Protein, Amino Acid, Fatty Acid Composition, and in Vitro Digestibility of Bread Fortified with Oncorhynchus tschawytscha Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fish Powder Preparation
2.3. Preparation of Bread
2.4. Proximate Chemical Composition Analysis of Bread
2.5. Moisture Content
2.6. Volume, Density, and Texture Properties of Bread
2.7. Color Measurements
2.8. In Vitro Starch Digestion Process
2.9. Amino Acid Profile and Scoring
2.10. In Vitro Protein Digestibility, Protein Digestibility Corrected Amino Acid Score (PDCAAS), and Nutritional Index
2.11. Fatty Acid Profile
2.12. Determination of Total Phenolic Content
2.13. Analysis of Antioxidant Properties
2.14. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Physical Properties of Bread
3.2. Texture and Color Measurement of Bread
3.3. Protein Quality of Bread
3.3.1. Amino Acid Profile and Amino Acid Scoring of Bread Samples
3.3.2. In Vitro Protein Digestibility (IVPD), Protein Digestibility Corrected Amino Acid Score (PDCAAS), and Nutritional Index of Bread
3.4. Fatty Acid Profile of Bread
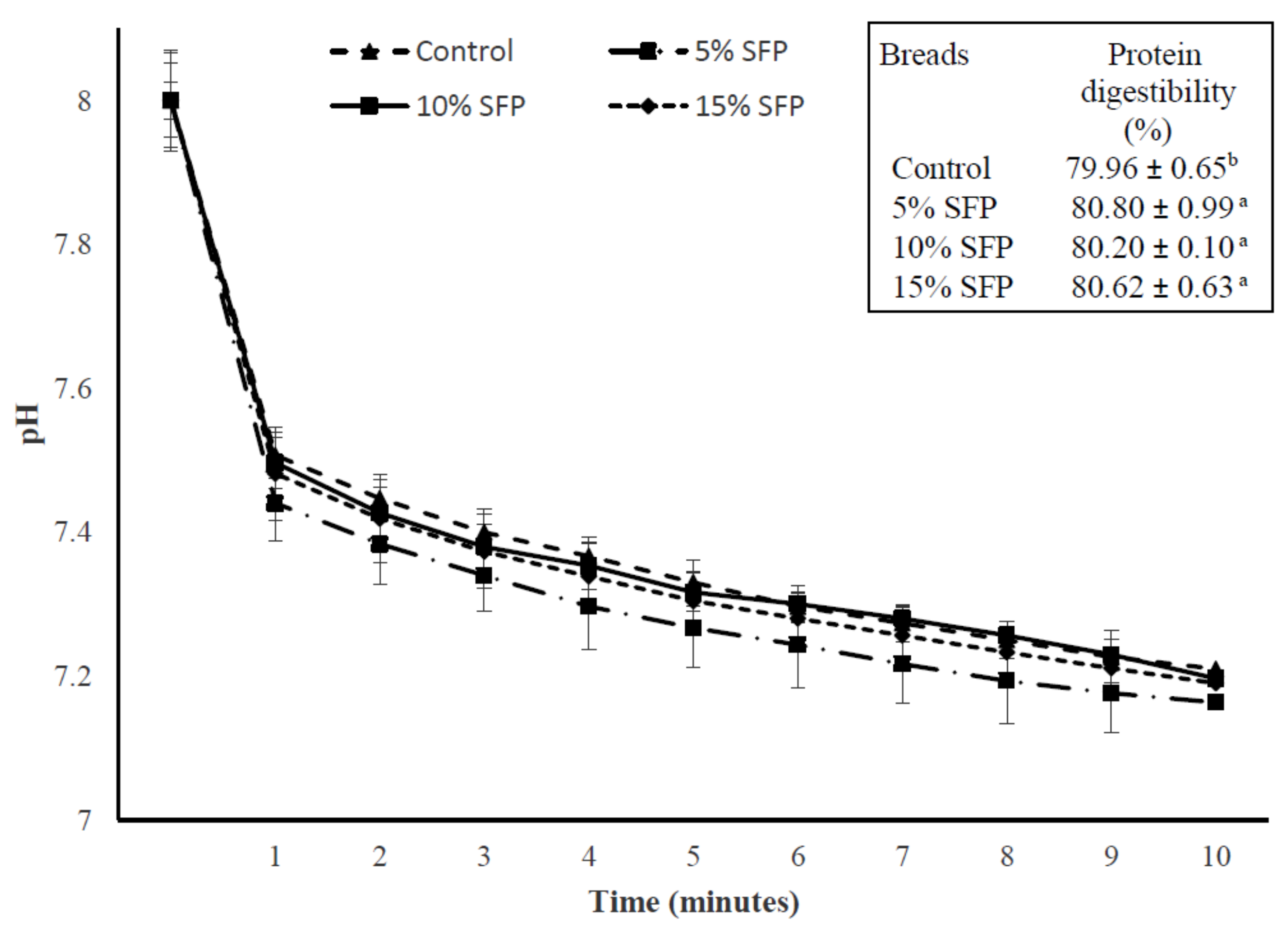
3.5. In Vitro Starch Digestion Analysis
3.6. Total Phenolic Content (TPC) and Antioxidant Capacity of Bread
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Jiang, Q.; Xu, Y.; Xia, W. Effects of washing and membrane removal pretreatments on th eantioxidant properties of grass carp (Ctenopharyngodon idella) protein hydrolysates produced by in vitro digestion. Int. J. Food Sci. Technol. 2017, 52, 1260–1268. [Google Scholar] [CrossRef]
- Berríos, M.M.; Rodriguez, A.; Rivera, M.; Pando, M.E.; Valenzuela, M.A.; Aubourg, S.P. Optimisation of rancidity stability in long-chain PUFA concentrates obtained from a rainbow trout (Oncorhynchus mykiss) by-product. Int. J. Food Sci. Technol. 2017, 52, 1463–1472. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Abril, R. Production and nutraceutical properties of breads fortified with DHA- and Omega-3-containing oils. Flour Breads Fortif. Heal. Dis. Prev. 2011. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wandersleben, T.; Morales, E.; Burgos-Díaz, C.; Barahona, T.; Labra, E.; Rubilar, M.; Salvo-Garrido, H. Enhancement of functional and nutritional properties of bread using a mix of natural ingredients from novel varieties of flaxseed and lupine. LWT Food Sci. Technol. 2018, 91, 48–54. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Brennan, C.S. Incorporation of mushroom powder into bread dough-effects on dough rheology and bread properties. Cereal. Chem. 2018. [Google Scholar] [CrossRef]
- Parvathy, U.; Bindu, J.; Joshy, C.G. Development and optimization of fish-fortified instant noodles using response surface methodology. Int. J. Food Sci. Technol. 2017, 53, 608–616. [Google Scholar] [CrossRef]
- Graca, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT Food Sci. Technol. 2018, 89, 466–474. [Google Scholar] [CrossRef]
- Freitas, D.; Le Feunteun, S.; Panouille, M.; Souchon, I. The important role of salivary α-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef]
- Liu, W.; Brennan, M.; Serventi, L.; Brennan, C. Buckwheat flour inclusion in Chinese steamed bread: Potential reduction in glycemic response and effects on dough quality. Eur. Food Res. Technol. 2017, 243, 727–734. [Google Scholar] [CrossRef]
- Seczyk, Ł.; Swieca, M.; Dziki, D.; Anders, A.; Gawlik-Dziki, U. Antioxidant, nutritional and functional characteristics of wheat bread enriched with ground flaxseed hulls. Food Chem. 2017, 214, 32–38. [Google Scholar] [CrossRef]
- Turfani, V.; Narducci, V.; Durazzo, A.; Galli, V.; Carcea, M. Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT Food Sci. Technol. 2017, 78, 361–366. [Google Scholar] [CrossRef]
- Fagundes, G.A.; Rocha, M.; Salas-Mellado, M.M. Improvement of protein content and effect on technological properties of wheat. Food Res. 2018, 2, 221–227. [Google Scholar] [CrossRef]
- Desai, A.; Brennan, M.A.; Brennan, C.S. The effect of semolina replacement with protein powder from fish (Pseudophycis bachus) on the physicochemical characteristics of pasta. LWT Food Sci. Technol. 2018, 89, 52–57. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Senousi, N.A.; Ali, Z.A.; Omran, A.A. The impact of using chickpea flour and dried carp fish powder on pizza quality. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Lemus-Mondaca, R.; Vega-Galvez, A.; Ah-Hen, K.; Puente-Diaz, L.; Zura-Bravo, L.; Aubourg, S. Influence of air-drying temperature on drying kinetics, colour, firmness and biochemical characteristics of Atlantic salmon (Salmo salar L.) fillets. Food Chem. 2013, 139, 162–169. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- AACC Approved methods of the American Association of Cereal Chemists, 10th ed.; American Association of Cereal Chemistis: St. Paul, MN, USA, 2000.
- Lu, X.; Brennan, M.A.; Serventi, L.; Mason, S.; Brennan, C.S. How the inclusion of mushroom powder can affect the physicochemical characteristics of pasta. Int. J. Food Sci. Technol. 2016, 51, 2433–2439. [Google Scholar] [CrossRef]
- Heems, D.; Luck, G.; Fraudeau, C.; Verette, E. Fully automated precolumn derivatization, on-line dialysis and high- performance liquid chromatographic analysis of amino acids in food, beverages and feedstuff. J. Chromatogr. A. 1998, 798, 9–17. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Protein and amino acid requirements in human nutrition. Rep. Jt. WHO/FAO/UNU Expert Consult; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A.A. Multienzyme technique for estimating protein digestibility. J. Food Sci 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Sarwar, G. The protein digestibility-corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. J. Nutr. 1997, 127, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L.; Jenkins, T.C. Challenges with fats and fatty acid methods. J. Anim. Sci. 2003, 81, 3250–3254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pickard, M.D.; Beta, T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple wheat grain. J. Agric. Food Chem. 2007, 55, 8958–8966. [Google Scholar] [CrossRef]
- Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of astaxanthin-rich microalgae “Haematococcus pluvialis” and wholemeal flours incorporation in improving the physical and functional properties of cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Sayed Ahmad, B.; Talou, T.; Straumite, E.; Sabovics, M.; Kruma, Z.; Saad, Z.; Hijazi, A.; Merah, O. Protein bread fortification with cumin and caraway Seeds and by-product flour. Foods 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishnaraj, M.; Roopa, B.S.; Prabhasankar, P. Preparation of gluten free bread enriched with green mussel (Perna canaliculus) protein hydrolysates and characterization of peptides responsible for mussel flavour. Food Chem. 2016, 211, 715–725. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Wronkowska, M.; Soral-Smietana, M.; Haros, M. Effect of whole amaranth flour on bread properties and nutritive value. LWT Food Sci. Technol. 2013, 50, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Reshmi, S.K.; Sudha, M.L.; Shashirekha, M.N. Starch digestibility and predicted glycemic index in the bread fortified with pomelo (Citrus maxima) fruit segments. Food Chem. 2017, 237, 957–965. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT Food Sci. Technol. 2017, 82, 296–302. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Foley, R.; Fanning, K.; Johnson, S.K. The effects of lupin (Lupinus angustifolius) addition to wheat bread on its nutritional, phytochemical and bioactive composition and protein quality. Food Res. Int. 2015, 76, 58–65. [Google Scholar] [CrossRef]
- Lorusso, A.; Verni, M.; Montemurro, M.; Coda, R.; Gobbetti, M.; Rizzello, C.G. Use of fermented quinoa flour for pasta making and evaluation of the technological and nutritional features. LWT Food Sci. Technol. 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Coelho, M.S.; Salas-Mellado, M.d.l.M. Effects of substituting chia (Salvia hispanica L.) flour or seeds for wheat flour on the quality of the bread. LWT Food Sci. Technol. 2015, 60, 729–736. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Food, E.; Authority, S. Outcome of the Public consultation on the Draft Opinion of the Scientific Panel on Dietetic Products, Nutrition, and Allergies ( NDA ) on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. Eur. Food Safety Authority 2010, 8, 1–232. [Google Scholar]
- Ramya, N. S.; Prabhasankar, P.; Gowda, L.R.; Modi, V.K.; Bhaskar, N. Influence of freeze-dried shrimp meat in pasta processing qualities of Indian T. durum wheat. J. Aquat. Food Prod. Technol. 2014. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Siripatrawan, U.; Teekachunhatean, S.; Wangtueai, S.; Seesuriyachan, P.; Surawang, S.; Laokuldilok, T.; Regenstein, J.M.; Henry, C.J. Technological properties, in virto starch digestibility and in vivo glycaemic index of bread containing crude malva nut gum. Int. J. Food Sci. Technol. 2017, 52, 1035–1041. [Google Scholar] [CrossRef]
- Annor, G.A.; Marcone, M.; Corredig, M.; Bertoft, E.; Seetharaman, K. Effects of the amount and type of fatty acids present in millets on their in vitro starch digestibility and expected glycemic index (eGI). J. Cereal. Sci. 2015, 64, 76–81. [Google Scholar] [CrossRef]
- Sopade, P.A. Cereal processing and glycaemic response. Int. J. Food Sci. Technol. 2017, 52, 22–37. [Google Scholar] [CrossRef]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Enrichment of extruded snack products with coproducts from chestnut mushroom (Agrocybe aegerita) production: Interactions between dietary fiber, physicochemical characteristics, and glycemic load. J. Agric. Food Chem. 2012, 60, 4396–4401. [Google Scholar] [CrossRef]
- Swieca, M.; Seczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves-The influence of protein-phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyz, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]





| A | |||||||
| Sample code | |||||||
| Ingredients (g) | Control | 5% SFP | 10% SFP | 15% SFP | |||
| Wheat flour | 150 | 142.5 | 135 | 127.5 | |||
| Salmon powder | - | 7.5 | 15 | 22 | |||
| Salt | 2.25 | 2.25 | 2.25 | 2.25 | |||
| Sugar | 9 | 9 | 9 | 9 | |||
| Yeast | 2.25 | 2.25 | 2.25 | 2.25 | |||
| Butter | 7.5 | 7.5 | 7.5 | 7.5 | |||
| Water | 90 | 90 | 90 | 90 | |||
| B | |||||||
| Sample code | SFP | Control | 5% SFP | 10% SFP | 15% SFP | ||
| Protein (%) | 58.06 ± 0.13 | 13.91 ± 0.19 d | 16.27 ± 0.08 c | 18.22 ± 0.06 b | 20.04 ± 0.10 a | ||
| Fat (%) | 38.55 ± 0.09 | 3.86 ± 0.02 d | 6.03 ± 0.06 c | 7.26 ± 0.08 b | 9.13 ± 0.02 a | ||
| Ash (%) | 1.37 ± 0.06 | 2.13 ± 0.02 b | 2.36 ± 0.05 a | 2.42 ± 0.09 a | 2.42 ± 0.09 a | ||
| Moisture (%) | 2.15 ± 0.03 | 34.38 ± 0.55 a | 31.42 ± 0.42 a | 32.90 ± 0.47 a | 33.33 ± 0.73 a | ||
| Carbohydrate (%) | - | 80.10 ± 0.18 a | 75.35 ± 0.11 b | 72.10 ± 0.19 c | 68.42 ± 0.11 d | ||
| Energy (Kcal) | 579.55 ± 0.30 | 410.8 ± 0.18 d | 420.71 ± 0.17 c | 426.62 ± 0.44 b | 435.96 ± 0.36 a | ||
| C | |||||||
| Sample code | Width/height ratio (mm) | Volume (mL) | Specific volume (mL/g) | ||||
| Control | 1.80 ± 0.12 b | 111 ± 1.00 a | 2.47± 0.02 c | ||||
| 5% SFP | 1.77 ± 0.38 a | 103 ± 1.15 b | 2.35 ± 0.01 b | ||||
| 10% SFP | 1.74 ± 0.15 a | 97 ± 0.58 c | 2.18 ± 0.00 a | ||||
| 15% SFP | 1.77 ± 0.10 a | 96 ± 1.05 c | 2.16 ± 0.02 a | ||||
| A | ||||
| Parameter | Control | 5% SFP | 10% SFP | 15% SFP |
| Hardness (g) | 1082.77 ± 0.20 b | 1648.48 ± 44.88 a | 1525.91 ± 60.81 a | 1198.41 ± 78.59 b |
| Springiness (mm) | 0.935 ± 0.03 a | 0.892 ± 0.01 b | 0.882 ± 0.03 b | 0.879 ± 0.01 b |
| Gumminess (g) | 788.99 ± 5.01 b | 946.54 ± 5.26 a | 962.11 ± 86.19 a | 776.15 ± 33.23 b |
| Chewiness (g) | 737.25 ± 10.79 b | 835.58 ± 18.70 a | 886.89 ± 76.60 a | 674.79 ± 42.26 c |
| Cohesiveness (ratio) | 0.73 ± 0.01 a | 0.58 ± 0.02 b | 0.62 ± 0.02 b | 0.62 ± 0.02 b |
| Resilience (ratio) | 0.35 ± 0.01 a | 0.27 ± 0.01 b | 0.29 ± 0.02 b | 0.29 ± 0.01 b |
| B | ||||
| Crust Color | ||||
| Sample code | Control | 5% SFP | 10% SFP | 15% SFP |
| L* | 91.45 ± 0.32 a | 85.46 ± 0.11 b | 86.05 ± 0.38 b | 85.95 ± 0.22 b |
| a* | 9.97 ± 0.27 a | 2.17 ±0.63 b | 1.58 ± 0.37 bc | 0.66 ± 0.23 c |
| b* | 36.65 ±0.07 a | 32.57 ± 0.09 b | 33.42 ± 0.91 b | 33.73 ± 0.41 b |
| ΔE | - | 10.67 ± 0.34 a | 10.49 ± 0.34 a | 11.26 ± 1.08 a |
| Crumb Color | ||||
| Sample code | Control | 5% SFP | 10% SFP | 15% SFP |
| L* | 95.31 ± 0.12 b | 96.54 ± 0.11 a | 96.59 ± 0.16 a | 96.31 ± 0.06 a |
| a* | 12.23 ± 0.02 c | 12.96 ± 0.23 b | 13.23 ± 0.12 ab | 13.53 ± 0.12 a |
| b* | 30.92 ± 0.08 d | 32.55 ± 0.56 c | 33.92 ± 0.26 b | 35.54 ± 0.03 a |
| ΔE | - | 2.23 ± 0.69 a | 3.41 ± 0.27 b | 4.89 ± 0.23 c |
| A | ||||
| Amino acid | Control | 5% SFP | 10% SFP | 15% SFP |
| Phenylalanine | 42.24 ± 0.19 a | 38.12 ± 2.30 ab | 40.33 ± 2.50 ab | 37.48 ± 0.92 b |
| Tyrosine | 18.66 ± 2.08 b | 23.62 ± 1.20 ab | 25.81 ± 3.37 a | 26.47 ± 0.33 a |
| Histidine | 20.75 ± 1.33 a | 17.79 ± 1.12 a | 20.42 ± 0.89 a | 19.42 ± 0.75 a |
| Isoleucine | 30.31 ± 0.81 b | 30.63 ± 1.84 b | 34.58 ± 1.87 a | 34.64 ± 0.68 a |
| Leucine | 62.18 ± 0.57 a | 61.13 ± 3.68 a | 68.25 ± 3.72 b | 66.50 ± 1.41 b |
| Lysine | 9.69 ± 1.14 c | 16.42 ± 1.10 b | 23.76 ± 1.68 a | 26.94 ± 1.00 a |
| Methionine | 10.88 ± 0.65 c | 13.71 ± 0.63 b | 16.93 ± 1.19 a | 17.42 ± 0.22 a |
| Cysteine | 11.69 ± 0.98 a | 10.35 ± 0.57 ab | 9.56 ± 0.61 b | 8.64 ± 0.52 b |
| Threonine | 23.22 ± 0.22 b | 24.46 ± 1.62 b | 29.78 ± 1.36 a | 30.52 ± 0.59 a |
| Valine | 32.17 ± 0.90 b | 32.86 ± 1.93 b | 37.50 ± 2.07 a | 37.57 ± 0.71 a |
| ΣEAAs | 261.75 ± 9.23 a | 269.14 ± 8.69 b | 306.96 ± 7.89 c | 305.65 ± 6.76 c |
| B. Amino acid score a | ||||
| Histidine | 1.15 ± 0.08 a | 0.99 ± 0.06 a | 1.13 ± 0.04 a | 1.07 ± 0.04 a |
| Isoleucine | 0.98 ± 0.03 b | 0.99 ± 0.05 b | 1.12 ± 0.06 a | 1.12 ± 0.02 a |
| Leucine | 0.99 ± 0.01 a | 0.97 ± 0.05 a | 1.08 ± 0.05 a | 1.05 ± 0.02 a |
| Lysine | 0.19 ± 0.02 c | 0.32 ± 0.02 b | 0.46 ± 0.03 a | 0.52 ± 0.01 a |
| Phenylalanine + Tyrosine | 1.32 ± 0.05 a | 1.34 ± 0.05 a | 1.43 ± 0.06 a | 1.39 ± 0.02 a |
| Methionine + Cysteine | 0.87 ± 0.06 b | 0.92 ± 0.02 ab | 1.01 ± 0.01 a | 1.00 ± 0.03 a |
| Threonine | 0.86 ± 0.02 b | 0.91 ± 0.06 b | 1.10 ± 0.05 a | 1.13 ± 0.02 a |
| Valine | 0.77 ± 0.03 b | 0.78 ± 0.04 b | 0.89 ± 0.04 a | 0.89 ± 0.01 a |
| C. Nutritional parameters | ||||
| IVPD (%) | 79.96 ± 0.65 a | 80.80 ± 0.99 a | 80.20 ± 0.10 a | 80.60 ± 0.73 a |
| PDCAAS b | 0.15 ± 0.06 a | 0.26 ± 0.05 b | 0.37 ± 0.02 c | 0.42 ± 0.06 d |
| EAAI | 62.51 ± 1.15 a | 71.44 ± 1.28 b | 76.76 ± 1.95 c | 76.68 ± 1.40 c |
| BV | 56.44 ± 1.05 a | 66.18 ± 1.22 b | 71.97 ± 1.56 c | 71.68 ± 1.10 c |
| NI | 8.69 ± 0.10 a | 11.62 ± 0.19 b | 13.98 ± 0.38 c | 15.36 ± 0.21 d |
| Fatty acid | Control | 5% SFP | 10% SFP | 15% SFP |
|---|---|---|---|---|
| Saturated Fatty Acids (SFA) | ||||
| C12:0 | 3.68 ± 0.01 a | 2.69 ± 0.02 b | 1.99 ± 0.01 c | 1.70 ± 0.00 d |
| C14:0 | 9.74 ± 0.06 a | 7.64 ± 0.07 b | 6.11 ± 0.04 c | 5.56 ± 0.01 d |
| C15:0 | 1.77 ± 0.01 a | 1.36 ± 0.01 b | 1.08 ± 0.01 c | 0.95 ± 0.01 d |
| C16:0 | 35.48 ± 1.44 a | 31.87 ± 0.08 b | 29.14 ± 0.05 c | 28.01 ± 0.03 d |
| C17:0 | 0.96 ± 0.08 a | 0.75 ± 0.08 b | 0.62 ± 0.04 bc | 0.56 ± 0.04 c |
| C18:0 | 8.27 ± 0.11 a | 7.25 ± 0.10 b | 6.50 ± 0.01 c | 6.20 ± 0.01 d |
| C19:0 | 0.29 ± 0.01 a | 0.20 ± 0.01 b | 0.15 ± 0.01 b | 0.19 ± 0.01 b |
| C20:0 | 0.11 ± 0.01 a | 0.12 ± 0.00 a | 0.12 ± 0.00 a | 0.12 ± 0.00 a |
| C22:0 | - | 0.07 ± 0.00 c | 0.14± 0.01 b | 0.16 ± 0.01 a |
| C24:0 | - | 0.06 ± 0.01 b | 0.11 ± 0.02 a | 0.13 ± 0.01 a |
| Monounsaturated Fatty Acids (MUFA) | ||||
| C14:1 | 0.83 ± 0.01 a | 0.63 ± 0.00 b | 0.50 ± 0.00 b | 0.43 ± 0.00 d |
| C16:1 ω-7 | 1.37 ± 0.02 d | 2.52 ± 0.01 c | 3.31 ± 0.01 b | 3.73 ± 0.00 a |
| C17:1 | 0.14 ± 0.01 b | 0.17 ± 0.00 a | 0.18 ± 0.01 a | 0.18 ± 0.00 a |
| C18:1 ω-9 | 17.28 ± 0.08 d | 23.67 ± 0.12 c | 28.41 ± 0.01 b | 31.09 ± 0.04 a |
| C20:1 | 0.03 ± 0.04 d | 0.46 ± 0.03 c | 0.88 ± 0.02 b | 1.05 ± 0.01 a |
| C22:1 ω-9 | - | 0.10 ± 0.00 c | 0.15 ± 0.01 b | 0.17 ± 0.00 a |
| C24:1 ω-9 | - | - | 0.07 ± 0.00 b | 0.08 ± 0.01 a |
| Polyunsaturated fatty acids (PUFA) | ||||
| C18:2 ω-6 | 12.08 ± 0.09 c | 12.68 ± 0.06 b | 12.97 ± 0.04 a | 12.91 ±0.01 c |
| C18:3 ω-3 | 1.35 ± 0.00 d | 1.40 ± 0.01 c | 1.50 ± 0.01 b | 1.45 ± 0.00 a |
| C20:2 ω-6 | - | 0.10 ± 0.01 c | 0.15 ± 0.00 b | 0.17 ± 0.00 a |
| C20:3 ω-6 | - | 0.09 ± 0.01 c | 0.14 ± 0.00 b | 0.17 ± 0.00 a |
| C20:4 ω-6 | - | 0.14 ± 0.01 c | 0.22 ± 0.01 b | 0.25 ± 0.00 a |
| C20:5 ω-3 | - | 0.29 ± 0.00 c | 0.46 ± 0.00 b | 0.58 ± 0.01 a |
| C22:2 ω-6 | - | - | - | 0.04 ± 0.01 |
| C22:5 ω-3 | 0.09 ± 0.04 d | 0.18 ± 0.01 c | 0.25 ± 0.00 b | 0.28 ± 0.00 a |
| C22:6 ω-3 | - | 0.78 ± 0.03 c | 1.31 ± 0.01 b | 1.54 ± 0.01 a |
| ΣSFA | 60.31 ± 0.45 a | 49.33 ± 0.41 b | 43.96 ± 0.27 c | 43.60± 0.12 c |
| ΣMUFA | 19.65 ± 0.17 | 27.56 ± 0.18 | 33.50 ± 0.12 | 36.75 ± 0.06 |
| ΣPUFA | 13.51 ± 0.14 | 15.68 ± 0.13 | 17.00 ± 0.08 | 16.62 ± 0.04 |
| Σω-6 | 12.08 ± 0.01 | 13.01 ±0.02 | 13.48 ± 0.05 | 12.83 ± 0.01 |
| Σω-3 | 1.43 ± 0.01 | 2.66 ± 0.04 | 3.52 ± 0.03 | 3.80 ± 0.02 |
| PUFA/SFA | 0.22 | 0.31 | 0.38 | 0.38 |
| EPA + DHA | - | 1.07 ± 0.03 | 1.77 ± 0.01 | 2.06 ± 0.01 |
| ω-6/ω-3 | 12.08 | 4.89 | 3.82 | 3.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, A.S.; Beibeia, T.; Brennan, M.A.; Guo, X.; Zeng, X.-A.; Brennan, C.S. Protein, Amino Acid, Fatty Acid Composition, and in Vitro Digestibility of Bread Fortified with Oncorhynchus tschawytscha Powder. Nutrients 2018, 10, 1923. https://doi.org/10.3390/nu10121923
Desai AS, Beibeia T, Brennan MA, Guo X, Zeng X-A, Brennan CS. Protein, Amino Acid, Fatty Acid Composition, and in Vitro Digestibility of Bread Fortified with Oncorhynchus tschawytscha Powder. Nutrients. 2018; 10(12):1923. https://doi.org/10.3390/nu10121923
Chicago/Turabian StyleDesai, Ajay S., Tang Beibeia, Margaret A. Brennan, Xinbo Guo, Xin-An Zeng, and Charles S. Brennan. 2018. "Protein, Amino Acid, Fatty Acid Composition, and in Vitro Digestibility of Bread Fortified with Oncorhynchus tschawytscha Powder" Nutrients 10, no. 12: 1923. https://doi.org/10.3390/nu10121923






