Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism
Abstract
:1. Introdution
2. Materials and Methods
2.1. Human Study
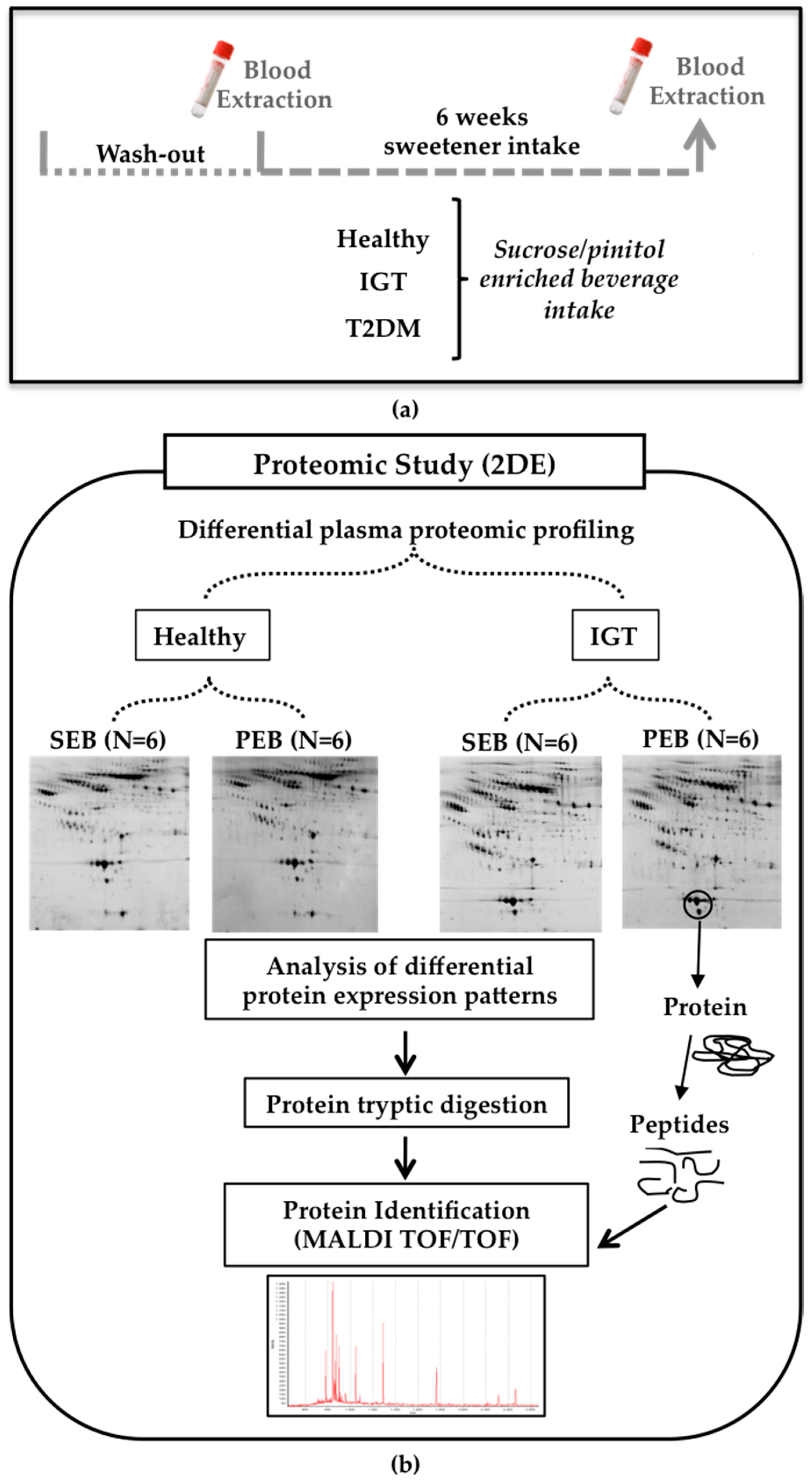
2.1.1. Subjects and Study Design
2.1.2. Blood Collection and Sample Preparation
2.1.3. Sample Preparation
2.1.4. Proteomic Analysis
Two-Dimensional Gel Electrophoresis
Mass Spectrometry Analysis
Quantification of Protein Serum Levels
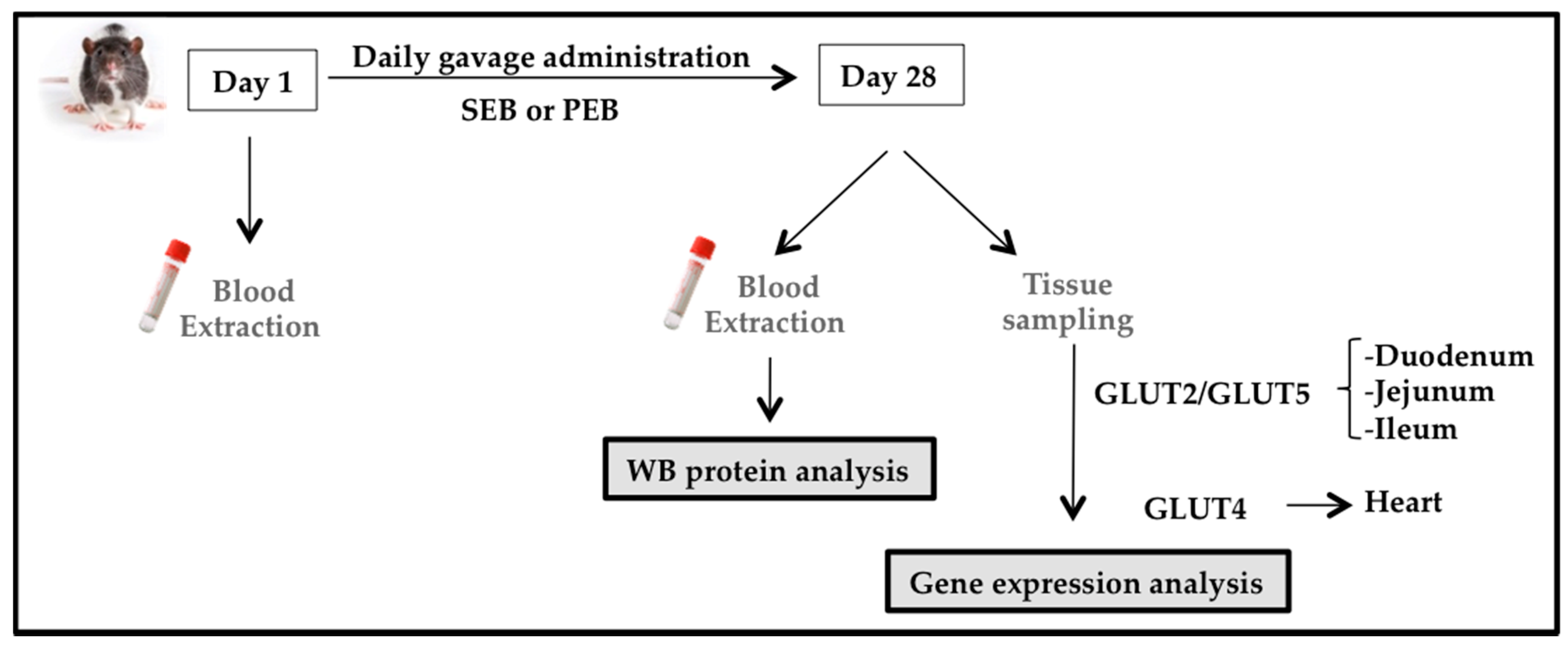
2.2. Animal Study
2.2.1. Animal Model
2.2.2. Experimental Design
2.2.3. Western Blot Analysis
2.2.4. Glucose Transporter mRNA Expression
2.3. Statistical Analysis
3. Results
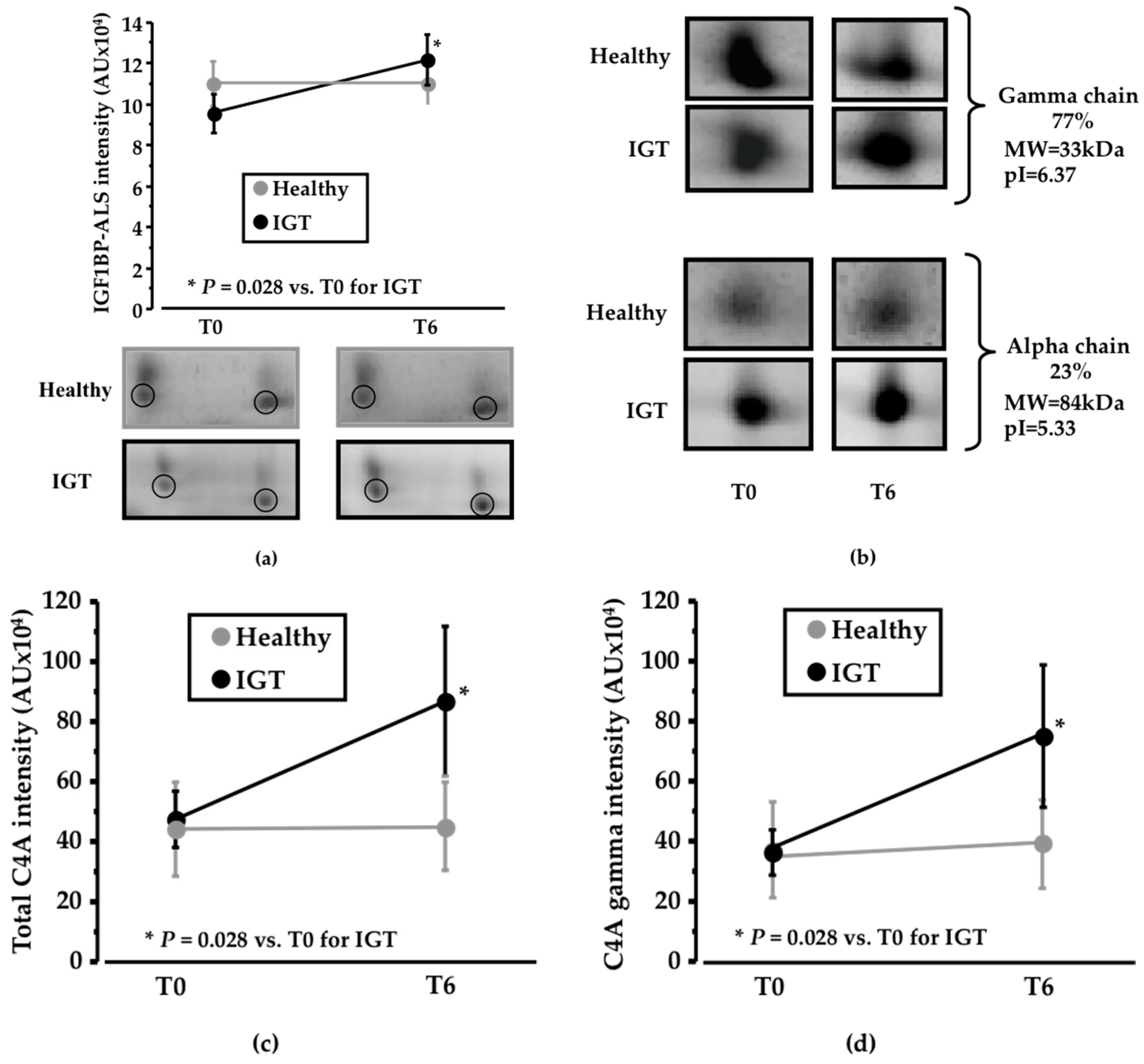
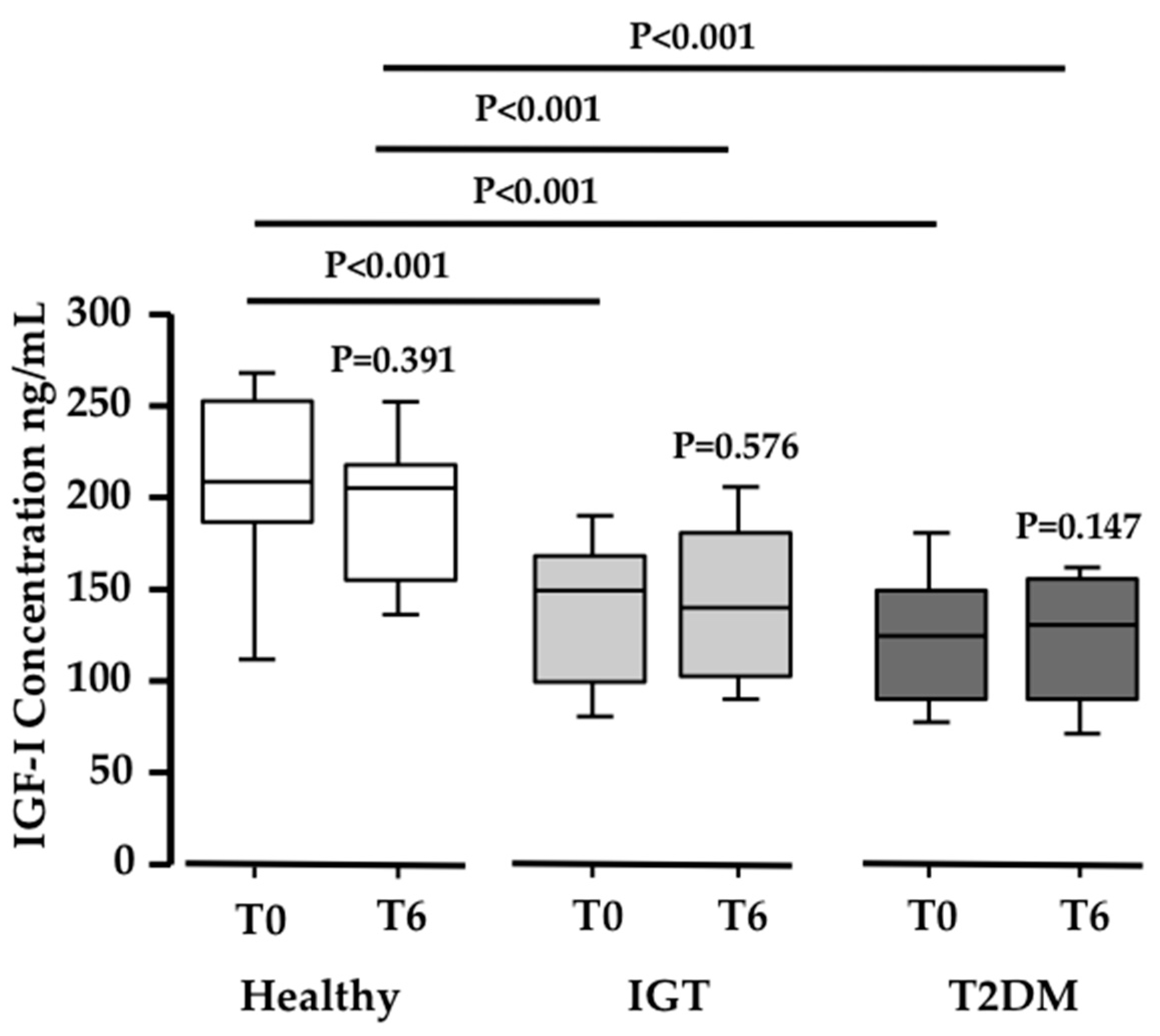
3.1. Pinitol-Enriched Beverage Intake Induces Serum Proteomic Changes in Proteins Related to the Insulin Secretion Pathway
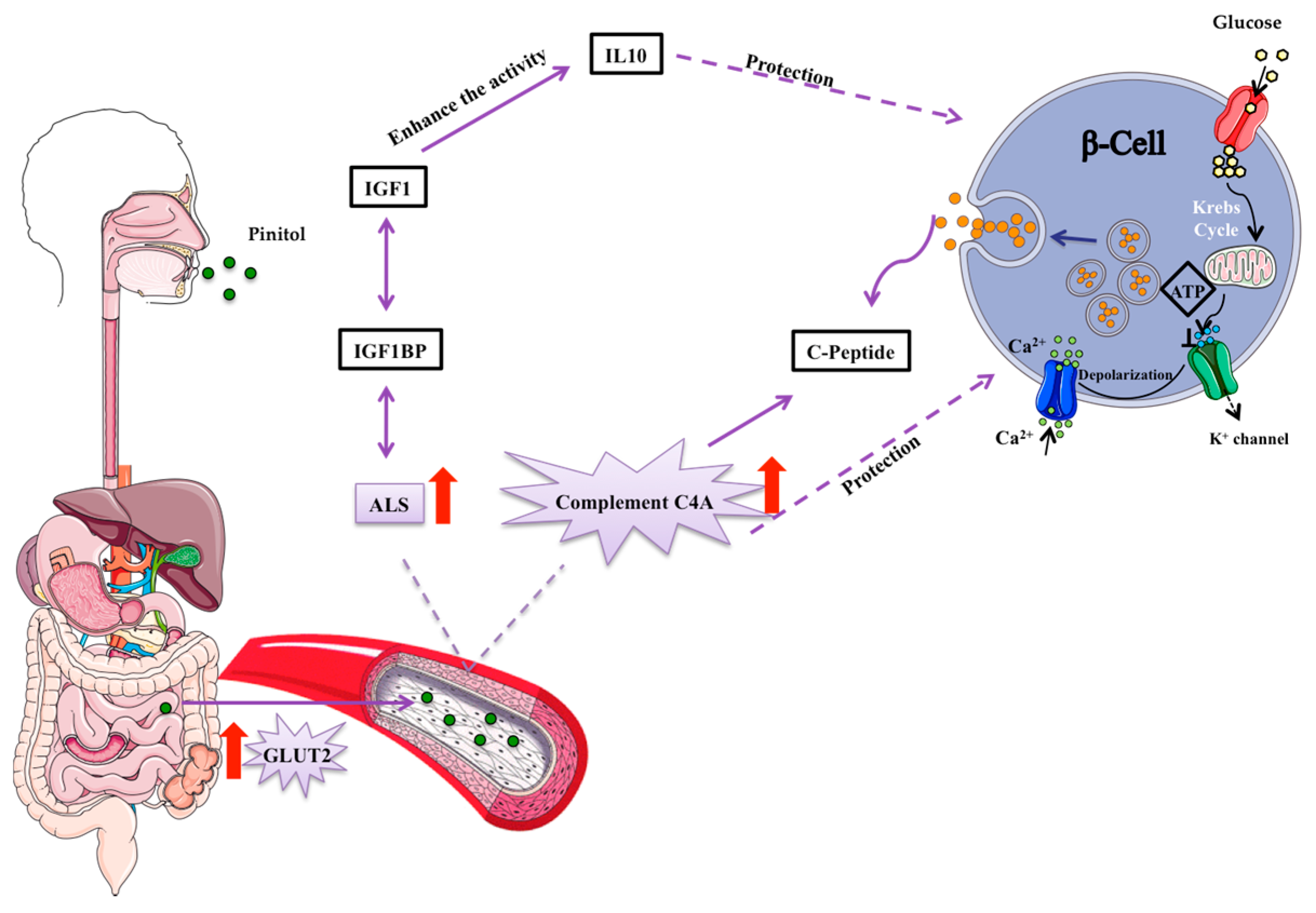
3.2. Potential Mechanisms Involved in the Peb Glucose Modulating Effect
4. Discussion
Limitations of the Study
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nathan, D.M. Diabetes: Advances in Diagnosis and Treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34 (Suppl. S1), S062–S069. [Google Scholar]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world: A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; Defronzo, R.A.; Abdul-ghani, M.A. Treatment of prediabetes. World J. Prediabetes 2015, 6, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, E. Diabetes and cardiovascular risk markers. Curr. Med. Res. Opin. 2005, 21, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Ahmed, H.M.; Michos, E.D. Preventing cardiovascular disease in patients with diabetes: Use of aspirin for primary prevention. Curr. Cardiol. Rep. 2015, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Morenga, L. Te Sugar and Type 2 diabetes. Sugar Diabetes 2016, 10, 43–53. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 6736, 1–13. [Google Scholar] [CrossRef]
- De Koning, L.; Malik, V.S.V.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011, 93, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Sweeteners and risk of obesity and type 2 diabetes: The role of sugar-sweetened beverages. Curr. Diabetes Rep. 2012, 12, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Cunningham, S.A.; Vos, M.B. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011, 123, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Swithers, S.E. Artificial sweeteners and metabolic dysregulation: Lessons learned from agriculture and the laboratory. Rev. Endocr. Metab. Disord. 2016, 17, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Connor, H.; Annan, F.; Bunn, E.; Frost, G.; McGough, N.; Sarwar, T.; Thomas, B. The implementation of nutritional advice for people with diabetes. Diabet. Med. 2003, 20, 786–807. [Google Scholar] [PubMed]
- Hernández-Mijares, A.; Bañuls, C.; Peris, J.E.; Monzó, N.; Jover, A.; Bellod, L.; Victor, V.M.; Rocha, M. A single acute dose of pinitol from a naturally-occurring food ingredient decreases hyperglycaemia and circulating insulin levels in healthy subjects. Food Chem. 2013, 141, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Bañuls, C.; Rovira-Llopis, S.; Falcón, R.; Veses, S.; Monzó, N.; Víctor, V.M.; Rocha, M.; Hernández-Mijares, A. Chronic consumption of an inositol-enriched carob extract improves postprandial glycaemia and insulin sensitivity in healthy subjects: A randomized controlled trial. Clin. Nutr. 2016, 35, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; Schindler, A.E. Myoinositol as a Safe and Alternative Approach in the Treatment of Infertile PCOS Women: A German Observational Study. Int. J. Endocrinol. 2016, 2016, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Christiansen, M.A.; Horowitz, J.F.; Klein, S.; Hellerstein, M.K.; Ostlund, R.E. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mijares, A.; Bañuls, C.; Rovira-Llopis, S.; Álvarez, Á.; Orden, S.; Rubio-Puchol, O.; Víctor, V.M.; Rocha, M. Chronic consumption of an inositol-enriched beverage ameliorates endothelial dysfunction and oxidative stress in type 2 diabetes. J. Funct. Foods 2015, 18, 598–607. [Google Scholar] [CrossRef]
- Bañuls, C.; Rovira-Llopis, S.; López-Doménech, S.; Veses, S.; Víctor, V.M.; Rocha, M.; Hernández-Mijares, A. Effect of consumption of a carob pod inositol-enriched beverage on insulin sensitivity and inflammation in middle-aged prediabetic subjects. Food Funct. 2016, 7, 4379–4387. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padró, T.; García-Moll, X.; Pintó, X.; Cinca, J.; Badimon, L. Proteomic signature of apolipoprotein J in the early phase of new-onset myocardial infarction. J. Proteome Res. 2011, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padro, T.; Garcia-Moll, X.; Pinto, X.; Cinca, J.; Badimon, L. Serum proteome in acute myocardial infarction. Clin. Investig. Arterioscler. 2011, 23, 147–154. [Google Scholar] [CrossRef]
- Cubedo, J.; Padró, T.; Badimon, L. Coordinated proteomic signature changes in immune response and complement proteins in acute myocardial infarction: The implication of serum amyloid P-component. Int. J. Cardiol. 2013, 168, 5196–5204. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padró, T.; García-Arguinzonis, M.; Vilahur, G.; Miñambres, I.; Pou, J.M.; Ybarra, J.; Badimon, L. A novel truncated form of apolipoprotein A-I transported by dense LDL is increased in diabetic patients. J. Lipid Res. 2015, 56, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padro, T.; Badimon, L. Glycoproteome of human apolipoprotein A-I: N- and O-glycosylated forms are increased in patients with acute myocardial infarction. Transl. Res. 2014, 164, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Tofovic, S.P.; Jackson, E.K. Rat models of the metabolic syndrome. Methods Mol. Med. 2003, 86, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Angel, I.; Bidet, S.; Langer, S.Z. Pharmacological characterization of the hyperglycemia induced by alpha-2 adrenoceptor agonists. J. Pharmacol. Exp. Ther. 1988, 246, 1098–1103. [Google Scholar] [PubMed]
- Rensing, K.L.; van Duyvenvoorde, H.A.; Cramer, M.J.; Teske, A.J.; Prokop, M.; Stroes, E.S.; Wit, J.M.; Hermus, A.R.M.M.; Twickler, T.B. Case report: Low circulating IGF-I levels due to Acid-Labile Subunit deficiency in adulthood are not associated with early development of atherosclerosis and impaired heart function. Growth Horm. IGF Res. 2011, 21, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.; Popkin, B. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Huitrón-Bravo, G.; Talavera, J.O.; Castañón, S.; Gallegos-Carrillo, K.; Flores, Y.; Salmerón, J. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J. Nutr. Metab. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Health Promot. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Rafael, S.A.; Henry, C.J.K. FRUIT UP (new food ingredient from wild): Scientific review of its healthful properties. Nutrición Clínica y Dietética Hospitalaria 2010, 30, 15–25. [Google Scholar]
- Wallace, M.; Whelan, H.; Brennan, L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim. Biophys. Acta 2013, 1830, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Li, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2012, 9, 25–53. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renström, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Wong, X.; Hong, W.; Han, W. Molecular regulation of insulin granule biogenesis and exocytosis. Biochem. J. 2016, 473, 2737–2756. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Salojin, K.V.; Mi, Q.S.; Grattan, M.; Meagher, T.C.; Zucker, P.; Delovitch, T.L. Insulin-Like Growth Factor (IGF)-I/IGF-Binding Protein-3 Complex: Therapeutic Efficacy and Mechanism of Protection against Type 1 Diabetes. Endocrinology 2004, 145, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Eriksson, J. Serum Insulin-like Growth Factor (IGF)-I and IGF-Binding Protein-1 in Elderly People: Relationships with Cardiovascular Risk Factors, Body Composition, Size at Birth, and Childhood Growth. J. Clin. Endocrinol. Metab. 2003, 88, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Capalbo, D.; Marcovecchio, M.L.; Sleigh, A.; Jørgensen, S.W.; Hill, N.R.; Mooslehner, K.; Yeo, G.S.H.; Bluck, L.; Juul, A.; et al. Low circulating levels of IGF-1 in healthy adults are associated with reduced β-cell function, increased intramyocellular lipid, and enhanced fat utilization during fasting. J. Clin. Endocrinol. Metab. 2014, 99, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Domené, H.M.; Hwa, V.; Héctor, G. Jasper; Rosenfeld, R.G. Acid-labile subunit (ALS) deficiency. Best Pract. Res. Clin. Endocrinol. Metab. J. 2011, 25, 101–113. [Google Scholar] [CrossRef]
- Boisclair, Y.R.; Rhoads, R.P.; Ueki, I.; Wang, J.; Ooi, G.T. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: An important but forgotten component of the circulating IGF system. J. Endocrinol. 2001, 170, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Guler, H.P.; Zapf, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol. 1989, 121, 753–758. [Google Scholar] [PubMed]
- Gilmore, J.H. NIH Public Access. AJNR Am. J. Neuroradiol. 2008, 29, 1883–1889. [Google Scholar] [CrossRef]
- Charlesworth, J.A.; Timmermans, V.; Golding, J.; Campbell, L.V.; Peake, P.W.; Pussell, B.A.; Wakefield, D.; Howard, N. The complement system in Type 1 (insulin-dependent) diabetes. Diabetologia 1987, 30, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jenhani, F.; Bardi, R.; Gorgi, Y.; Ayed, K.; Jeddi, M. C4 polymorphism in multiplex families with insulin dependent diabetes in the Tunisian population: Standard C4 typing methods and RFLP analysis. J. Autoimmun. 1992, 5, 149–160. [Google Scholar] [CrossRef]
- Szilagyi, A.; Blasko, B.; Szilassy, D.; Fust, G.; Sasvari-szekely, M.; Ronai, Z. Real-time PCR quantification of human complement C4A and C4B genes. BMC Genet. 2006, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Y.; Chien, C.; Kan, W.; Liao, P.; Wu, H.; Su, S.; Lin, C. Differential proteomic characterization between normal peritoneal fluid and diabetic peritoneal dialysate. Nephrol. Dial. Transpl. 2010, 25, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M. Lower circulating levels of complement split proteins C3a and C4a in maternal plasma of women with gestational diabetes mellitus. Diabet. Med. 2011, 28, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Kingery, S.E.; Wu, Y.L.; Zhou, B.; Hoffman, R.P.; Yu, C.Y. Gene Copy-Number Variations (CNVs) and Protein Levels of Complement C4A and C4B as Novel Biomarkers for Partial Disease Remissions in New-Onset Type 1 Diabetes Patients. Pediatr. Diabetes 2012, 13, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K.E.; Wu, Y.L.; Yang, Y.; Spencer, C.H.; Hauptmann, G.; Hebert, L.A.; Atkinson, J.P.; Yu, C.Y. Early components of the complement classical activation pathway in human systemic autoimmune diseases. Front. Immunol. 2016, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nityanand, S.; Hamsten, A.; Lithell, H.; Holm, G.; Lefvert, A.K. C4 null alleles and myocardial infarction. Atherosclerosis 1999, 143, 377–381. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2014, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Holman, G.D. The glucose transporter family: Structure, function and tissue-specific expression. Biochem. J. 1993, 295, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I.; Kayano, T.; Buse, J.B.; Burant, C.F.; Takeda, J.; Lin, D.; Fukumoto, H.; Seino, S. Molecular biology of mammalian glucose transporters. Diabetes Care 1990, 13, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Simpson, A.M.; Swan, M.A.; Tao, C.; Tuch, B.E.; Crawford, R.M.; Jovanovic, A.; Martin, D.K. ATP-sensitive potassium channels induced in liver cells after transfection with insulin cDNA and the GLUT2 transporter regulate glucose-stimulated insulin secretion. FASEB J. 2003, 17, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Tobin, V.; Le Gall, M.; Fioramonti, X.; Stolarczyk, E.; Blazquez, A.G.; Klein, C.; Prlgent, M.; Serradas, P.; Cuif, M.H.; Magnan, C.; et al. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes 2008, 57, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamaoka, M.; Hasunuma, T.; Ashida, H.; Yoshida, K.I. Detection of orally administered inositol stereoisomers in mouse blood plasma and their effects on translocation of glucose transporter 4 in skeletal muscle cells. J. Agric. Food Chem. 2013, 61, 4850–4854. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.F.S.A.; Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; García-Ocaña, A.; Stewart, A.F. Diabetes mellitus—Advances and challenges in human β cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201–212. [Google Scholar] [CrossRef]

| PEB | SEB | |
|---|---|---|
| Pinitol (g) | 4.00 | - |
| Myoinositol + D-chiro-inositol (g) | 0.45 | - |
| Sugars(g) | 34.90 | 42.5 |
| -Glucose | 6.23 | - |
| -Fructose | 4.83 | - |
| -Sucrose | 37.29 | 42.5 |
| -Others | 0.58 | - |
| Oligosaccharides (g) | 0.05 | - |
| Soluble fibre (g) | 1.65 | - |
| Total carbohydrates (g) | 41.18 | 42.50 |
| Total available carbohydrates (g) | 39.12 | 42.50 |
| Total calories (kcal) | 155.0 | 170.0 |
| Healthy Subjects | IGT Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEB (N = 6) | PEB (N = 6) | SEB (N = 6) | PEB (N = 6) | |||||||||
| T0 | T6 | P Value | T0 | T6 | P Value | T0 | T6 | P Value | T0 | T6 | P Value | |
| Cholesterol (mg/dL) | 192 | 202 | 0.022 * | 183 | 178 | 0.398 | 197 | 203 | 0.602 | 189 | 194 | 0.798 |
| cLDL(mg/dL) | 112 | 125 | 0.004 * | 109 | 108 | 0.706 | 104 | 130 | 0.266 | 122 | 125 | 0.824 |
| cHDL (mg/dL) | 54 | 52 | 0.283 | 55 | 52 | 0.047 * | 54 | 53 | 0.734 | 42 | 43 | 0.812 |
| TG (mg/dL) | 126 | 125 | 0.934 | 88 | 89 | 0.802 | 111 | 101 | 0.379 | 121 | 128 | 0.797 |
| Urea (mg/dL) | 24 | 26 | 0.168 | 32 | 34 | 0.445 | 41 | 35 | 0.028 * | 43 | 35 | 0.01 * |
| Glucose (mg/dL) | 87 | 92 | 0.057 | 98 | 89 | 0.003 * | 100 | 103 | 0.003 * | 120 | 105 | <0.001* |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, C.; Cubedo, J.; Padró, T.; Vilahur, G.; López-Bernal, S.; Rocha, M.; Hernández-Mijares, A.; Badimon, L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients 2018, 10, 271. https://doi.org/10.3390/nu10030271
Lambert C, Cubedo J, Padró T, Vilahur G, López-Bernal S, Rocha M, Hernández-Mijares A, Badimon L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients. 2018; 10(3):271. https://doi.org/10.3390/nu10030271
Chicago/Turabian StyleLambert, Carmen, Judit Cubedo, Teresa Padró, Gemma Vilahur, Sergi López-Bernal, Milagros Rocha, Antonio Hernández-Mijares, and Lina Badimon. 2018. "Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism" Nutrients 10, no. 3: 271. https://doi.org/10.3390/nu10030271
APA StyleLambert, C., Cubedo, J., Padró, T., Vilahur, G., López-Bernal, S., Rocha, M., Hernández-Mijares, A., & Badimon, L. (2018). Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients, 10(3), 271. https://doi.org/10.3390/nu10030271









