Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Published in English.
- Conducted among humans.
- Being a case control study, a cohort study, or a randomized controlled trial (RCT).
- Studying the association between vitamin D status (including indicators such as circulating vitamin D, and vitamin D intake) and the risk of incident stroke.
- Containing information about contingency tables, the odds ratio (OR), or the risk ratio (RR), as well as their 95% confidence interval (CI).
- Repeating publications or duplicates.
- Insufficient data for analysis.
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Analysis and Statistical Methods
2.6. Sensitivity Analyses
2.7. Subgroup Analysis
2.8. Publication Bias
3. Results
3.1. Characteristics of Included Studies
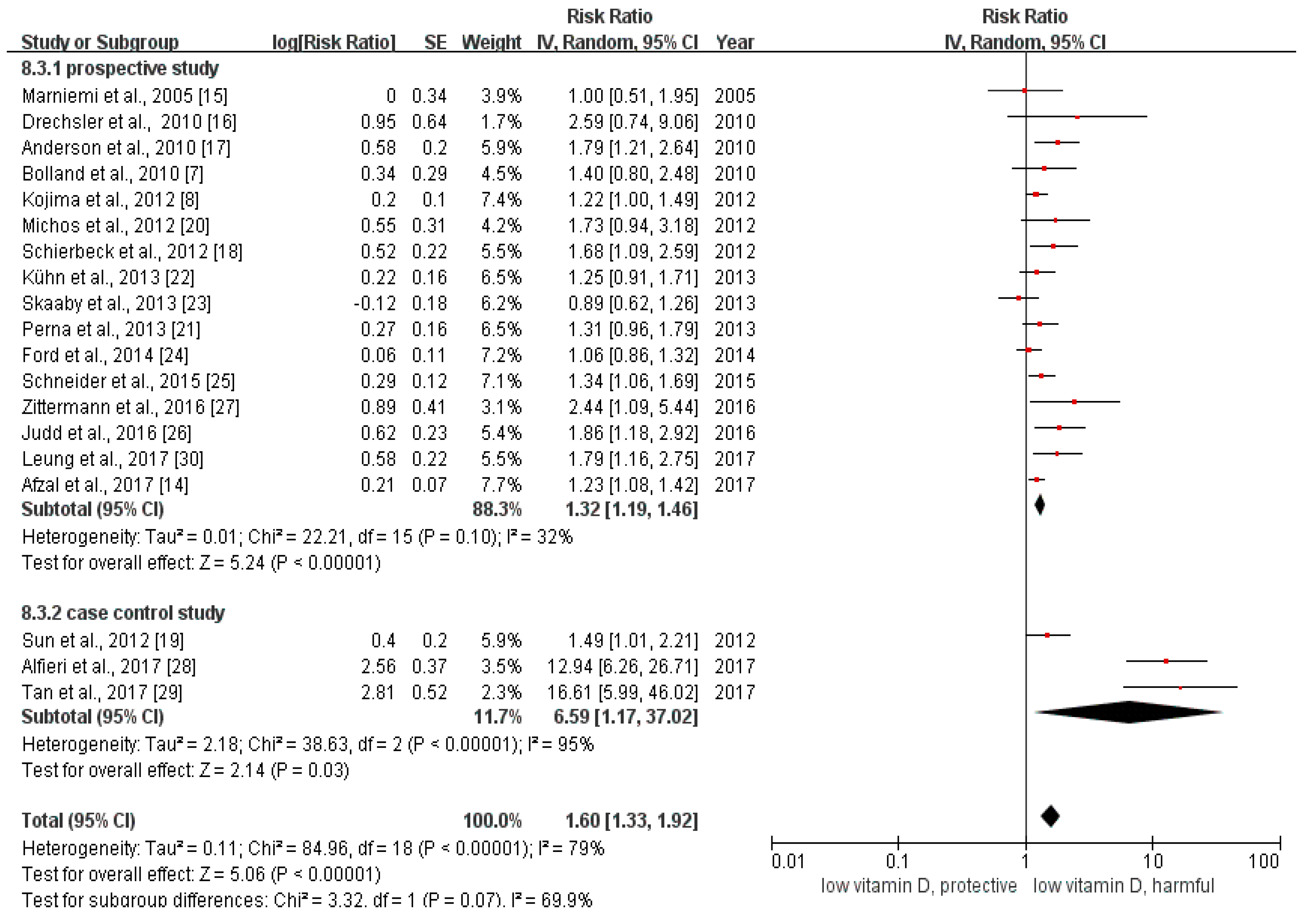
3.2. Overall Analysis
3.3. Subgroup Analysis
3.4. Risk of Bias Assessment
3.5. Sensitivity Analysis
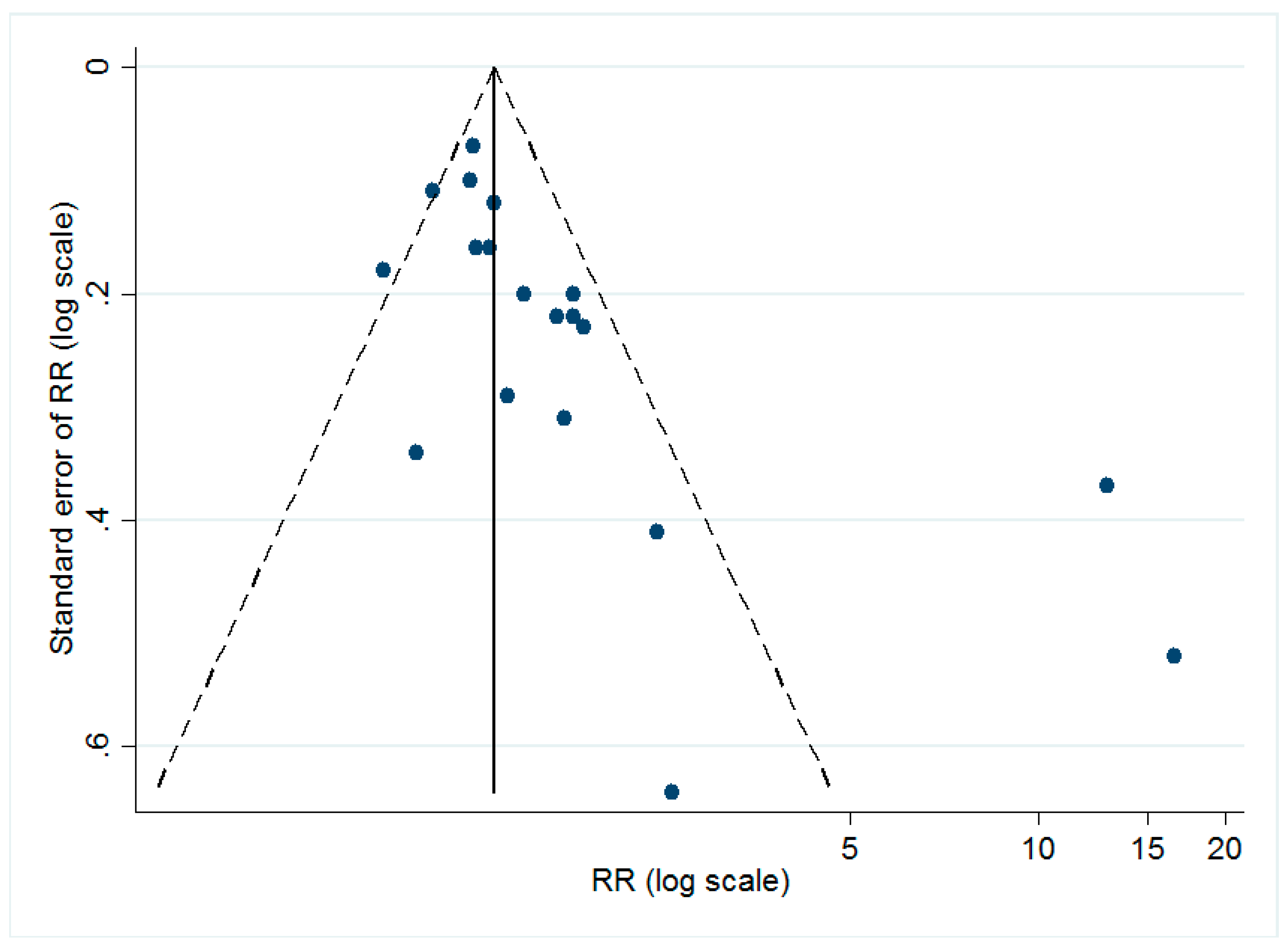
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Heart Federation. Available online: www.world-heart-federation.org (accessed on 18 January 2018).
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Schnatz, P.F.; Manson, J.E. Vitamin D and cardiovascular disease: An appraisal of the evidence. Clin. Chem. 2014, 60, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Bacon, C.J.; Horne, A.M.; Mason, B.H.; Ames, R.W.; Wang, T.K.; Grey, A.B.; Gamble, G.D.; Reid, I.R. Vitamin D insufficiency and health outcomes over 5 y in older women. Am. J. Clin. Nutr. 2010, 91, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Bell, C.; Abbott, R.D.; Launer, L.; Chen, R.; Motonaga, H.; Ross, G.W.; Curb, J.D.; Masaki, K. Low dietary vitamin D predicts 34-year incident stroke: The Honolulu Heart Program. Stroke 2012, 43, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Dobnig, H.; Fischer, J.E.; Wellnitz, B.; Seelhorst, U.; Boehm, B.O.; Marz, W. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke 2008, 39, 2611–2613. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Nordestgaard, B.G.; Schnohr, P.; Benn, M. 25-hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann. Neurol. 2013, 73, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Manson, J.E.; Pilz, S.; Marz, W.; Michaelsson, K.; Lundqvist, A.; Jassal, S.K.; Barrett-Connor, E.; Zhang, C.; et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T. The relationship of vitamin D status to risk of cardiovascular disease and mortality. Dan. Med. J. 2015, 62. pii: B5008. [Google Scholar]
- Wu, L.; Sun, D. Effects of calcium plus vitamin D supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2017, 31, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Nordestgaard, B.G. Vitamin D, Hypertension, and Ischemic Stroke in 116 655 Individuals from the General Population: A Genetic Study. Hypertension 2017. [Google Scholar] [CrossRef] [PubMed]
- Marniemi, J.; Alanen, E.; Impivaara, O.; Seppanen, R.; Hakala, P.; Rajala, T.; Ronnemaa, T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Pilz, S.; Obermayer-Pietsch, B.; Verduijn, M.; Tomaschitz, A.; Krane, V.; Espe, K.; Dekker, F.; Brandenburg, V.; Marz, W.; et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur. Heart J. 2010, 31, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; May, H.T.; Horne, B.D.; Bair, T.L.; Hall, N.L.; Carlquist, J.F.; Lappe, D.L.; Muhlestein, J.B.; Intermountain Heart Collaborative Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am. J. Cardiol. 2010, 106, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Schierbeck, L.L.; Rejnmark, L.; Tofteng, C.L.; Stilgren, L.; Eiken, P.; Mosekilde, L.; Kober, L.; Jensen, J.E. Vitamin D deficiency in postmenopausal, healthy women predicts increased cardiovascular events: A 16-year follow-up study. Eur. J. Endocrinol. 2012, 167, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Pan, A.; Hu, F.B.; Manson, J.E.; Rexrode, K.M. 25-Hydroxyvitamin D levels and the risk of stroke: A prospective study and meta-analysis. Stroke 2012, 43, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Reis, J.P.; Post, W.S.; Lutsey, P.L.; Gottesman, R.F.; Mosley, T.H.; Sharrett, A.R.; Melamed, M.L. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition 2012, 28, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Perna, L.; Schottker, B.; Holleczek, B.; Brenner, H. Serum 25-hydroxyvitamin D and incidence of fatal and nonfatal cardiovascular events: A prospective study with repeated measurements. J. Clin. Endocrinol. Metab. 2013, 98, 4908–4915. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Kaaks, R.; Teucher, B.; Hirche, F.; Dierkes, J.; Weikert, C.; Katzke, V.; Boeing, H.; Stangl, G.I.; Buijsse, B. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident myocardial infarction and stroke in the European prospective investigation into cancer and nutrition (EPIC)-Germany study. PLoS ONE 2013, 8, e69080. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jorgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and incident cardiovascular disease and all-cause mortality: A general population study. Endocrine 2013, 43, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; MacLennan, G.S.; Avenell, A.; Bolland, M.; Grey, A.; Witham, M.; Group, R.T. Cardiovascular disease and vitamin D supplementation: Trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.; Lutsey, P.L.; Selvin, E.; Mosley, T.H.; Sharrett, A.R.; Carson, K.A.; Post, W.S.; Pankow, J.S.; Folsom, A.R.; Gottesman, R.F.; et al. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: The Atherosclerosis Risk in Communities (ARIC) study. Eur. J. Neurol. 2015, 22, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.E.; Morgan, C.J.; Panwar, B.; Howard, V.J.; Wadley, V.G.; Jenny, N.S.; Kissela, B.M.; Gutierrez, O.M. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int. J. Stroke Off. J. Int. Stroke Soc. 2016, 11, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Morshuis, M.; Kuhn, J.; Pilz, S.; Ernst, J.B.; Oezpeker, C.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Milting, H. Vitamin D metabolites and fibroblast growth factor-23 in patients with left ventricular assist device implants: Association with stroke and mortality risk. Eur. J. Nutr. 2016, 55, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, D.F.; Lehmann, M.F.; Oliveira, S.R.; Flauzino, T.; Delongui, F.; de Araujo, M.C.; Dichi, I.; Delfino, V.D.; Mezzaroba, L.; Simao, A.N.; et al. Vitamin D deficiency is associated with acute ischemic stroke, C-reactive protein, and short-term outcome. Metab. Brain Dis. 2017, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.M.; Wang, L.; Chen, J.J.; Li, H.; Luo, W.B. Diagnostic performance of bone metabolic indexes for the detection of stroke. Saudi Med. J. 2017, 38, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.Y.; Han, Y.; Sing, C.W.; Cheung, B.M.; Wong, I.C.; Tan, K.C.; Kung, A.W.; Cheung, C.L. Serum 25-hydroxyvitamin D and the risk of stroke in Hong Kong Chinese. Thromb. Haemost. 2017, 117, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Somjen, D.; Weisman, Y.; Kohen, F.; Gayer, B.; Limor, R.; Sharon, O.; Jaccard, N.; Knoll, E.; Stern, N. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 2005, 111, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; De Vivo, E.; Attanasio, A.; Gallo, V.; Mazzucco, G.; Pescarmona, G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE 2010, 5, e8670. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, M.; Koyama, T.; Yamamoto, K.; Hirosawa, S.; Kamei, S.; Kamiyama, R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 2000, 102, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Williams, J.S.; Fisher, N.D. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Manabe, I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ. J. Off. J. Jpn. Circ. Soc. 2011, 75, 2739–2748. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Abnet, C.C.; Cross, A.J.; Gunter, M.; Pfeiffer, R.; Gail, M.; Lim, U.; Davey-Smith, G. Mendelian randomization: How it can—And cannot—Help confirm causal relations between nutrition and cancer. Cancer Prev. Res. 2009, 2, 104–113. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
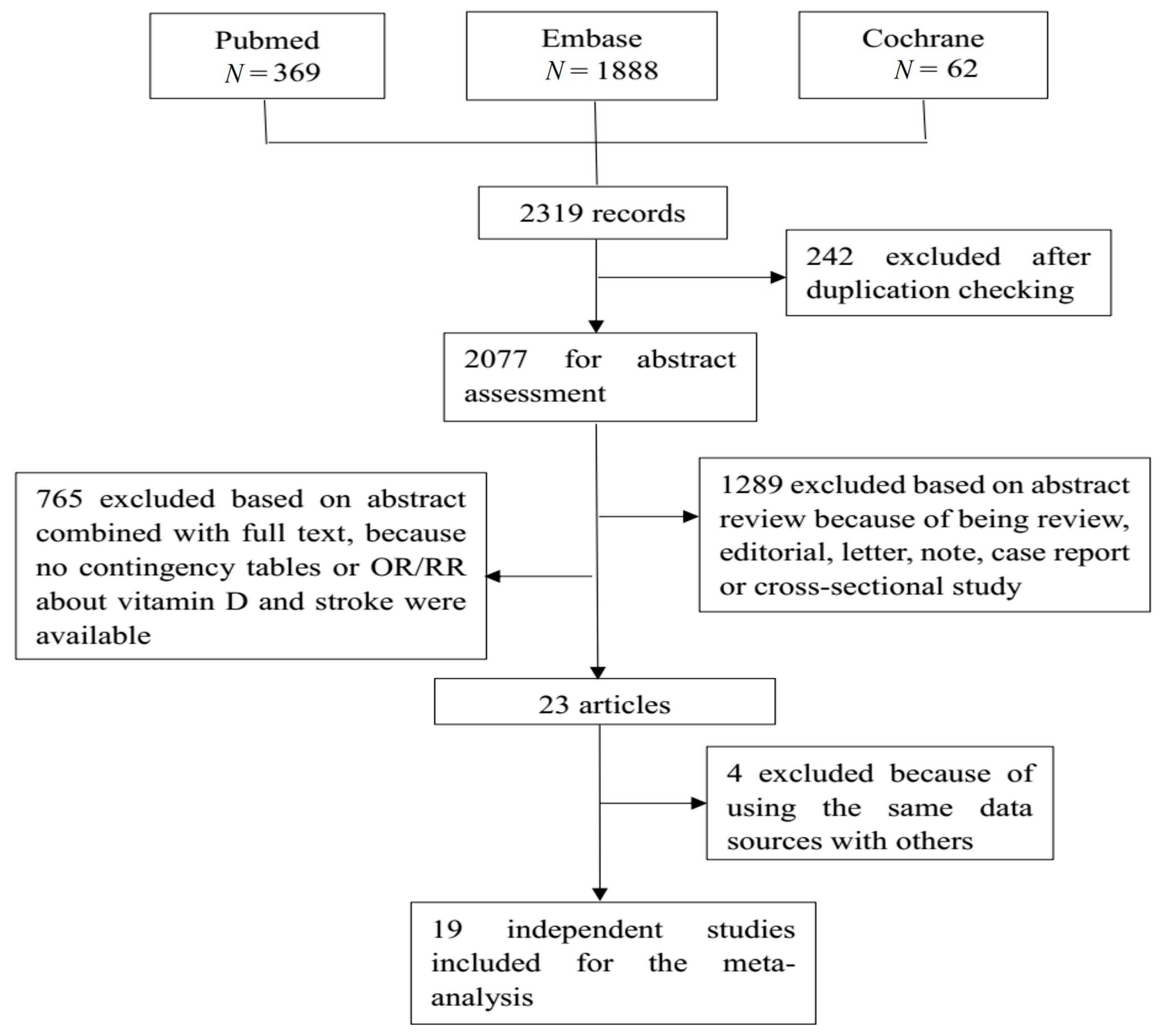
| Database | Search Strategy | Retrieved Records |
|---|---|---|
| Pubmed | ((stroke[MeSH Terms]) OR (cerebrovascular accident) OR (CVA) OR (brain vascular accident) OR (cerebrovascular stroke) OR (cerebral stroke)) AND ((vitamin D[MeSH Terms]) OR (ergocalciferol) OR (25(OH)D) OR (25-hydroxyvitamin D) OR (cholecalciferol)) | 369 |
| Embase | (‘cerebrovascular accident’/exp OR ‘stroke’ OR ‘CVA’ OR ‘brain ischemia’ OR ‘brain vascular accident’ OR ‘cerebrovascular stroke’ OR ‘cerebral stroke’) AND (‘vitamin D’/exp OR ‘ergocalciferol’ OR ‘25(OH)D’ OR ‘25-hydroxyvitamin D’ OR ‘cholecalciferol’) AND [english]/lim AND [embase]/lim | 1888 |
| Cochrane library | ((stroke[MeSH Terms]) OR (cerebrovascular accident) OR (CVA) OR (brain vascular accident) OR (cerebrovascular stroke) OR (cerebral stroke)) AND ((vitamin D[MeSH Terms]) OR (ergocalciferol) OR (25(OH)D) OR (25-hydroxyvitamin D) OR (cholecalciferol)) | 62 |
| Study | Countries/Districts | Sample Size | Event | Number of Cases | Study Design | OR/RR 95% CI |
|---|---|---|---|---|---|---|
| Marniemi et al., 2005 [15] | Finland | 755 | stroke | 70 | cohort study | 1.00 (0.52, 1.96), for serum 25(OH)D |
| 2.20 (1.11, 4.35), for vitamin intake | ||||||
| Bolland et al., 2010 [7] | New Zealand | 1471 | stroke | 59 | cohort study | 1.4 (0.8, 2.5) |
| Drechsler et al., 2010 [16] | Germany | 1108 | stroke | 89 | cohort study | 2.58 (0.74, 8.98) |
| Anderson et al., 2010 [17] | The US | 26,025 | stroke | 208 | cohort study | 1.78 (1.2, 2.66) |
| Schierbeck et al., 2012 [18] | Denmark | 2013 | stroke | 89 | cohort study | 1.68 (1.10, 2.56) |
| Kojima et al., 2012 [8] | Hawaii | 7385 | stroke | 960 | cohort study | 1.22 (1.01, 1.47) |
| ischemic stroke | 651 | 1.27 (1.01, 1.59) | ||||
| hemorrhagic stroke | 269 | 0.97 (0.68, 1.38) | ||||
| unknown | 40 | |||||
| Sun et al., 2012 [19] | The US | 928 | ischemic stroke | 464 | case control study | 1.49 (1.01, 2.18) |
| Michos et al., 2012 [20] | The US | 7981 | fatal stroke | 176 | cohort study | 1.74 (0.94, 3.2) |
| Perna et al., 2013 [21] | Germany | 7709 | stroke | 353 | cohort study | 1.31 (0.95, 1.81) |
| Kühn et al., 2013 [22] | Germany | 2603 | stroke | 471 | cohort study | 1.25 (0.92, 1.70) |
| Skaaby et al., 2013 [23] | Denmark | 8131 | stroke | 316 | cohort study | 0.88 (0.63, 1.25) |
| Ford et al., 2014 [24] | The UK | 5292 | stroke | 309 | RCT | 1.06 (0.85, 1.32) |
| Schneider et al., 2015 [25] | The US | 12,158 | stroke | 804 | cohort study | 1.34 (1.06, 1.71) |
| Judd et al., 2016 [26] | The US | 1547 | stroke | 610 | cohort study | 1.85 (1.17, 2.93) |
| ischemic stroke | 536 | 1.84 (1.14, 2.97) | ||||
| hemorrhagic stroke | 74 | 1.82 (0.91, 3.65) | ||||
| Zittermann et al., 2016 [27] | Germany | 154 | stroke | 27 | cohort study | 2.44 (1.09, 5.45) |
| ischemic stroke | 13 | 2.36 (0.78, 7.19) | ||||
| hemorrhagic stroke | 14 | 1.91 (0.67, 5.46) | ||||
| Alfieri et al., 2017 [28] | Brazil | 286 | ischemic stroke | 168 | case control study | 16.64 (5.66, 42.92) |
| Tan et al., 2017 [29] | China | 404 | stroke | 224 | case control study | 12.92 (6.23, 26.82) |
| ischemic stroke | 121 | 11.67 (4.82, 28.27) | ||||
| hemorrhagic stroke | 103 | 14.67 (5.38, 40.02) | ||||
| Leung et al., 2017 [30] | Hong Kong | 3458 | stroke | 244 | cohort study | 1.78 (1.16, 2.74) |
| Afzal et al., 2017 [14] | Denmark | 35,152 | ischemic stroke | 1660 | cohort study | 1.23 (1.06, 1.42) |
| Study | Selection of the Study Groups | Comparability of the Groups | Ascertainment of the Exposure or Outcome | Total Score | Risk of Bias |
|---|---|---|---|---|---|
| Case control study 1 | |||||
| Sun et al., 2012 [19] | 4 | 2 | 3 | 9 | low |
| Alfieri et al., 2017 [28] | 4 | 2 | 3 | 9 | low |
| Tan et al., 2017 [29] | 2 | 2 | 3 | 7 | low |
| Cohort study 1 | |||||
| Marniemi et al., 2005 [15] | 4 | 1 | 3 | 8 | low |
| Bolland et al., 2010 [7] | 4 | 2 | 3 | 9 | low |
| Anderson et al., 2010 [17] | 4 | 2 | 3 | 9 | low |
| Drechsler et al., 2010 [16] | 3 | 2 | 3 | 8 | low |
| Schierbeck et al., 2012 [18] | 3 | 2 | 3 | 8 | low |
| Kojima et al., 2012 [8] | 4 | 2 | 3 | 9 | low |
| Michos et al., 2012 [20] | 4 | 1 | 3 | 8 | low |
| Judd et al., 2016 [26] | 4 | 2 | 3 | 9 | low |
| Perna et al., 2013 [21] | 4 | 2 | 3 | 9 | low |
| Kühn et al., 2013 [22] | 4 | 2 | 3 | 9 | low |
| Skaaby et al., 2013 [23] | 3 | 1 | 3 | 7 | low |
| Schneider et al., 2015 [25] | 4 | 1 | 3 | 8 | low |
| Zittermann et al., 2016 [27] | 3 | 1 | 2 | 6 | medium |
| Leung et al., 2017 [30] | 3 | 1 | 3 | 7 | low |
| Afzal et al., 2017 [14] | 4 | 2 | 3 | 9 | low |
| RCT 2 | |||||
| Ford et al., 2014 [24] | 6 | low |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Wang, M.; Huang, H.; Li, W.; Hu, Y.; Wu, T. Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 277. https://doi.org/10.3390/nu10030277
Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T. Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Nutrients. 2018; 10(3):277. https://doi.org/10.3390/nu10030277
Chicago/Turabian StyleZhou, Ren, Mengying Wang, Hui Huang, Wenyong Li, Yonghua Hu, and Tao Wu. 2018. "Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis" Nutrients 10, no. 3: 277. https://doi.org/10.3390/nu10030277





