Modifiable “Predictors” of Zinc Status in Toddlers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcome Assessment
2.2.1. Questionnaire Data
2.2.2. Anthropometric Assessment
2.2.3. Dietary Assessment
2.2.4. Biochemical Assessment
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics at Baseline
3.2. Participant Characteristics at 12 Months of Age
3.3. Univariate Associations between Potential “Predictor” Variables and Plasma Zinc Concentrations
3.4. Multiple Regression Analysis of “Predictors” of Plasma Zinc Concentrations at 12 Months of Age
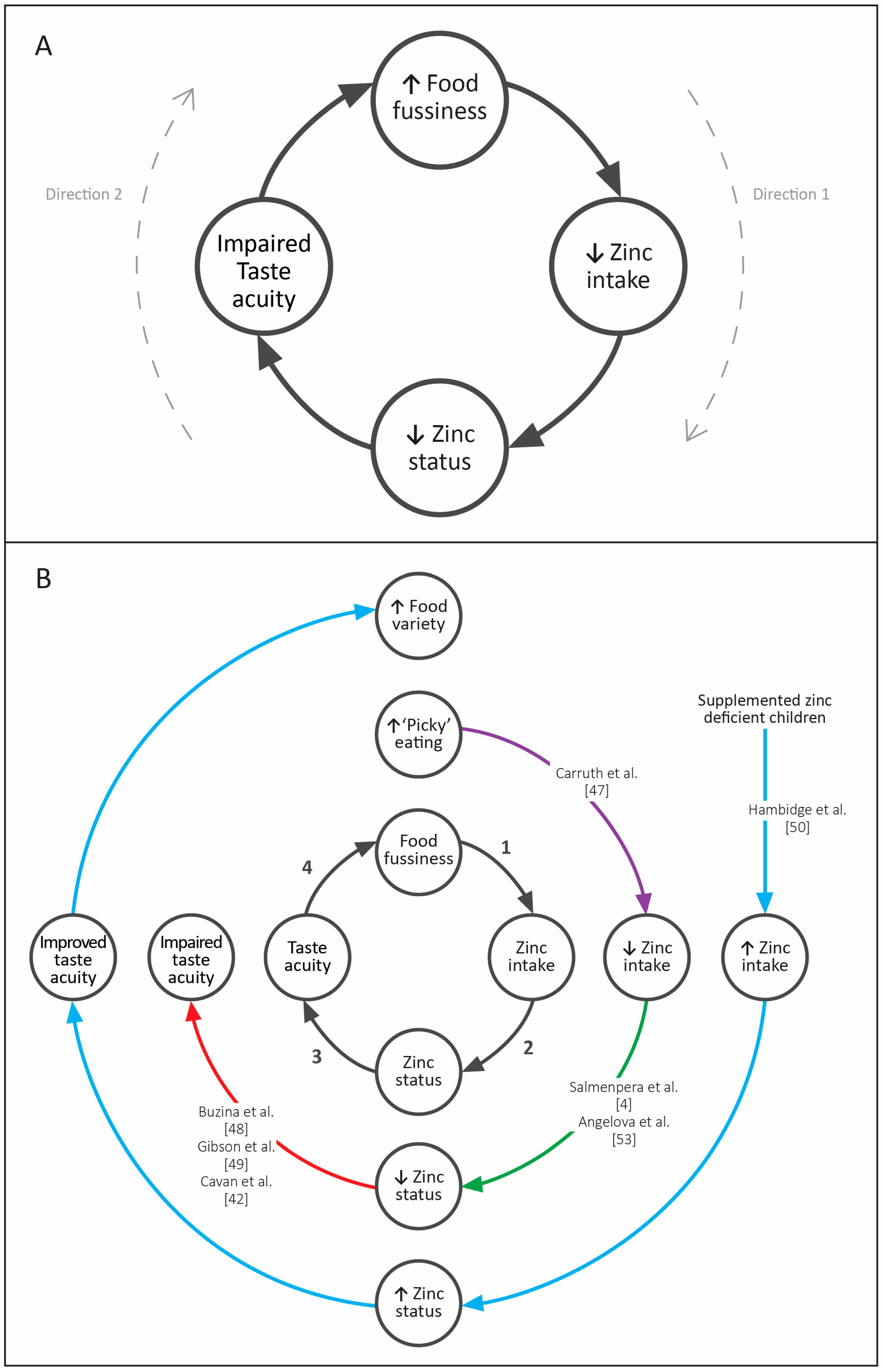
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gibson, R.; Heath, A.-L.M. Population groups at risk of zinc deficiency in Australia and New Zealand. Nutr. Diet. 2011, 68, 97–108. [Google Scholar] [CrossRef]
- Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001.
- World Health Organization. Guiding Principles of Complementary Feeding of the Breastfed Child; World Health Organization: Geneva, Switzerland, 2004; pp. 1–38. [Google Scholar]
- Salmenperä, L.; Perheentupa, J.; Näntö, V.; Siimes, M.A. Low Zinc Intake during Exclusive Breast-Feeding Does Not Impair Growth. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Samuelson, G.; Graham, T.W.; Lönnerdal, B. Zinc intake, zinc status and growth in a longitudinal study of healthy Danish infants. Acta Paediatr. Scand. 1994, 83, 1115–1121. [Google Scholar] [CrossRef]
- Persson, L.Å.; Lundström, M.; Lönnerdal, B.; Hernell, O. Are weaning foods causing impaired iron and zinc status in 1-year-old Swedish infants? A cohort study. Acta Paediatr. Scand. 1998, 87, 618–622. [Google Scholar] [CrossRef]
- Bouglé, D.; Laroche, D.; Bureau, F. Zinc and iron status and growth in healthy infants. Eur. J. Clin. Nutr. 2000, 54, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Kattelmann, K.K.; Ho, M.; Specker, B.L. Effect of Timing of Introduction of Complementary Foods on Iron and Zinc Status of Formula Fed Infants at 12, 24 and 36 Months of Age. J. Am. Diet. Assoc. 2001, 101, 443–447. [Google Scholar] [CrossRef]
- Taylor, A.; Redworth, E.W.; Morgan, J.B. Influence of diet on iron, copper, and zinc status in children under 24 months of age. Biol. Trace Elem. Res. 2004, 97, 197–214. [Google Scholar] [CrossRef]
- Torrejón, C.S.; Castillo-Durán, C.; Hertrampf, E.D.; Ruz, M. Zinc and iron nutrition in Chilean children fed fortified milk provided by the Complementary National Food Program. Nutr. Rev. 2004, 20, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Westcott, J.E.; Butler, T.; Robinson, C.; Bell, M.; Hambidge, K.M. Meat as a first complementary food for breastfed infants: Feasibility and impact on zinc intake and status. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 207–214. [Google Scholar] [PubMed]
- Morgan, E.J.; Heath, A.-L.M.; Szymlek-Gay, E.; Gibson, R.S.; Gray, A.R.; Bailey, K.B.; Ferguson, E.L. Red meat and a fortified manufactured toddler milk drink increase dietary zinc intakes without affecting zinc status of New Zealand toddlers. J. Nutr. 2010, 140, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Yon, M.; Han, H.-S.; Johnston, K.E.; Tamura, T.; Hyun, T. Zinc status and growth of Korean infants fed human milk, casein-based, or soy-based formula: Three-year longitudinal study. Nutr. Res. Pract. 2011, 5, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E.; Laakko, T.; Vollmer, T.L. The dynamic link between the integrity of the immune system and zinc status. J. Nutr. 2000, 130, 1399S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Heath, A.; Ferguson, E.L. Rates of common communicable illnesses in non-anaemic 12–24 month old South Island, New Zealand children. N. Z. Med. J. 2009, 122, 24–35. [Google Scholar] [PubMed]
- Nissensohn, M.; Sánchez-Villegas, A. Effect of zinc intake on growth in infants: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2016, 56, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Lanata, C.F.; Yuen, M.L.; Peerson, J.M.; Butron, B.; Lönnerdal, B. Potential magnitude of the misclassification of a population’s trace element status due to infection: example from a survey of young Peruvian children. Am. J. Clin. Nutr. 1993, 58, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, P.; Bhaskaram, P.; Kumar, P.A.; Khan, M.M.; Islam, M.A. Zinc status of breastfed and formula-fed infants of different gestational ages. J. Trop. Pediat. 1997, 43, 52–54. [Google Scholar] [CrossRef]
- Gibson, R.S.; Ferguson, E.F.; Vanderkooy, P.; MacDonald, A.C. Seasonal variations in hair zinc concentrations in Canadian and African children. Sci. Total Environ. 1989, 84, 291–298. [Google Scholar] [CrossRef]
- Wilhelm, M.; Hafner, D.; Lombeck, I.; Ohnesorge, F.K. Monitoring of cadmium, copper, lead and zinc status in young children using toenails: Comparison with scalp hair. Sci. Total Environ. 1991, 103, 199–207. [Google Scholar] [CrossRef]
- Arsenault, J.E.; Wuehler, S.E.; de Romaña, D.L.; Penny, M.E.; Sempertegui, F.; Brown, K.H. The time of day and the interval since previous meal are associated with plasma zinc concentrations and affect estimated risk of zinc deficiency in young children in Peru and Ecuador. Eur. J. Clin. Nutr. 2011, 65, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Bailey, K.B.; Parnell, W.R.; Wilson, N.; Ferguson, E.L. Higher risk of zinc deficiency in New Zealand Pacific school children compared with their Māori and European counterparts: A New Zealand national survey. Br. J. Nutr. 2010, 105, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Ndjebayi, A.O.; Nankap, M.; Killilea, D.W.; Brown, K.H. Stunting Prevalence, Plasma Zinc Concentrations, and Dietary Zinc Intakes in a Nationally Representative Sample Suggest a High Risk of Zinc Deficiency among Women and Young Children in Cameroon. J. Nutr. 2014, 144, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Galetti, V.; Mitchikpè, C.E.S.; Kujinga, P.; Tossou, F.; Hounhouigan, D.J.; Zimmermann, M.B.; Moretti, D. Rural Beninese Children Are at Risk of Zinc Deficiency According to Stunting Prevalence and Plasma Zinc Concentration but Not Dietary Zinc Intakes. J. Nutr. 2016, 146, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Heath, A.-L.M.; Williams, S.M.; Cameron, S.L.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Gibson, R.S.; Taylor, R.W. Baby-Led Introduction to SolidS (BLISS) study: A randomised controlled trial of a baby-led approach to complementary feeding. BMC Pediatr. 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Williams, S.M.; Fangupo, L.J.; Wheeler, B.J.; Taylor, B.J.; Daniels, L.; Fleming, E.A.; McArthur, J.; Morison, B.; Erickson, L.W.; et al. Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight. JAMA Pediatr. 2017, 171, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Taylor, R.W.; Williams, S.M.; Gibson, R.S.; Fleming, E.A.; Wheeler, B.J.; Taylor, B.J.; Haszard, J.J.; Heath, A.-L.M. Impact of a modified version of Baby-Led Weaning on iron intake and status: A randomised controlled trial. BMJ Open 2018, in press. [Google Scholar]
- Daniels, L.; Taylor, R.W.; Williams, S.M.; Gibson, R.S.; Samman, S.; Wheeler, B.J.; Taylor, B.J.; Fleming, E.A.; Heath, A.-L.M. Modified version of Baby-Led Weaning does not result in lower zinc intake or status in infants: A randomized controlled trial. J. Acad. Nutr. Diet. 2018, in press. [Google Scholar]
- Fangupo, L.J.; Heath, A.-L.M.; Williams, S.M.; Erickson Williams, L.W.; Morison, B.J.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Taylor, R.W. A Baby-Led Approach to Eating Solids and Risk of Choking. Pediatrics 2016, 138, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Salmond, C.; Crampton, P. NZDep2013 Index of Deprivation User’s Manual; Department of Public Health, University of Otago: Wellington, New Zealand; Division of Health Sciences, University of Otago: Dunedin, New Zealand, 2014. [Google Scholar]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the children’s eating behaviour questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Van den Broeck, J.; Chumlea, W.C.; Martorell, R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr. Bull. 2004, 25, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Martorell, R.; Garza, C.; Lartey, A.; Reference, W.M.G. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Scand. 2006, 95, 76–85. [Google Scholar]
- Ministry of Health New Zealand Food Composition Database (FOODfiles). Available online: www.foodcomposition.co.nz/foodfiles (accessed on 4 July 2014).
- Barris, A. Meat and Haem Iron Intakes of New Zealand Toddlers Aged 12–24 Months. Master of Dietetics, University of Otago: Dunedin, New Zealand, 2012. Available online: http://hdl.handle.net/10523/3839 (accessed on 15 September 2017).
- International Zinc Nutrition Consultative Group (IZiNCG). IZiNCG Technical Brief 2007: Assessing Population Zinc Status with Serum Zinc Concentration; International Zinc Nutrition Consultative Group (IZiNCG): Davis, CA, USA, 2007.
- Larson, L.M.; Addo, O.Y.; Sandalinas, F.; Faigao, K.; Kupka, R.; Flores-Ayala, R.; Suchdev, P.S. Accounting for the influence of inflammation on retinol-binding protein in a population survey of Liberian preschool-age children. Matern. Child Nutr. 2017, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gorstein, J.; Sullivan, K.; Yip, R.; de Onis, M.; Trowbridge, F.; Fajans, P.; Clugston, G. Issues in the assessment of nutritional status using anthropometry. Bull. World Health Organ. 1994, 72, 273–283. [Google Scholar] [PubMed]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandström, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) Technical Document #1 Assessment of the Risk of Zinc Deficiency in Populations and Options for its Control. Food Nutr. Bull. 2004, 25 (Suppl. 2), S91–S203. [Google Scholar]
- Hotz, C.; Peerson, J.M.; Brown, K.H. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980). Am. J. Clin. Nutr. 2003, 78, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Cavan, K.R.; Gibson, R.S.; Grazioso, C.F.; Isalgue, A.M.; Ruz, M.; Solomons, N.W. Growth and Body Composition of Periurban Guatemalan Children in Relation to Zinc Status—A Cross-Sectional Study. Am. J. Clin. Nutr. 1993, 57, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.L.; Parackal, S.; Gibson, R.S. Zinc status of 6–24 month old New Zealand children and their mothers. In Proceedings of the IZiNCG Symposium—Moving Zinc into the Micronutrient Program Agenda, Lima, Peru, 19 November 2004. [Google Scholar]
- Szymlek-Gay, E.A.; Ferguson, E.L.; Heath, A.-L.M.; Gray, A.R.; Gibson, R.S. Food-based strategies improve iron status in toddlers: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research (AICR): Washington, DC, USA, 2007. [Google Scholar]
- Roussell, M.A.; Hill, A.M.; Gaugler, T.L.; West, S.G.; Vanden Heuvel, J.P.; Alaupovic, P.; Gillies, P.J.; Kris-Etherton, P.M. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am. J. Clin. Nutr. 2012, 95, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Carruth, B.R.; Ziegler, P.J.; Gordon, A.; Barr, S.I. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J. Am. Diet. Assoc. 2004, 104, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Buzina, R.; Jusić, M.; Sapunar, J.; Milanović, N. Zinc nutrition and taste acuity in school children with impaired growth. Am. J. Clin. Nutr. 1980, 33, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Vanderkooy, P.; MacDonald, A.C.; Goldman, A.; Ryan, B.A.; Berry, M. A Growth-Limiting, Mild Zinc-Deficiency Syndrome in Some Southern Ontario Boys with Low Height Percentiles. Am. J. Clin. Nutr. 1989, 49, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Hambidge, C.; Jacobs, M.; Baum, J.D. Low-Levels of Zinc in Hair, Anorexia, Poor Growth, and Hypogeusia in Children. Pediatr. Res. 1972, 6, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L.; Marlin, D.W. I don’t like it; I never tried it: Effects of exposure on two-year-old children’s food preferences. Appetite 1982, 3, 353–360. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A Systematic Review of the Validity of Dietary Assessment Methods in Children when Compared with the Method of Doubly Labeled Water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.G.; Petkova-Marinova, T.V.; Pogorielov, M.V.; Loboda, A.N.; Nedkova-Kolarova, V.N.; Bozhinova, A.N. Trace Element Status (Iron, Zinc, Copper, Chromium, Cobalt, and Nickel) in Iron-Deficiency Anaemia of Children under 3 Years. Anemia 2014, 2014, 718089. [Google Scholar]
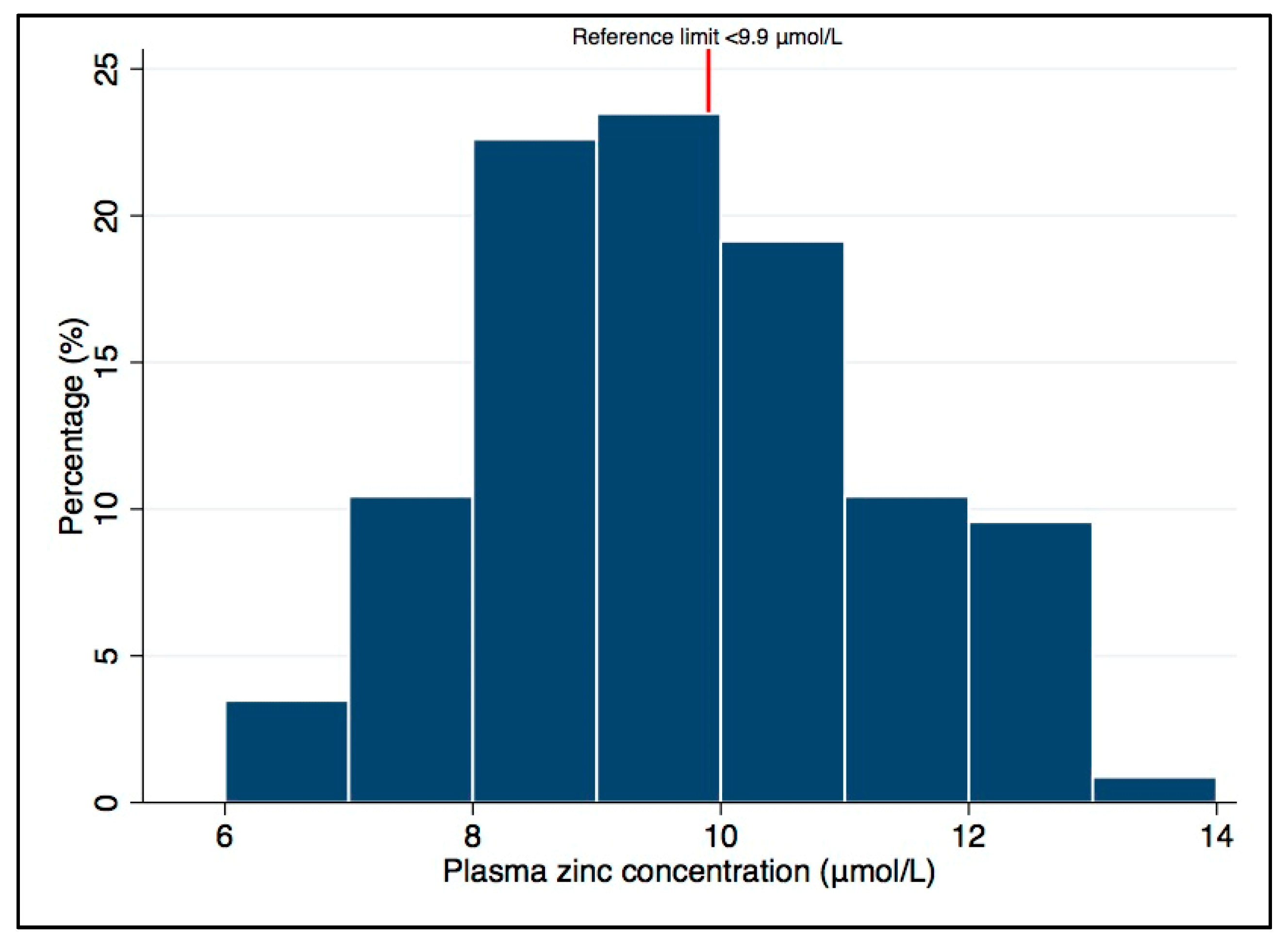
| Total (n = 115) | |
|---|---|
| Maternal and Household Variables at Baseline | |
| Maternal parity | |
| First child | 44 (38) |
| Two children | 47 (41) |
| Three or more children | 24 (21) |
| Maternal ethnicity | |
| New Zealand European | 84 (73) |
| Māori | 22 (19) |
| Other 2 | 9 (8) |
| Maternal education | |
| School only | 33 (29) |
| Post-secondary | 22 (19) |
| University | 60 (52) |
| Household deprivation 3 | |
| 1–3 (Low) | 30 (26) |
| 4–7 | 59 (51) |
| 8–10 (High) | 26 (23) |
| Infant variables at baseline | |
| Sex | |
| Female | 61 (53) |
| Male | 54 (47) |
| Group 4 | |
| Control | 58 (50) |
| BLISS | 57 (50) |
| Median (25th, 75th) 1 | |
|---|---|
| Biochemical Variables | |
| Unadjusted plasma zinc, μmol/L (mean (SD)) | 9.7 (1.5) |
| Adjusted plasma zinc, μmol/L (mean (SD)) 2 | 9.7 (1.5) |
| Hemoglobin, g/L (mean (SD)) 3 | 117 (8.7) |
| C-reactive protein, mg/L | 0.1 (0.0, 0.5) |
| α1-acid glycoprotein, g/L | 0.61 (0.47, 0.87) |
| Dietary variables 4 | |
| Energy, kJ/day | 3543 (3090, 4168) |
| Zinc, mg/day | 4.4 (3.7, 5.4) |
| Phytate, mg/day | 230 (150, 318) |
| Phytate:zinc molar ratio 5 | 5.0 (3.4, 7.1) |
| “Meat, fish, poultry”, g/day | 18.9 (9.5, 30.5) |
| Red meat, g/day | 4.4 (0, 11.5) |
| Cow’s milk, g/day | 21.6 (5.9, 132) |
| Dairy, g/day 6 | 39.9 (9.5, 73.9) |
| Breast milk (n (%)) | |
| No | 47 (45) |
| Yes | 57 (55) |
| Infant formula (n (%)) | |
| No | 59 (57) |
| Yes | 45 (43) |
| Other variables | |
| Weight, kg (mean (SD)) 7 | 9.8 (1.1) |
| Length, cm (mean (SD)) 7 | 75.8 (2.6) |
| Weight-for-age z-score (mean (SD)) 7,8 | 0.37 (0.96) |
| Length-for-age z-score (mean (SD)) 7,9 | 0.26 (0.93) |
| Food fussiness score (mean (SD)) 7,10 | 2.1 (0.6) |
| Age complementary foods introduced, weeks (mean (SD)) | 23.5 (3.6) |
| Topical zinc preparation use in the past month (n (%)) | |
| No | 56 (49) |
| Yes | 59 (51) |
| Change in Plasma Zinc Concentration (μmol/L) 1 for Each Unit Change in the Potential “Predictor” | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for Group | ||||
| n (%) | B (95% CI) | p | B (95% CI) | p | |
| Biochemical variables | |||||
| Hemoglobin, g/L | 114 (99) | 0.05 (0.01, 0.08) | 0.005 | 0.05 (0.02, 0.08) | 0.004 |
| Dietary variables | |||||
| Energy, kJ/day | 104 (90) | 0.00 (−0.00, 0.00) | 0.07 | 0.00 (−0.00, 0.00) | 0.07 |
| Zinc, mg/day | 104 (90) | 0.23 (0.07, 0.39) | 0.005 | 0.23 (0.07, 0.39) | 0.005 |
| Phytate, mg/day | 104 (90) | 0.00 (−0.00, 0.00) | 0.70 | 0.00 (−0.00, 0.00) | 0.71 |
| “Meat, fish, poultry”, g/day | 104 (90) | 0.02 (0.00, 0.03) | 0.015 | 0.02 (0.00, 0.03) | 0.016 |
| Red meat, g/day | 104 (90) | 0.02 (0.00, 0.03) | 0.015 | 0.02 (0.00, 0.03) | 0.016 |
| Cow’s milk, g/day | 104 (90) | 0.00 (−0.00, 0.00) | 0.66 | 0.00 (−0.00, 0.00) | 0.69 |
| Dairy, g/day 2 | 104 (90) | −0.00 (−0.01, 0.00) | 0.50 | −0.00 (−0.01, 0.00) | 0.54 |
| Breast milk | |||||
| No | 47 (45) | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 57 (55) | −0.53 (−1.11, 0.06) | 0.077 | −0.52 (−1.11, 0.06) | 0.080 |
| Infant formula | |||||
| No | 59 (57) | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 45 (43) | 0.76 (0.18, 1.33) | 0.010 | 0.77 (0.19, 1.34) | 0.010 |
| Other variables | |||||
| Maternal parity | |||||
| First child | 44 (38) | 1.00 (reference) | - | 1.00 (reference) | - |
| Two children | 47 (41) | −0.17 (−0.81, 0.47) | 0.60 | −0.16 (−0.80, 0.48) | 0.62 |
| Three or more children | 24 (21) | −0.41 (−1.19, 0.36) | 0.29 | −0.39 (−1.18, 0.39) | 0.32 |
| Maternal education | |||||
| School only | 33 (29) | 1.00 (reference) | - | 1.00 (reference) | - |
| Post-secondary | 22 (19) | −0.33 (−1.15, 0.49) | 0.43 | −0.35 (−1.18, 0.48) | 0.41 |
| University | 60 (52) | −0.77 (−1.42, −0.12) | 0.020 | −0.77 (−1.42, −0.11) | 0.022 |
| Household deprivation 3 | |||||
| 1–3 (Low) | 30 (26) | 1.00 (reference) | - | 1.00 (reference) | - |
| 4–7 | 59 (51) | 0.09 (−0.60, 0.78) | 0.80 | 0.07 (−0.61, 0.76) | 0.83 |
| 8–10 (High) | 26 (23) | 0.80 (0.02, 1.56) | 0.044 | 0.80 (0.03, 1.57) | 0.043 |
| Sex | |||||
| Male | 54 (47) | 1.00 (reference) | - | 1.00 (reference) | - |
| Female | 61 (53) | −0.21 (−0.78, 0.36) | 0.47 | −0.23 (−0.81, 0.34) | 0.42 |
| Weight-for-age z-score 4 | 114 (99) | 0.02 (−0.28, 0.32) | 0.91 | 0.02 (−0.28, 0.32) | 0.90 |
| Length-for-age z-score 5 | 114 (99) | 0.14 (−0.16, 0.45) | 0.35 | 0.16 (−0.15, 0.47) | 0.32 |
| Food fussiness score 6 | 114 (99) | −0.44 (−0.89, 0.02) | 0.06 | −0.41 (−0.88, 0.06) | 0.09 |
| Age complementary foods introduced, weeks | 115 (100) | 0.03 (−0.05, 0.11) | 0.39 | 0.03 (−0.05, 0.11) | 0.47 |
| Topical zinc preparation use in the past month | |||||
| No | 59 (51) | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 56 (49) | −0.07 (−0.64, 0.50) | 0.82 | −0.07 (−0.64, 0.50) | 0.81 |
| Potentially Modifiable “Predictor” Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plasma Zinc | Energy | Zinc | Red Meat | MFP | Infant Formula | Food Fussiness | ||
| Potentially modifiable “predictor” variables | Plasma zinc | - | ||||||
| Energy | 0.18 | - | ||||||
| Zinc | 0.27 * | 0.82 ** | - | |||||
| Red meat | 0.24 * | 0.59 ** | 0.73 ** | - | ||||
| MFP | 0.24 * | 0.53 ** | 0.70 ** | 0.70 ** | - | |||
| Food fussiness | −0.18 | −0.15 | −0.07 | −0.01 | 0.01 | 0.10 | - | |
| Change in Plasma Zinc Concentration (μmol/L) 1 for Each Unit Change in the “Predictor” | ||
|---|---|---|
| B (SE) | p | |
| Red meat intake, 10 g/day | 0.12 (0.04) | 0.004 |
| Infant formula | ||
| No | 1.00 (reference) | - |
| Yes | 0.64 (0.28) | 0.026 |
| Food fussiness score 2 | −0.49 (0.22) | 0.028 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, L.; Williams, S.M.; Gibson, R.S.; Taylor, R.W.; Samman, S.; Heath, A.-L.M. Modifiable “Predictors” of Zinc Status in Toddlers. Nutrients 2018, 10, 306. https://doi.org/10.3390/nu10030306
Daniels L, Williams SM, Gibson RS, Taylor RW, Samman S, Heath A-LM. Modifiable “Predictors” of Zinc Status in Toddlers. Nutrients. 2018; 10(3):306. https://doi.org/10.3390/nu10030306
Chicago/Turabian StyleDaniels, Lisa, Sheila M. Williams, Rosalind S. Gibson, Rachael W. Taylor, Samir Samman, and Anne-Louise M. Heath. 2018. "Modifiable “Predictors” of Zinc Status in Toddlers" Nutrients 10, no. 3: 306. https://doi.org/10.3390/nu10030306






