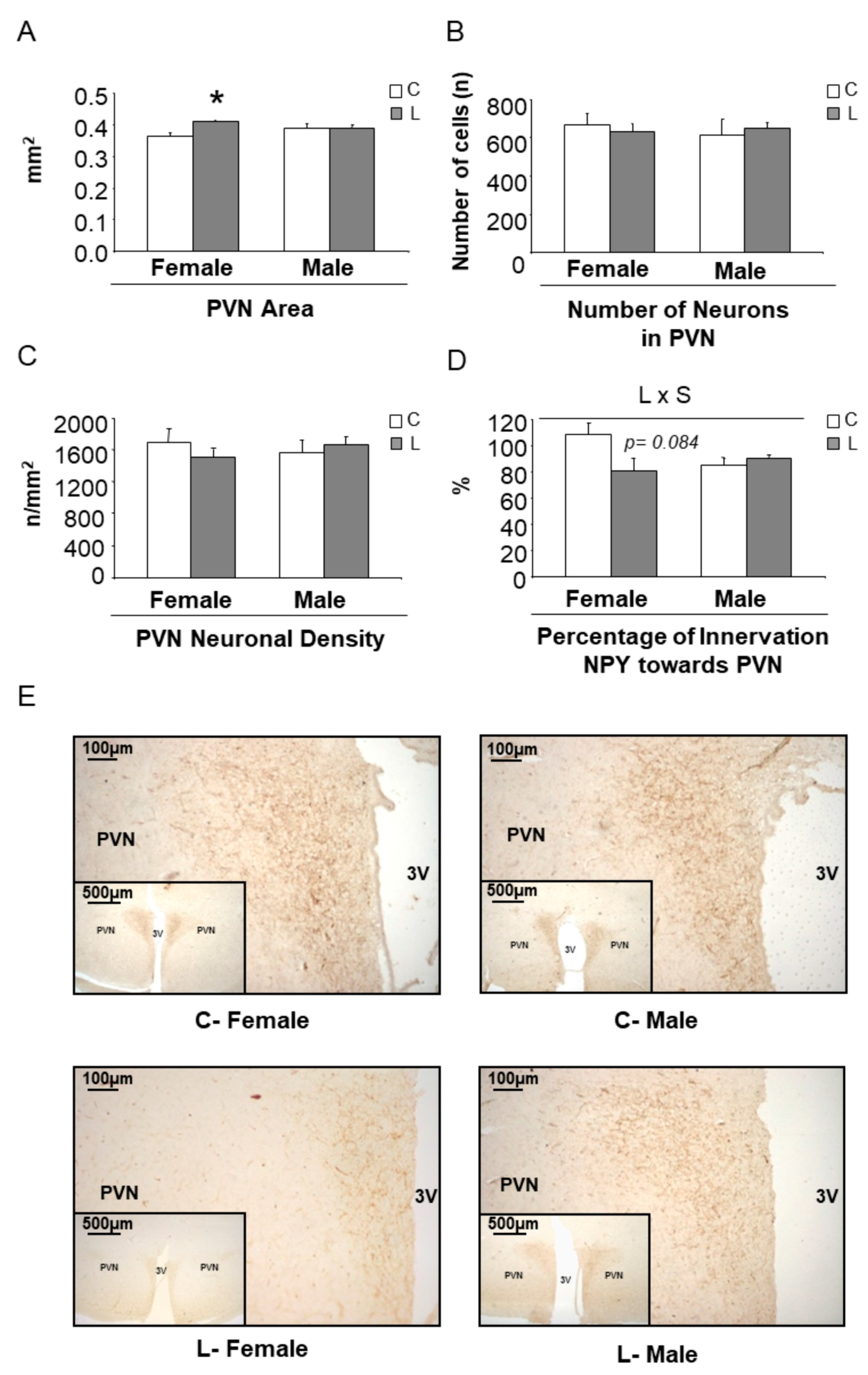
3.4. Early Leu Supplementation Was Associated with Long-Term Perturbations of Energy Balance Regulatory Signals in Hypothalamus
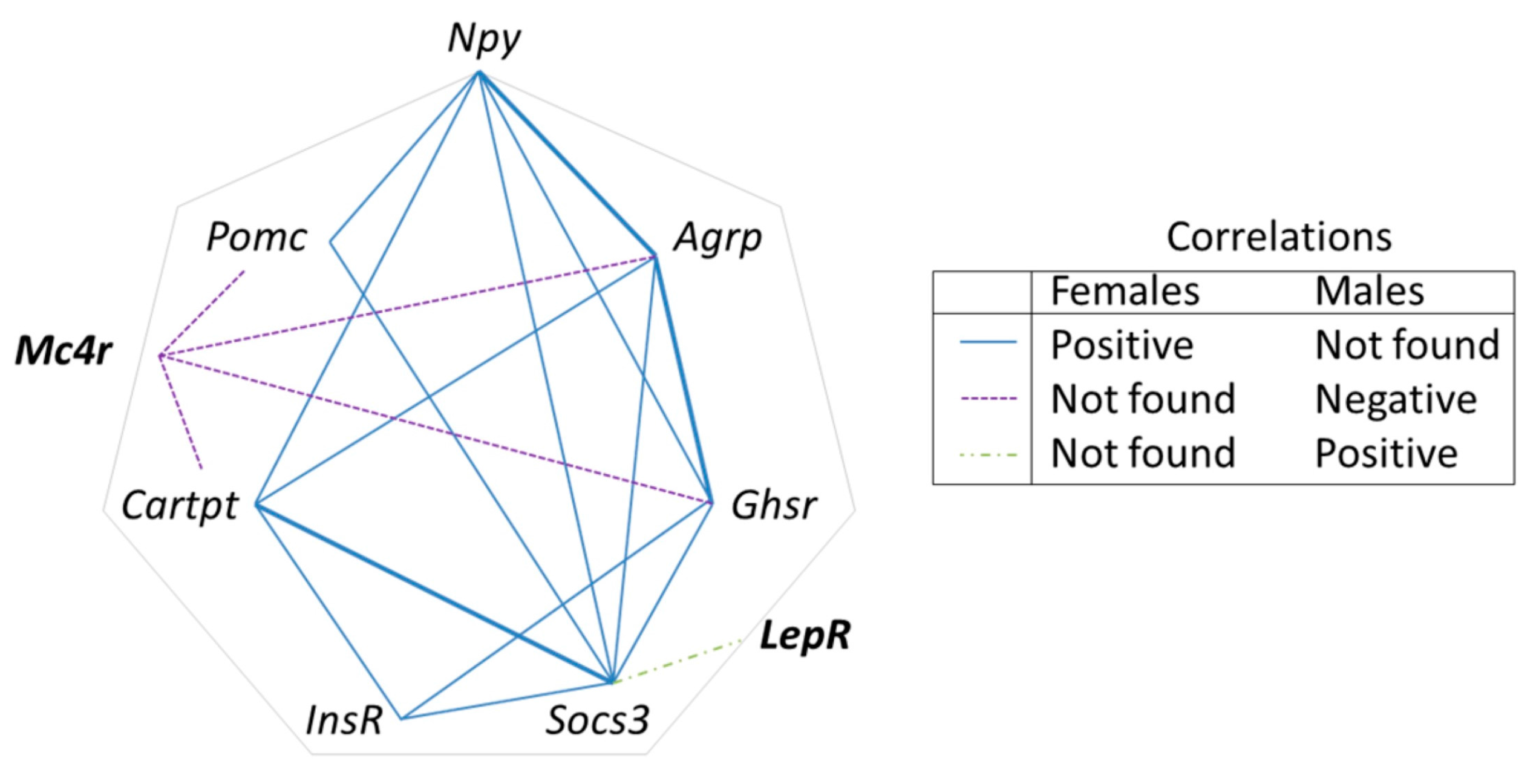
3.5. Selected Early Biomarkers at Weaning May Allow Identification of Susceptibility to Obesity in Adulthood
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Branca, F.; Nikogosian, H.; Lobstein, T. The Challenge of Obesity in the WHO European Region and the Strategies for Response, 1st ed.; WHO: Copenhagen, Denmark, 2007; ISBN 978-92-890-1408-3. [Google Scholar]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine—An environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottrell, E.C.; Ozanne, S.E. Early life programming of obesity and metabolic disease. Physiol. Behav. 2008, 94, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Early life origins of obesity: Role of hypothalamic programming. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. S1), S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E. Early determinants of development: A lipid perspective. Am. J. Clin. Nutr. 2009, 89, 1523S–1529S. [Google Scholar] [CrossRef] [PubMed]
- Pico, C.; Palou, A. Perinatal programming of obesity: An introduction to the topic. Front. Physiol. 2013, 4, 255. [Google Scholar] [CrossRef] [PubMed]
- Caspó, J.; Salamon, S. Composition of the mother’s milk i. Protein contents, amino acid composition, biological value. A review. Acta Univ. Sapientiae Aliment. 2009, 2, 174–195. [Google Scholar]
- Chavalittamrong, B.; Suanpan, S.; Boonvisut, S.; Chatranon, W.; Gershoff, S.N. Protein and amino acids of breast milk from thai mothers. Am. J. Clin. Nutr. 1981, 34, 1126–1130. [Google Scholar] [PubMed]
- Pedroso, J.A.; Zampieri, T.T.; Donato, J. Reviewing the effects of l-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [PubMed]
- Shah, S.H.; Crosslin, D.R.; Haynes, C.S.; Nelson, S.; Turer, C.B.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Laferrère, B.; et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rachdi, L.; Aïello, V.; Duvillié, B.; Scharfmann, R. L-leucine alters pancreatic β-cell differentiation and function via the mtor signaling pathway. Diabetes 2012, 61, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: Mtor signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Duan, Y.; Li, F.; Tan, B.; Hou, Y.; Wu, G.; Yin, Y. Leucine in obesity: Therapeutic prospects. Trends Pharmacol. Sci. 2016, 37, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Nairizi, A.; She, P.; Vary, T.C.; Lynch, C.J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr. 2009, 139, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Hutson, S.M.; Patson, B.J.; Vaval, A.; Vary, T.C. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E824–E835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (ucp1) in brown adipose tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, Q.; Meng, Q.; Xia, T.; Huang, Z.; Wang, C.; Liu, B.; Chen, S.; Xiao, F.; Du, Y.; et al. Leucine deprivation stimulates fat loss via increasing crh expression in the hypothalamus and activating the sympathetic nervous system. Mol. Endocrinol. 2011, 25, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine deprivation increases hepatic insulin sensitivity via gcn2/mtor/s6k1 and ampk pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Cheng, Y.; Zhang, Q.; Xiao, F.; Liu, B.; Chen, S.; Guo, F. S6k1 in the central nervous system regulates energy expenditure via mc4r/crh pathways in response to deprivation of an essential amino acid. Diabetes 2012, 61, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, B.; Cheng, Y.; Meng, Q.; Xia, T.; Jiang, L.; Chen, S.; Liu, Y.; Guo, F. Leptin signaling is required for leucine deprivation-enhanced energy expenditure. J. Biol. Chem. 2014, 289, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Sánchez, J.; Picó, C.; Palou, A.; Serra, F. Dietary l-leucine supplementation of lactating rats results in a tendency to increase lean/fat ratio associated to lower orexigenic neuropeptide expression in hypothalamus. Peptides 2010, 31, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Priego, T.; Palou, M.; Tobaruela, A.; Palou, A.; Picó, C. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 2008, 149, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (abc) in immunoperoxidase techniques: A comparison between abc and unlabeled antibody (pap) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A. Flavor preferences conditioned by sucrose depend upon training and testing methods: Two-bottle tests revisited. Physiol. Behav. 2002, 76, 633–644. [Google Scholar] [CrossRef]
- Takeda, M.; Imaizumi, M.; Fushiki, T. Preference for vegetable oils in the two-bottle choice test in mice. Life Sci. 2000, 67, 197–204. [Google Scholar] [CrossRef]
- Lamming, D.W.; Sabatini, D.M. A central role for mtor in lipid homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.D.; Baum, J.I.; Kimball, S.R.; Jefferson, L.S. Mechanisms involved in the coordinate regulation of mtorc1 by insulin and amino acids. J. Biol. Chem. 2011, 286, 8287–8296. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Heni, M.; Moss, A.; von Schnurbein, J.; Fritsche, A.; Häring, H.U.; Farooqi, S.; Preissl, H.; Wabitsch, M. Leptin therapy in a congenital leptin-deficient patient leads to acute and long-term changes in homeostatic, reward, and food-related brain areas. J. Clin. Endocrinol. Metab. 2011, 96, E1283–E1287. [Google Scholar] [CrossRef] [PubMed]
- Szostaczuk, N.; Priego, T.; Palou, M.; Palou, A.; Picó, C. Oral leptin supplementation throughout lactation in rats prevents later metabolic alterations caused by gestational calorie restriction. Int. J. Obes. 2017, 41, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, J.; García, A.P.; Sánchez, J.; Palou, M.; Palou, A.; Picó, C. Oral leptin treatment in suckling rats ameliorates detrimental effects in hypothalamic structure and function caused by maternal caloric restriction during gestation. PLoS ONE 2013, 8, e81906. [Google Scholar] [CrossRef] [PubMed]
- Resto, M.; O’Connor, D.; Leef, K.; Funanage, V.; Spear, M.; Locke, R. Leptin levels in preterm human breast milk and infant formula. Pediatrics 2001, 108, E15. [Google Scholar] [CrossRef] [PubMed]
- Mušinović, H.; Bonet, M.L.; Granados, N.; Amengual, J.; von Lintig, J.; Ribot, J.; Palou, A. B-carotene during the suckling period is absorbed intact and induces retinoic acid dependent responses similar to preformed vitamin a in intestine and liver, but not adipose tissue of young rats. Mol. Nutr. Food Res. 2014, 58, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Palou, M.; Priego, T.; Sánchez, J.; Palou, A. Metabolic programming of obesity by energy restriction during the perinatal period: Different outcomes depending on gender and period, type and severity of restriction. Front. Physiol. 2012, 3, 436. [Google Scholar] [CrossRef] [PubMed]
- Heerwagen, M.J.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R711–R722. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004, 20, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Travisano, M.; Tahiliani, K.G.; Rached, M.T.; Mirza, S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. 2008, 32, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Priego, T.; Romero, M.; Szostaczuk, N.; Konieczna, J.; Cabrer, C.; Remesar, X.; Palou, A.; Pico, C. Moderate calorie restriction during gestation programs offspring for lower bat thermogenic capacity driven by thyroid and sympathetic signaling. Int. J. Obes. 2015, 39, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Pomar, C.A.; Palou, A.; Picó, C.; Sánchez, J. Offspring predisposition to obesity due to maternal-diet-induced obesity in rats is preventable by dietary normalization before mating. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.A.; van Nes, R.; Sánchez, J.; Picó, C.; Keijer, J.; Palou, A. Maternal consumption of a cafeteria diet during lactation in rats leads the offspring to a thin-outside-fat-inside phenotype. Int. J. Obes. 2017, 41, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lin, S.P.; Lee, H.C.; Wang, T.J.; Shih, Y.S.; Huang, F.Y.; Yeung, C.Y. Free amino acids in full-term and pre-term human milk and infant formula. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Carratù, B.; Boniglia, C.; Riva, E.; Sanzini, E. Free amino acid content in standard infant formulas: Comparison with human milk. J. Am. Coll. Nutr. 2000, 19, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Knowledge gained from studies of leucine consumption in animals and humans. J. Nutr. 2012, 142, 2212S–2219S. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Excessive leucine-mtorc1-signalling of cow milk-based infant formula: The missing link to understand early childhood obesity. J. Obes. 2012, 2012, 197653. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2007; p. 1. [Google Scholar]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Individual amino acid requirements in humans: An update. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. Protein quantity and quality at levels above the rda improves adult weight loss. J. Am. Coll. Nutr. 2004, 23, 631S–636S. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Evans, E.M.; Erickson, D.; Seyler, J.; Weber, J.; Bagshaw, D.; Griel, A.; Psota, T.; Kris-Etherton, P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J. Nutr. 2009, 139, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Layman, D.K. Protein metabolic roles in treatment of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.C.; Thorpe, M.P.; Karampinos, D.C.; Johnson, C.L.; Layman, D.K.; Georgiadis, J.G.; Evans, E.M. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermúdez-Silva, F.J.; André, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, L.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, E74705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases sirt1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef] [PubMed]
- Eller, L.K.; Saha, D.C.; Shearer, J.; Reimer, R.A. Dietary leucine improves whole-body insulin sensitivity independent of body fat in diet-induced obese sprague-dawley rats. J. Nutr. Biochem. 2013, 24, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Simar, D.; Ting, J.H.; Erkelens, J.R.; Morris, M.J. Leucine improves glucose and lipid status in offspring from obese dams, dependent on diet type, but not caloric intake. J. Neuroendocrinol. 2012, 24, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Noatsch, A.; Petzke, K.J.; Millrose, M.K.; Klaus, S. Body weight and energy homeostasis was not affected in c57bl/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur. J. Nutr. 2011, 50, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J. Nutr. Biochem. 2012, 23, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Torrens, J.M.; Priego, T.; Sánchez, J.; Palou, A.; Picó, C. Moderate caloric restriction in lactating rats programs their offspring for a better response to HF diet feeding in a sex-dependent manner. J. Nutr. Biochem. 2011, 22, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Konieczna, J.; Torrens, J.M.; Sánchez, J.; Priego, T.; Fernandes, M.L.; Palou, A.; Picó, C. Impaired insulin and leptin sensitivity in the offspring of moderate caloric-restricted dams during gestation is early programmed. J. Nutr. Biochem. 2012, 23, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Catania, C.; Binder, E.; Cota, D. Mtorc1 signaling in energy balance and metabolic disease. Int. J. Obes. 2011, 35, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Priego, T.; Sánchez, J.; Palou, A.; Picó, C. Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr. Metab. 2010, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, J.; Sánchez, J.; van Schothorst, E.M.; Torrens, J.M.; Bunschoten, A.; Palou, M.; Picó, C.; Keijer, J.; Palou, A. Identification of early transcriptome-based biomarkers related to lipid metabolism in peripheral blood mononuclear cells of rats nutritionally programmed for improved metabolic health. Genes Nutr. 2014, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Torrens, J.M.; Konieczna, J.; Palou, M.; Sánchez, J.; Picó, C.; Palou, A. Early biomarkers identified in a rat model of a healthier phenotype based on early postnatal dietary intervention may predict the response to an obesogenic environment in adulthood. J. Nutr. Biochem. 2014, 25, 208–218. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, N.; Sánchez, J.; Palou, A.; Serra, F. Gender-Associated Impact of Early Leucine Supplementation on Adult Predisposition to Obesity in Rats. Nutrients 2018, 10, 76. https://doi.org/10.3390/nu10010076
López N, Sánchez J, Palou A, Serra F. Gender-Associated Impact of Early Leucine Supplementation on Adult Predisposition to Obesity in Rats. Nutrients. 2018; 10(1):76. https://doi.org/10.3390/nu10010076
Chicago/Turabian StyleLópez, Nora, Juana Sánchez, Andreu Palou, and Francisca Serra. 2018. "Gender-Associated Impact of Early Leucine Supplementation on Adult Predisposition to Obesity in Rats" Nutrients 10, no. 1: 76. https://doi.org/10.3390/nu10010076
APA StyleLópez, N., Sánchez, J., Palou, A., & Serra, F. (2018). Gender-Associated Impact of Early Leucine Supplementation on Adult Predisposition to Obesity in Rats. Nutrients, 10(1), 76. https://doi.org/10.3390/nu10010076






