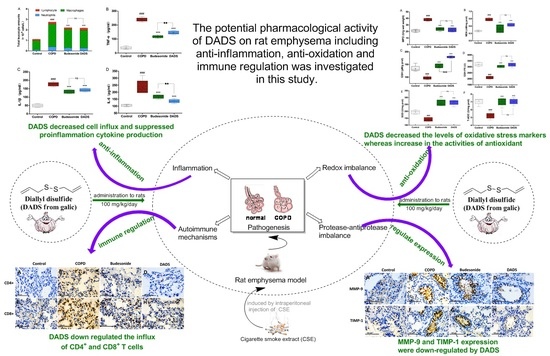
Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Preparation of Cigarette Smoke Extract (CSE)
2.4. Animal Model
2.5. Preparation and Analysis of Bronchoalveolar Lavage Fluid (BALF)
2.6. Sampling the Lung Tissue and Homogenization
2.7. Hematoxylin and Eosin Stain for the Morphology Assessment of Lung Tissues
2.8. Enzyme-Linked Immunosorbent Assay (ELISA) for Inflammatory Mediators
2.9. Antioxidant Defense and Oxidative Stress Biomarkers in Lung Homogenate
2.10. Western Blotting Analysis for Expression of Proteins
2.11. Immunohistochemistry for the Expression of CD4+, CD8+ T cells, MMP-9 and TIMP-1
2.12. Statistical Analysis
3. Results
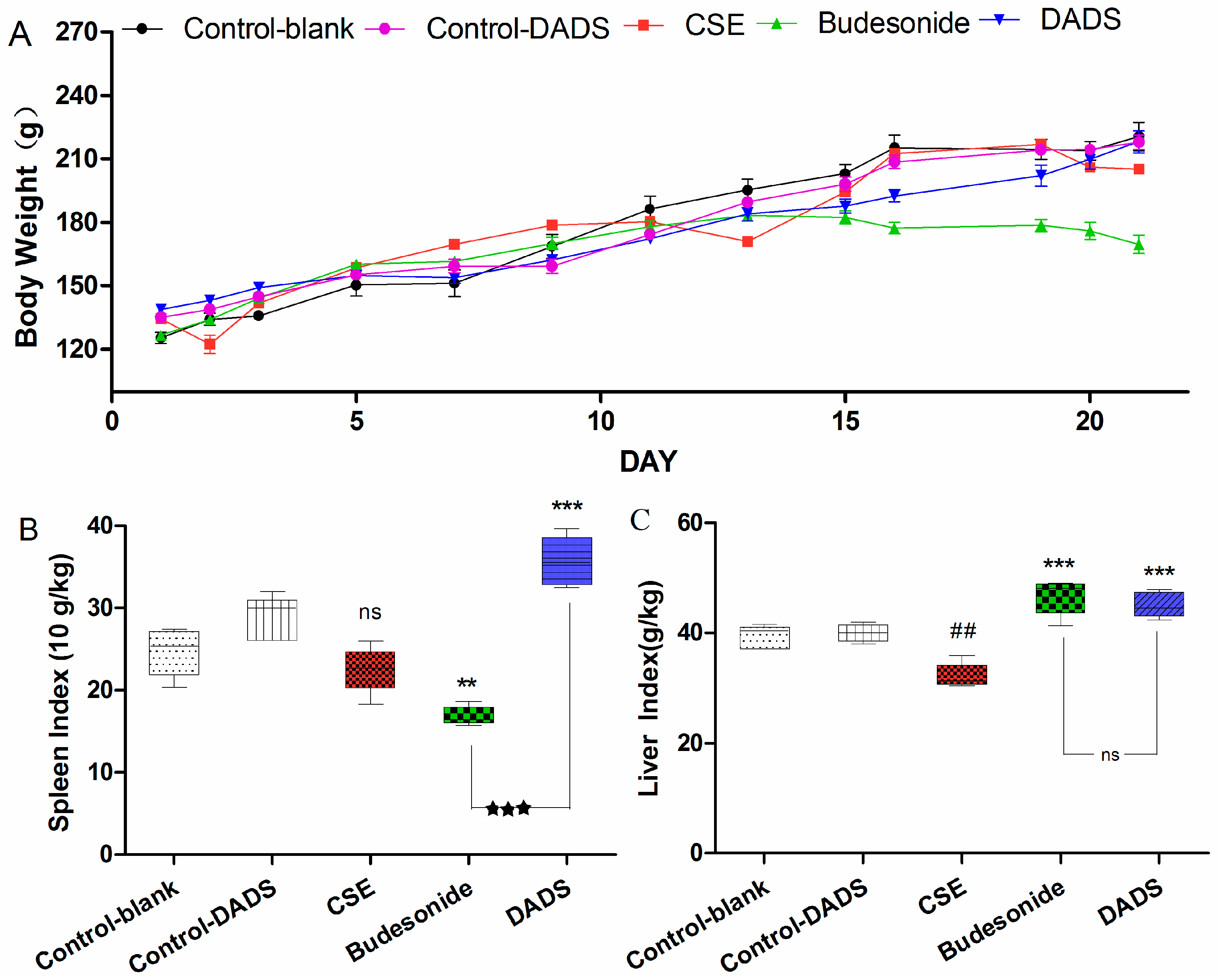
3.1. General Observation
3.2. Body Weight and Spleen/Liver Index
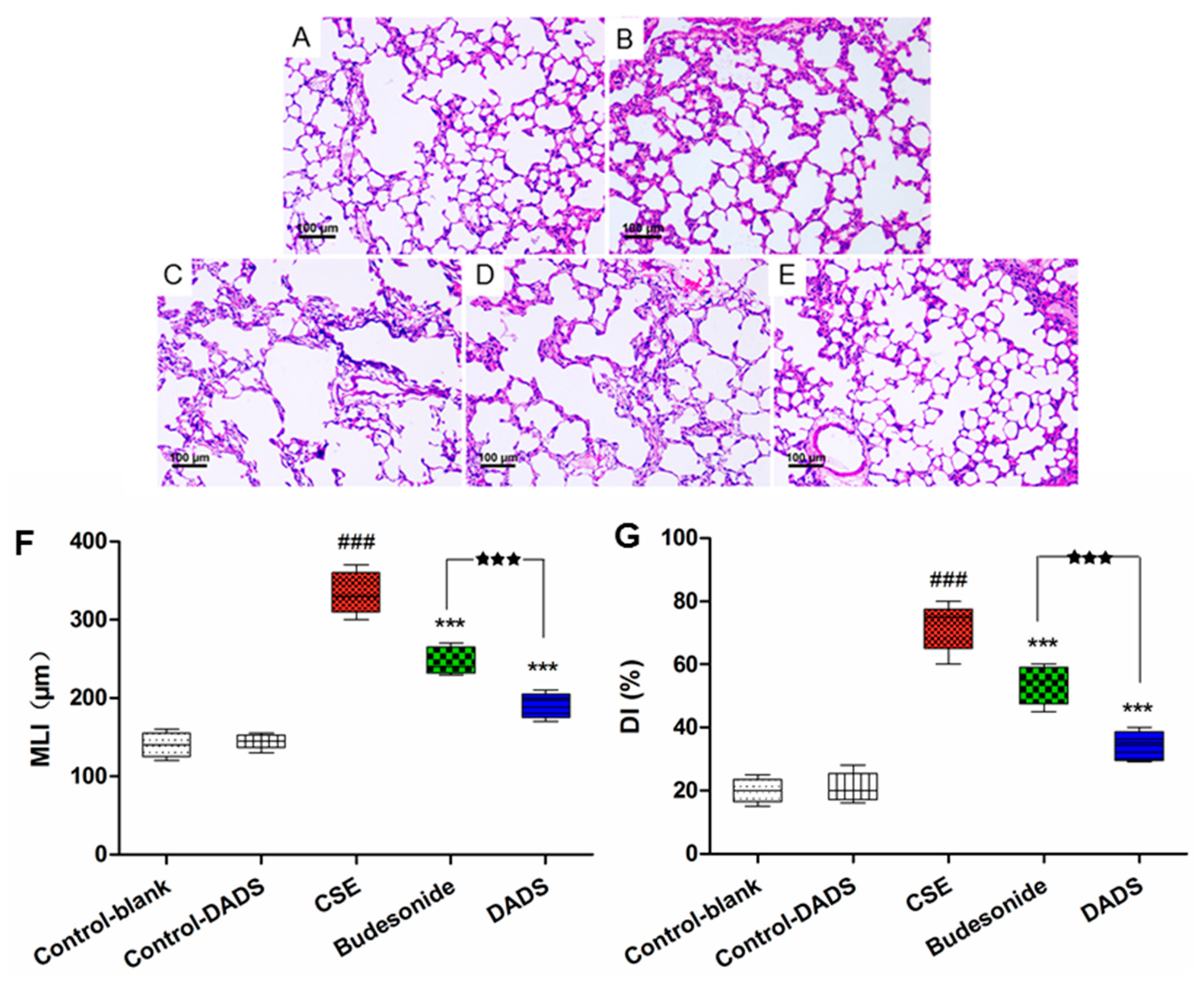
3.3. Morphological Findings of Lung Tissues
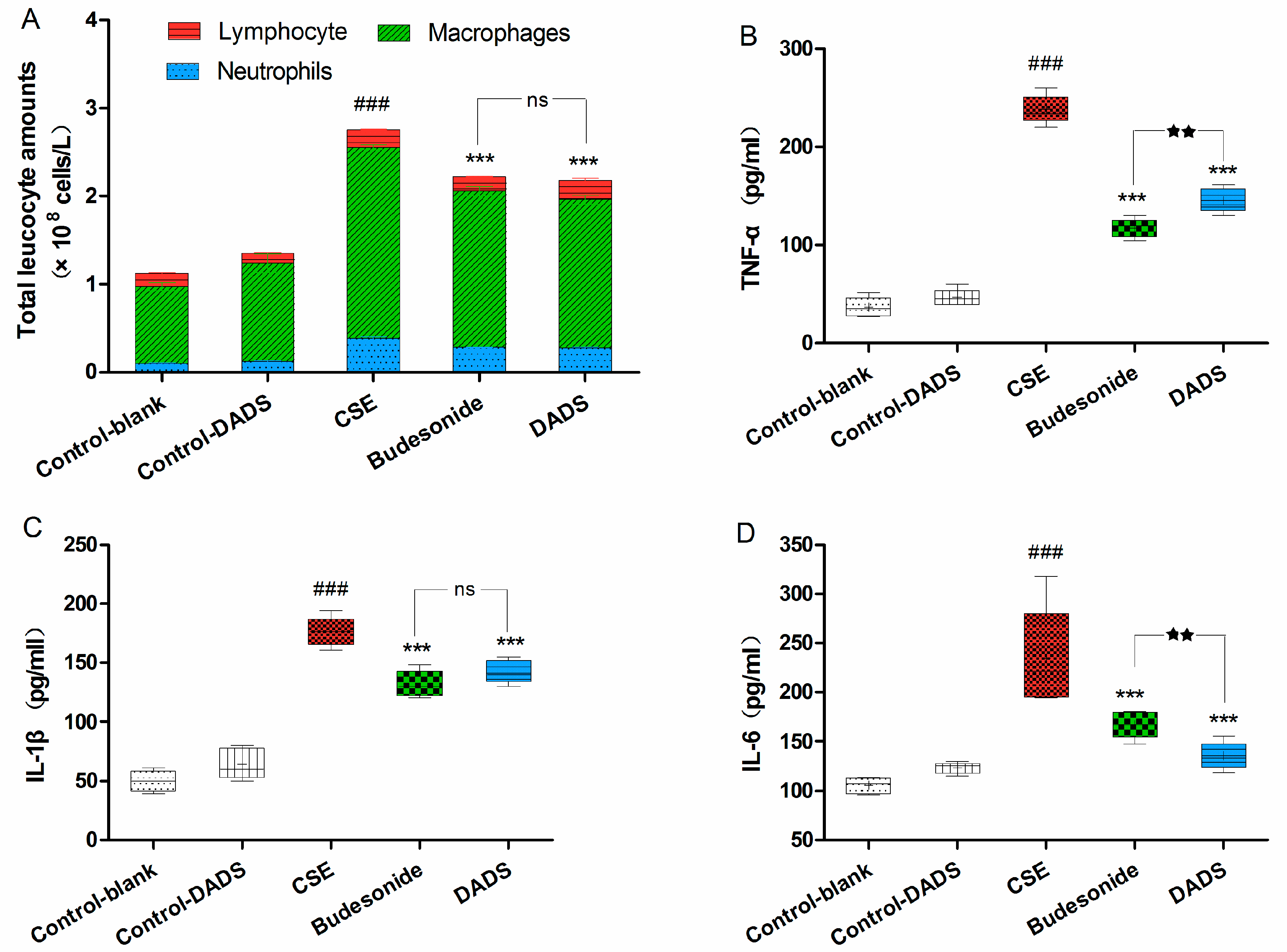
3.4. Cellular Influx in BALF
3.5. Evaluation of the Levels of Inflammatory Cytokines in Lung Homogenate
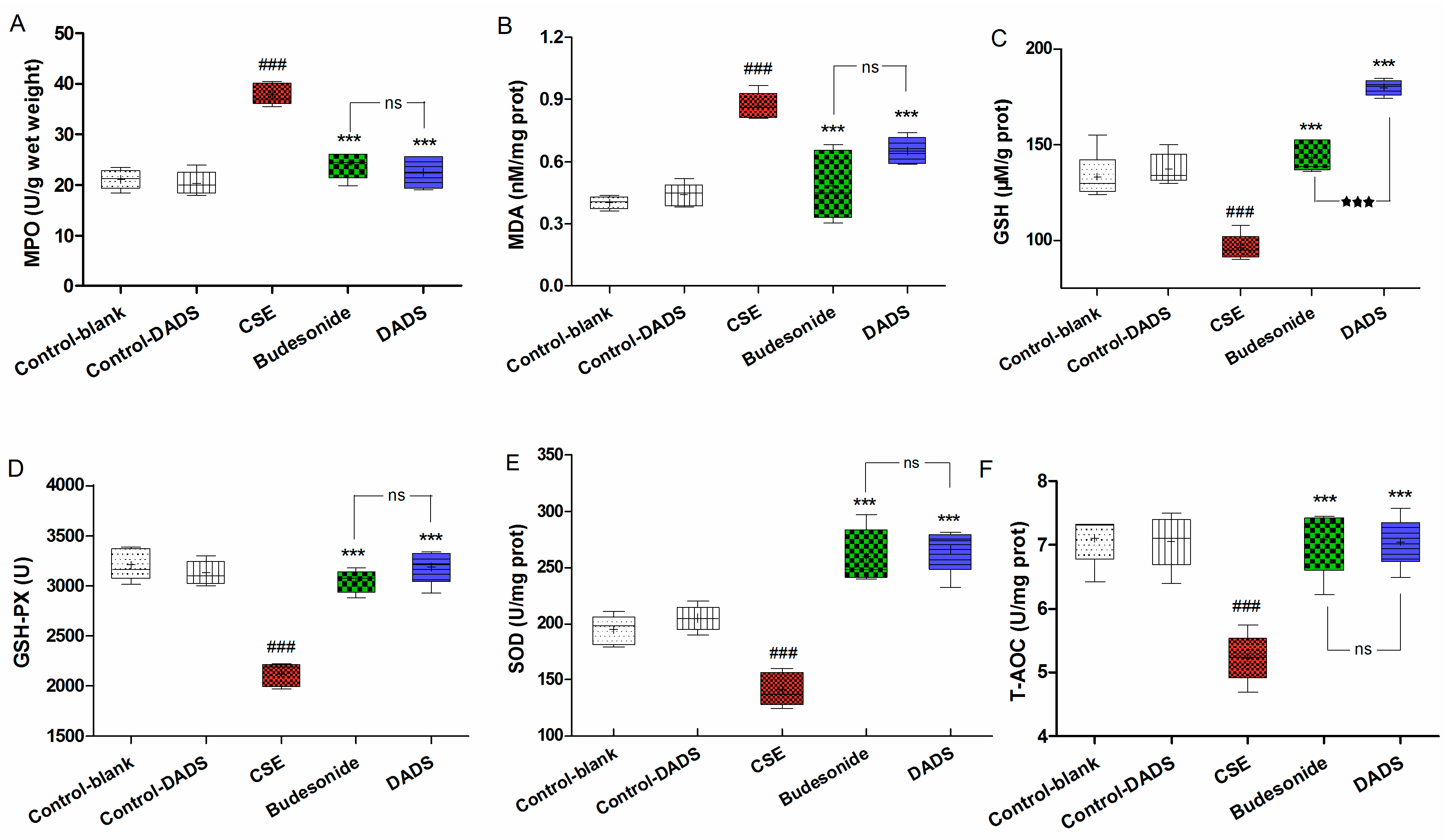
3.6. Effects of DADS on Oxidative Stress Markers (MPO, MDA) and Antioxidants (GSH, GSH-PX, SOD, T-AOC) in Lung Parenchyma
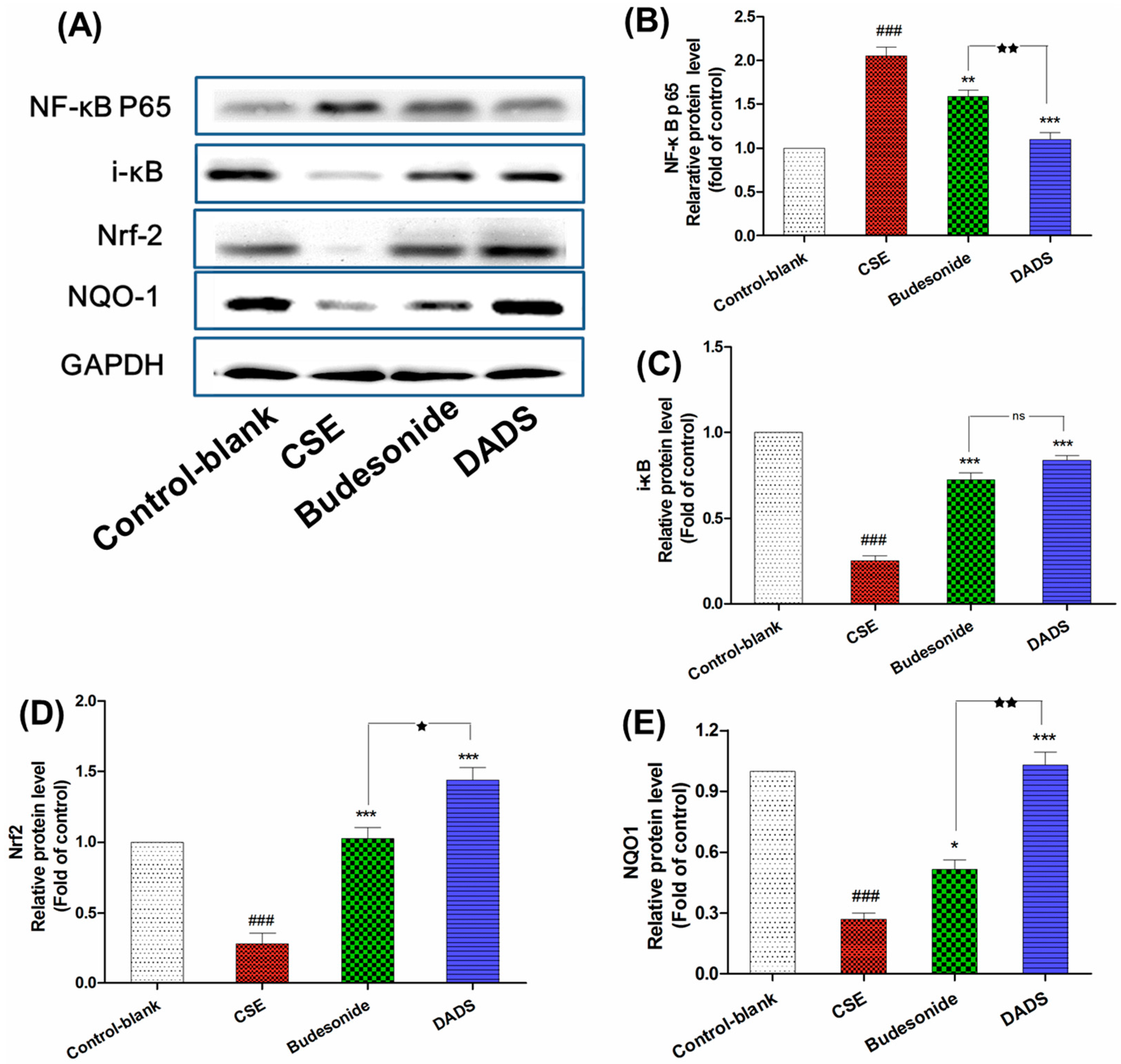
3.7. Effect of DADS on the Protein Expression of NF-κB p65, i-κB, Nrf2 and NQO1
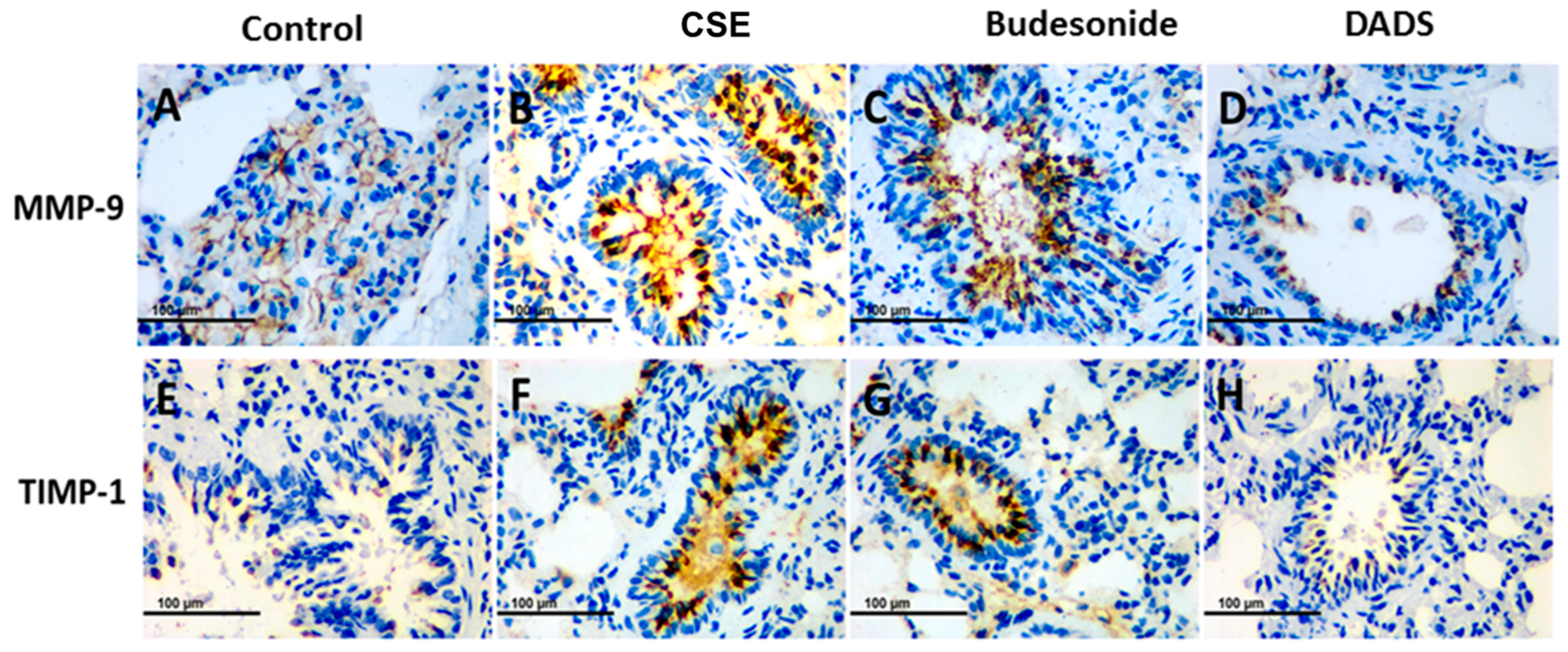
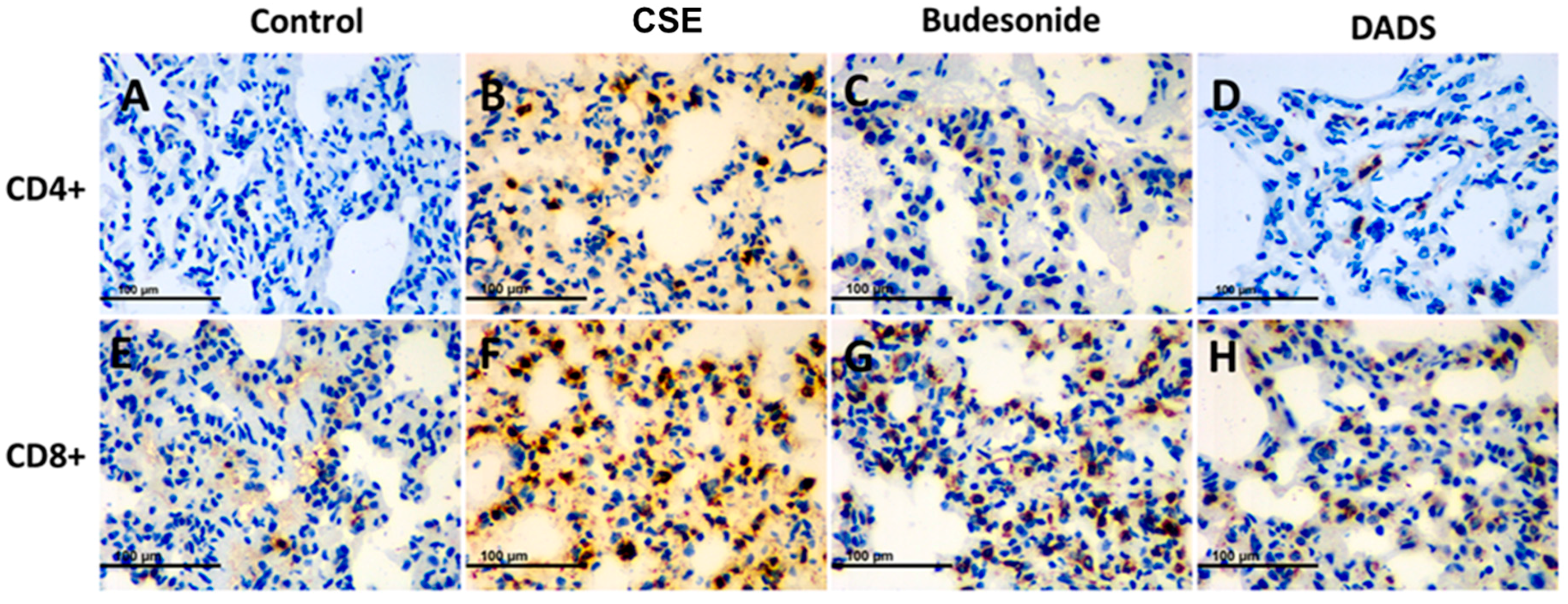
3.8. Immunohistochemistry for the Expression of the MMP-9, TIMP-1, CD4+ and CD8+ T cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.D.; Hersh, C.P. Integrative genomics of chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 2014, 452, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Wang, L.; Tao, Y.X.; Suo, X.L.; Li, Y.C.; Liu, F.; Zhao, Y.; Zhang, Q. Psychometric properties of the anxiety inventory for respiratory disease in patients with COPD in China. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluna, J.J. Gold in 2017: A view from the Spanish COPD guidelines (GesCOPD). Arch. Bronconeumol. 2017, 53, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Xie, J.G.; Zhao, J.P.; Xu, Y.J.; Yang, S.F.; Ni, W. Involvement of Bcl-2 family in apoptosis and signal pathways induced by cigarette smoke extract in the human airway smooth muscle cells. DNA Cell Biol. 2009, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Rahman, I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: Cellular and molecular mechanisms. Cell Biochem. Biophys. 2005, 43, 167–188. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2009, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Cosio, M.G.; Hoidal, J.R. Cigarette smoke-induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, X.; Lu, Y.J.; Rong, G.S.; Yang, R.Q.; Zhang, R.X.; Wang, G.Q.; Wei, X.Q.; Ye, Y.Q.; Qian, Z.X.; et al. A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respir. Med. 2016, 121, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Luo, Y.; Nian, Y.; Liu, D.; Yin, X.; Wu, J.; Di, J.; Zhang, R.; Zhang, J. Diallyl disulfide suppresses the inflammation and apoptosis resistance induced by DCA through ROS and the NF-κB signaling pathway in human barrett’s epithelial cells. Inflammation 2017, 40, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Hong, J.; Jeon, C.M.; Shin, N.R.; Kwon, O.K.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappab suppression. Food Chem. Toxicol. 2013, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Jafari, D.; Kamarul, T.; Bagheri, A.; Sharifi, A.M. Evaluating the protective effects and mechanisms of diallyl disulfide on interlukin-1β-induced oxidative stress and mitochondrial apoptotic signaling pathways in cultured chondrocytes. J. Cell. Biochem. 2017, 118, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim. Biophys. Acta 2013, 1830, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kwon, H.; Park, K.H.; Ko, J.K.; Kim, J.H.; Hwang, M.S.; Yum, Y.N.; Kim, O.H.; Kim, J.; Kim, H.T.; et al. Protective effect of diallyl disulfide on oxidative stress-injured neuronally differentiated PC12 cells. Mol. Brain Res. 2005, 133, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Gonzalez-Orozco, A.E.; Maldonado, P.D.; Barrera, D.; Medina-Campos, O.N.; Hernandez-Pando, R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur. J. Pharmacol. 2003, 473, 71–78. [Google Scholar] [CrossRef]
- Fanelli, S.L.; Castro, G.D.; de Toranzo, E.G.; Castro, J.A. Mechanisms of the preventive properties of some garlic components in the carbon tetrachloride-promoted oxidative stress. Diallyl sulfide; diallyl disulfide; allyl mercaptan and allyl methyl sulfide. Res. Commun. Mol. Pathol. Pharmacol. 1998, 102, 163–174. [Google Scholar] [PubMed]
- Horn, N.; Miller, G.; Ajuwon, K.M.; Adeola, O. Garlic diallyl disulfide and diallyl trisulfide mitigates effects of pro-oxidant induced cellular stress and has immune modulatory function in LPS-stimulated porcine epithelial cells. J. Anim. Sci. 2017, 95, 4045–4051. [Google Scholar] [PubMed]
- Lee, I.C.; Kim, S.H.; Baek, H.S.; Moon, C.; Kang, S.S.; Kim, S.H.; Kim, Y.B.; Shin, I.S.; Kim, J.C. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem. Toxicol. 2014, 63, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, N.D.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, W.J.; Choi, Y.H. Inhibitory effects of diallyl disulfide on the production of inflammatory mediators and cytokines in lipopolysaccharide-activated BV2 microglia. Toxicol. Appl. Pharmacol. 2012, 262, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Identification and In-Vitro and In-Vivo Metabolic Studies of Active Compounds of Novel Garlic Nasal Inhalants. Master’s Thesis, Shandong University, Jinan, China, 2014. (In Chinese). [Google Scholar]
- He, Z.H.; Chen, P.; Chen, Y.; He, S.D.; Ye, J.R.; Zhang, H.L.; Cao, J. Comparison between cigarette smoke-induced emphysema and cigarette smoke extract-induced emphysema. Tob. Induc. Dis. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Li, J.; Gan, Y.; Chen, Y.; Chen, P. Changes in the number of CD31−CD45−Sca-1+ cells and Shh signaling pathway involvement in the lungs of mice with emphysema and relevant effects of acute adenovirus infection. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, J.; Chen, Y.; Chen, P.; Peng, H.; Cai, S.; Luo, H.; Wu, S.J. Intraperitoneal injection of cigarette smoke extract induced emphysema, and injury of cardiac and skeletal muscles in BALB/C mice. Exp. Lung Res. 2013, 39, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.H.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Gera, L.; Tuder, R.M.; Voelkel, N.F. Methylprednisolone causes matrix metalloproteinase-dependent emphysema in adult rats. Am. J. Respir. Crit. Care Med. 2003, 167, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Shiner, R.J.; Angus, G.E.; Kim, W.D.; Wang, N.S.; King, M.; Ghezzo, H.; Cosio, M.G. Destructive index: A measurement of lung parenchymal destruction in smokers. Am. Rev. Respire. Dis. 1985, 131, 764–769. [Google Scholar]
- Zhu, X.; Jiang, X.; Li, A.; Sun, Y.; Liu, Y.; Sun, X.; Feng, X.; Li, S.; Zhao, Z. S-allylmercaptocysteine suppresses the growth of human gastric cancer xenografts through induction of apoptosis and regulation of MAPK and PI3K/Akt signaling pathways. Biochem. Biophys. Res. Commun. 2017, 491, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Legora-Machado, A.; Cardilo-Reis, L.; Valenca, S.; Porto, L.C.; Walker, C.; Zuany-Amorim, C.; Koatz, V.L.G. Inhibition of interleukin-1β reduces mouse lung inflammation induced by exposure to cigarette smoke. Eur. J. Pharmacol. 2004, 498, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Gosu, V.; Choi, S. Evolutionary, structural and functional interplay of the IκB family members. PLoS ONE 2013, 8, e54178. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Voynow, J.A.; Ghio, A.J. COPD: Balancing oxidants and antioxidants. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Piesiak, P.; Brzecka, A.; Kosacka, M.; Passowicz-Muszynska, E.; Dyla, T.; Jankowska, R. Concentrations of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in serum of patients with chronic obstructive pulmonary disease. Polski Merkur. Lek. 2011, 31, 270–273. [Google Scholar]
- Curtis, J.L.; Freeman, C.M.; Hogg, J.C. The immunopathogenesis of chronic obstructive pulmonary disease: Insights from recent research. Proc. Am. Thorac. Soc. 2007, 4, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Roos-Engstrand, E.; Ekstrand-Hammarstrom, B.; Pourazar, J.; Behndig, A.F.; Bucht, A.; Blomberg, A. Influence of smoking cessation on airway T lymphocyte subsets in COPD. COPD 2009, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Willen, R.; Brattsand, R.; Erlansson, M.; Svensjo, E. Topical anticolitic efficacy and selectivity of the glucocorticoid budesonide in a new model of acetic acid-induced acute colitis in the rat. Aliment. Pharmacol. Therap. 1994, 8, 433–441. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D. The macrophage in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Hazewindus, M.; Haenen, G.R.; Weseler, A.R.; Bast, A. Protection against chemotaxis in the anti-inflammatory effect of bioactives from tomato ketchup. PLoS ONE 2014, 9, e114387. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.L.; Wang, Y.X.; Li, X.P.; Qian, Z.B. Up-regulation of ICAM-1mRNA and IL-1βmRNA in lung tissues of a rat model of COPD. Int. J. Clin. Exp. Med. 2015, 8, 21956–21963. [Google Scholar] [PubMed]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.W.; Lee, K.W.; Kim, D.; Lee, Y.; Jeon, O.H.; Kwon, H.J.; Kim, D.S. Nf-κB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line raw 264.7. J. Biochem. Mol. Biol. 2007, 40, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kluchova, Z.; Petrasova, D.; Joppa, P.; Dorkova, Z.; Tkacova, R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol. Res. 2007, 56, 51–56. [Google Scholar] [PubMed]
- MacNee, W. Oxidants/antioxidants and COPD. Chest 2000, 117, 303S–317S. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.R.; Phadke, M.S. Plasma oxidant-antioxidant status in different respiratory disorders. Indian J. Clin. Biochem. 2006, 21, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Costarelli, L. Metalloproteases/anti-metalloproteases imbalance in chronic obstructive pulmonary disease: Genetic factors and treatment implications. Curr. Opin. Pulm. Med. 2011, 17, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Muroski, M.E.; Roycik, M.D.; Newcomer, R.G.; Van den Steen, P.E.; Opdenakker, G.; Monroe, H.R.; Sahab, Z.J.; Sang, Q.X. Matrix metalloproteinase-9/gelatinase B is a putative therapeutic target of chronic obstructive pulmonary disease and multiple sclerosis. Curr. Pharm. Biotechnol. 2008, 9, 34–46. [Google Scholar] [PubMed]
- Pardo, A.; Selman, M. Proteinase-antiproteinase imbalance in the pathogenesis of emphysema: The role of metalloproteinases in lung damage. Histol. Histopathol. 1999, 14, 227–233. [Google Scholar] [PubMed]
- Zhuo, S.; Li, N.; Zheng, Y.; Peng, X.; Xu, A.; Ge, Y. Expression of the lymphocyte chemokine XCL1 in lung tissue of COPD mice, and its relationship to CD4+/CD8+ ratio and IL-2. Cell Biochem. Biophys. 2015, 73, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Bajoriunas, V.; Sakalauskas, R. Distribution of CD4+ and CD8+ T cells in tumor islets and stroma from patients with non-small cell lung cancer in association with COPD and smoking. Medicina (Kaunas) 2015, 51, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Godet, Y.; Fabre, E.; Dosset, M.; Lamuraglia, M.; Levionnois, E.; Ravel, P.; Benhamouda, N.; Cazes, A.; Le Pimpec-Barthes, F.; Gaugler, B.; et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: Potential synergistic effect with chemotherapy response. Clin. Cancer Res. 2012, 18, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- OShaughnessy, T.C.; Ansari, T.W.; Barnes, N.C.; Jeffery, P.K. Inflammation in bronchial biopsies of subjects with chronic bronchitis: Inverse relationship of CD8+ T lymphocytes with FEV1. Am. J. Respir. Crit. Care Med. 1997, 155, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Larypoor, M.; Bayat, M.; Zuhair, M.H.; Akhavan Sepahy, A.; Amanlou, M. Evaluation of the number of CD4+ CD25+ FoxP3+ treg cells in normal mice exposed to AFB1 and treated with aged garlic extract. Cell J. 2013, 15, 37–44. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, A.; Feng, X.; Sun, X.; Zhu, X.; Zhao, Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients 2018, 10, 79. https://doi.org/10.3390/nu10010079
Liu Y, Li A, Feng X, Sun X, Zhu X, Zhao Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients. 2018; 10(1):79. https://doi.org/10.3390/nu10010079
Chicago/Turabian StyleLiu, Yan, Ang Li, Xiuli Feng, Xiao Sun, Xiaosong Zhu, and Zhongxi Zhao. 2018. "Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract" Nutrients 10, no. 1: 79. https://doi.org/10.3390/nu10010079