Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sample
2.3. Cooking Methods
2.4. Sample Preparation and Determination of Total Selenium
2.5. Sample Preparation and Determination of Selenium Speciation
3. Results and Discussion
3.1. Se Concentrations of Bio-Fortified Cereals and Soybeans
3.2. Se Speciation of Bio-Fortified Cereals and Soybeans
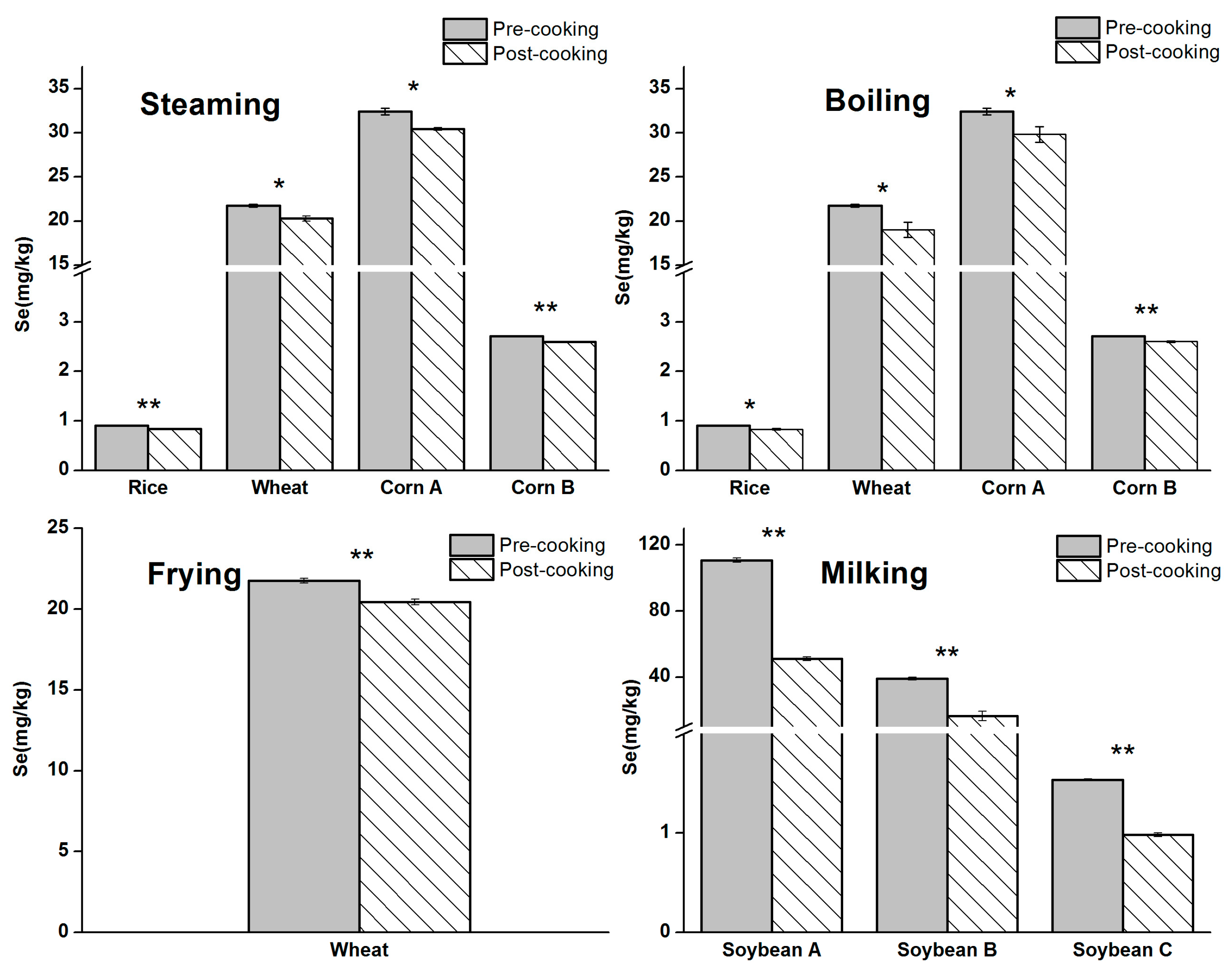
3.3. Effects of Cooking on Se Content
3.4. Effects of Cooking on Se Speciation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska-Durczynska, K.; Lewinski, A. Search for relevant indications for selenium supplementation in thyroid diseases. Neuroendocrinol. Lett. 2017, 38, 237–241. [Google Scholar] [PubMed]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Walker, G. Selenium, immune function and resistance to viral infections. Nutr. Diet. 2008, 65, 41–47. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.Y.; Xue, A.; Bai, J.; Wang, S.Q. Keshan disease—An endemic cardiomyopathy in China. Virchows Arch. A 1983, 401, 1–15. [Google Scholar] [CrossRef]
- Loscalzo, J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, Z.; Luo, D.; Tang, X.; Chen, D.; Hu, Y. Selenium level in the environment and the population of Zhoukoudian area, Beijing, China. Sci. Total Environ. 2007, 381, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Shimbo, S.; Qu, J.B.; Watanabe, T.; Nakatsuka, H.; Matsuda-Inoguchi, N.; Higashikawa, K.; Ikeda, M. Dietary selenium intake of Chinese adult women in the 1990s. Biol. Trace Elem. Res. 2001, 80, 125–138. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Huang, Y.; Lin, Z.Q.; Banuelos, G.S.; Lam, M.H.W.; Yin, X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011, 126, 1088–1093. [Google Scholar] [CrossRef]
- Li, S.; Banuelos, G.S.; Wu, L.; Shi, W. The Changing Selenium Nutritional Status of Chinese Residents. Nutrients 2014, 6, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Hardy, G. Can dietary selenium intake increase the risk of toxicity in healthy children? Nutrition 2016, 32, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nambi, R.W.; Won, S.; Katya, K.; Bai, S.C. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Bratakos, M.S.; Zafiropoulos, T.F.; Siskos, P.A.; Ioannou, P.V. Selenium losses on cooking Greek foods. Int. J. Food Sci. Technol. 1988, 23, 585–590. [Google Scholar] [CrossRef]
- Tanticharoenkiat, O.; Chastain, M.F.; Lane, H.W. Selenium content of chicken meat as affected by cooking. J. Food Sci. 1988, 53, 1294–1295. [Google Scholar] [CrossRef]
- Goenaga Infante, H.; Arias Borrego, A.; Peachey, E.; Hearn, R.; O’Connor, G.; Garcia Barrera, T.; Ariza, J.L.G. Study of the effect of sample preparation and cooking on the selenium speciation of selenized potatoes by HPLC with ICP-MS and electrospray ionization MS/MS. J. Agric. Food Chem. 2009, 57, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.J.; Morris, V.C.; Levander, O.A. Effect of cooking on selenium content of foods. J. Agric. Food Chem. 1972, 20, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.T.; Almeida, C.M.M.; Alvito, P.C. Selenium content of raw and cooked marine species consumed in Portugal. Food Anal. Method 2011, 4, 77–83. [Google Scholar] [CrossRef]
- Thiry, C.; Schneider, Y.J.; Pussemier, L.; De Temmerman, L.; Ruttens, A. Selenium bioaccessibility and bioavailability in Se-enriched food supplements. Biol. Trace Elem. Res. 2013, 152, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Jiang, S.J. Determination of selenium compounds in food supplements using reversed-phase liquid chromatography-inductively coupled plasma mass spectrometry. Microchem. J. 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Hu, L.; Dong, Z.Q.; Huang, X.H.; Li, Y.F.; Li, B.; Qu, L.Y.; Wang, G.P.; Gao, Y.X.; Chen, C.Y. Analysis of small molecular selenium species in serum samples from mercury-exposed people supplemented with selenium-enriched yeast by anion exchange-inductively coupled plasma mass spectrometry. Chin. J. Anal. Chem. 2011, 39, 466–470. [Google Scholar] [CrossRef]
- Da Silva, E.G.; Verola Mataveli, L.R.; Zezzi Arruda, M.A. Speciation analysis of selenium in plankton, Brazil nut and human urine samples by HPLC-ICP-MS. Talanta 2013, 110, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.I.; Yilmaz, O.; Gurkan, R. A micellar improved method for trace levels selenium quantification in food samples, alcoholic and nonalcoholic beverages through CPE/FAAS. Food Chem. 2013, 139, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.X.; Zhu, Y.Y.; Lin, Z.Q.; Banuelos, G.; Li, W.; Yin, X.B. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE 2013, 8, e65615. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.X.; Feng, C.C.; Ye, M.D.; Wang, C.J. Speciation determination of selenium in seafood by high-performance ion-exchange chromatography-hydride generation-atomic fluorescence spectrometry. Food Anal. Method 2015, 8, 1739–1745. [Google Scholar] [CrossRef]
- Mejutomarti, M.; Bollainrodriguez, M.; Herrerolatorre, C.; Bermejomartinez, F. Selenium content of vegetables, fruits, and cereals in Galicia (Northwest Spain). J. Agric. Food Chem. 1988, 36, 293–295. [Google Scholar] [CrossRef]
- DiazAlarcon, J.P.; NavarroAlarcon, M.; delaSerrana, H.L.G.; LopezMartinez, M.C. Determination of selenium in cereals, legumes and dry fruits from southeastern Spain for calculation of daily dietary intake. Sci. Total Environ. 1996, 184, 183–189. [Google Scholar] [CrossRef]
- Kadrabova, J.; Madaric, A.; Ginter, E. The selenium content of selected food from the Slovak Republic. Food Chem. 1997, 58, 29–32. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Gao, J.; Lin, Z.; Banuelos, G.S.; Yuan, L.; Yin, X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 2013, 5, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Eurola, M.; Ekholm, P.; Ylinen, M.; Koivistoinen, P.; Varo, P. Effects of selenium fertilization on the selenium content of cereal grains, flour, and bread produced in Finland. Cereal Chem. 1990, 67, 334–337. [Google Scholar]
- Huerta, V.D.; Reyes, L.H.; Marchante-Gayon, J.M.; Sanchez, M.L.F.; Sanz-Medel, A. Total determination and quantitative speciation analysis of selenium in yeast and wheat flour by isotope dilution analysis ICP-MS. J. Anal. At. Spectrom. 2003, 18, 1243–1247. [Google Scholar] [CrossRef]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- Sun, G.X.; Van de Wiele, T.; Alava, P.; Tack, F.M.G.; Laing, G.D. Bioaccessibility of selenium from cooked rice as determined in a simulator of the human intestinal tract (SHIME). J. Sci. Food Agric. 2017, 97, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
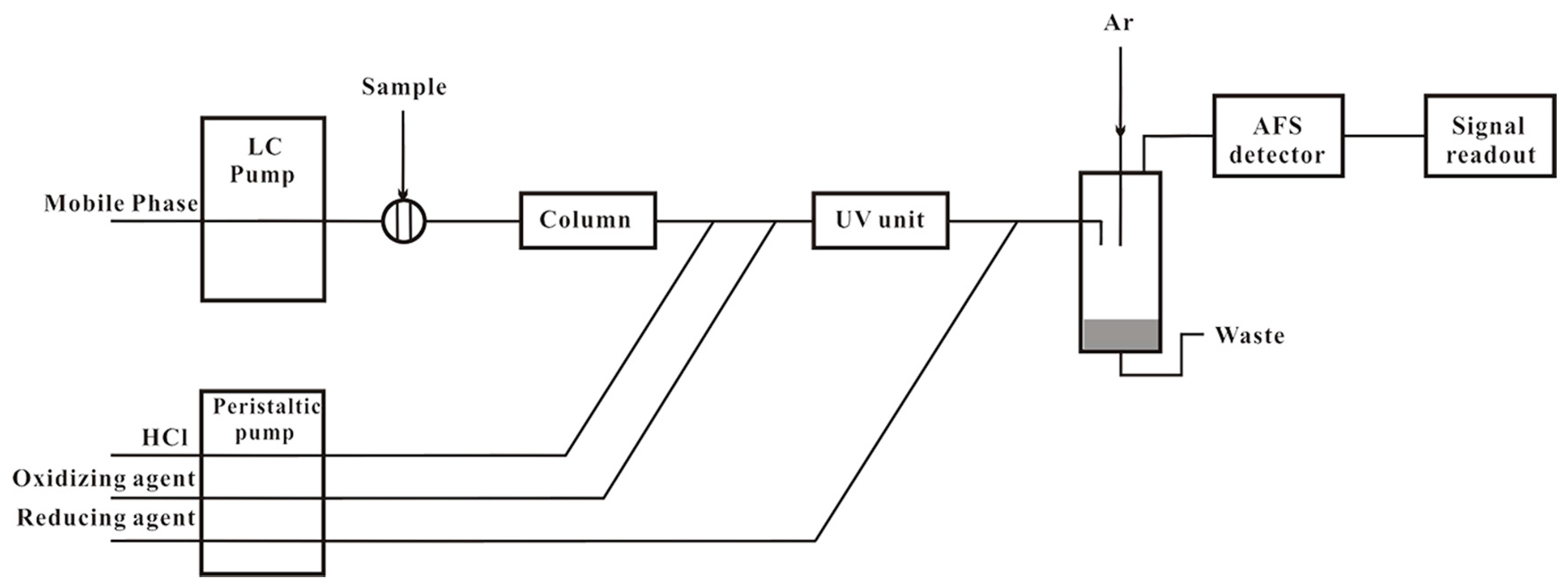

| Cooking Method | Process Description |
|---|---|
| Steaming | 100 g cereals in a container were placed in a steamer with boiling water vapor and heated for 30 min. |
| Boiling | 100 g cereals were put into a container with 1000 g boiling water and heated for 30 min. |
| Frying | Cereals were smashed to powder over a 0.425 mm sieve. 100 g cereals powder and 50 g cold water were mixed into dough. The dough was evenly divided into 30 equal parts and fried in soybean oil at a temperature of 200 °C for 5 min. |
| Milking | Soybean was smashed to powder over a 0.425 mm sieve. 100 g soybean powder were boiled in 2000 mL water at the temperature of 100 °C for 30 min. The liquid called as soy milk produced by filtering the mixture by using a 0.250 mm sieve. |
| LC Parameters | |
|---|---|
| Column | Hamilton PRP X-100 (250 mm × 4.1 mm × 10 μm) |
| Mobile phase | 40 mM NH4H2PO4 (pH 6.0) |
| Flow rate | 1 mL/min |
| Injection volume | 100 μL |
| Samples | Mean ± SD * (mg/kg) |
|---|---|
| Rice | 0.91 ± 0.01 |
| Wheat | 21.7 ± 0.25 |
| Corn A | 32.4 ± 0.38 |
| Corn B | 2.71 ± 0.01 |
| Soybean A | 110.8 ± 12.8 |
| Soybean B | 39.1 ± 1.0 |
| Soybean C | 1.54 ± 0.01 |
| Samples | Cooking | SeCys2 (Se, mg/kg) | SeMeCys (Se, mg/kg) | Selenite (Se, mg/kg) | SeMet (Se, mg/kg) |
|---|---|---|---|---|---|
| Wheat | Pre-cooking | 0.67 ± 0.13 | 0.14 ± 0.08 | ND * | 9.6 ± 0.1 |
| Steaming | 0.57 ± 0.12 | 0.09 ± 0.05 | ND | 8.3 ± 0.2 | |
| Boiling | ND | ND | ND | 7.3 ± 0.1 | |
| Frying | 0.29 ± 0.03 | 0.10 ± 0.03 | ND | 9.3 ± 0.1 | |
| Corn A | Pre-cooking | 0.72 ± 0.22 | 0.21 ± 0.05 | ND | 20.5 ± 2.5 |
| Steaming | 0.43 ± 0.03 | 0.18 ± 0.02 | ND | 19.6 ± 0.2 | |
| Boiling | ND | ND | ND | 17.7 ± 1.1 | |
| Corn B | Pre-cooking | 0.12 ± 0.02 | 0.11 ± 0.03 | ND | 2.2 ± 0.4 |
| Steaming | 0.08 ± 0.02 | 0.08 ± 0.01 | ND | 2.0 ± 0.1 | |
| Boiling | ND | ND | ND | 1.7 ± 0.1 | |
| Soybean A | Pre-cooking | 7.7 ± 0.9 | 1.4 ± 0.1 | 3.7 ± 0.9 | 55.7 ± 0.2 |
| Milking | 3.2 ± 0.5 | 0.79 ± 0.27 | ND | 29.6 ± 1.1 | |
| Soybean B | Pre-cooking | 0.64 ± 0.13 | 1.1 ± 0.1 | 0.35 ± 0.04 | 25.7 ± 3.5 |
| Milking | 0.21 ± 0.03 | 0.29 ± 0.16 | ND | 16.6 ± 2.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. https://doi.org/10.3390/nu10030317
Lu X, He Z, Lin Z, Zhu Y, Yuan L, Liu Y, Yin X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients. 2018; 10(3):317. https://doi.org/10.3390/nu10030317
Chicago/Turabian StyleLu, Xiaoqi, Zisen He, Zhiqing Lin, Yuanyuan Zhu, Linxi Yuan, Ying Liu, and Xuebin Yin. 2018. "Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans" Nutrients 10, no. 3: 317. https://doi.org/10.3390/nu10030317





