l-Carnitine Supplementation in Recovery after Exercise
Abstract
:1. Introduction
2. Methodology
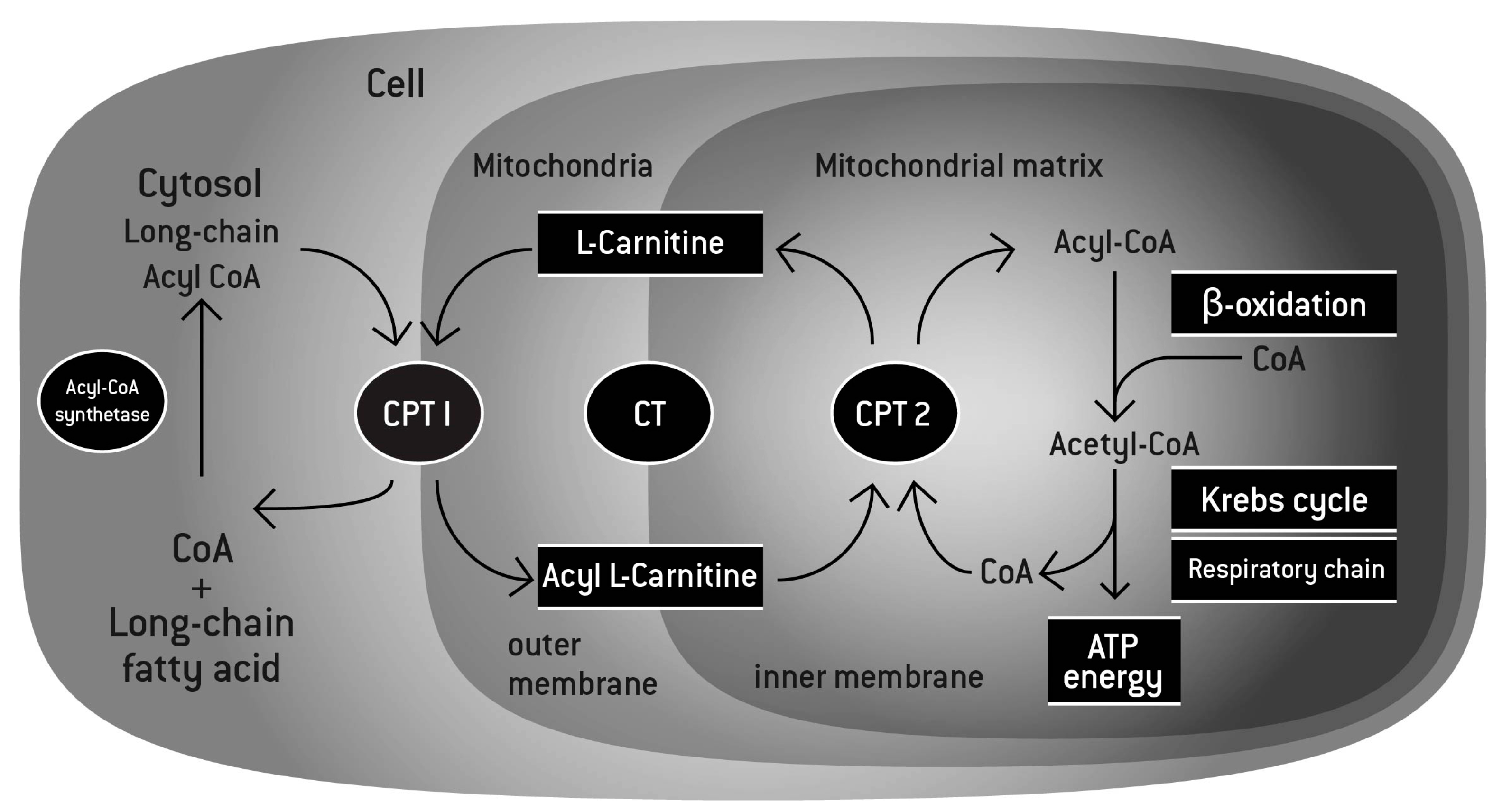
3. l-Carnitine and Exercise
4. Mechanisms Involved in the Effects of l-Carnitine on Recovery after Exercise
4.1. Effects of l-Carnitine on Muscle Injury during Exercise
4.2. Effects of l-Carnitine on Blood Flow and Endothelial Function
4.3. l-Carnitine as an Anti-Oxidant
5. l-Carnitine and Aging: Old Molecule, New Uses
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gulewitsch, W. Zur Kenntnis der Extraktionsstoffe der Muskeln. 2. Mitteilungen über das Carnitin (extracted substances in muscle, report on carnitine). Hoppe-Seyler Z. Physiol. Chem. 1905, 45, 326–330. [Google Scholar] [CrossRef]
- Tomita, M.; Senju, Y. Über die Aminoverbindungen, welche die Biuretreaktion zeigen. III. Spaltungen der gamma-amino-beta-Buttersäure in die optisch aktiven Komponenten. Hoppe-Seyler Z. Physiol. Chem. 1927, 169, 263–277. [Google Scholar] [CrossRef]
- Fritz, I.B. Action of carnitine on long chain fatty acid oxidation by liver. Am. J. Physiol. 1959, 197, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Lohninger, A. Supplementation of l-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine-metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, V.; Broquist, H.P. Role of lysine and e-N-trimethyllysine in carnitine biosynthesis. Ii. Studies in the rat. J. Biol. Chem. 1973, 248, 2176–2181. [Google Scholar] [PubMed]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef] [PubMed]
- Tein, I.; Bukovac, S.W.; Xie, Z.W. Characterization of the human plasmalemmal carnitine transporter in cultured skin fibroblasts. Arch. Biochem. Biophys. 1996, 329, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin. Ther. 1995, 17, 176–185, discussion 175. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Gandour, R.D.; Van der Leij, F.R. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta 2001, 1546, 21–43. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. (Lond.) 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.A.; DeLeeuw, S.; Coates, P.M.; Vianey-Liaud, C.; Divry, P.; Bonnefont, J.P.; Saudubray, J.M.; Haymond, M.; Trefz, F.K.; Breningstall, G.N.; et al. Chronic cardiomyopathy and weakness or acute coma in children with a defect in carnitine uptake. Ann. Neurol. 1991, 30, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rigault, C.; Mazue, F.; Bernard, A.; Demarquoy, J.; Le Borgne, F. Changes in l-carnitine content of fish and meat during domestic cooking. Meat Sci. 2008, 78, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Lombard, K.A.; Olson, A.L.; Nelson, S.E.; Rebouche, C.J. Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am. J. Clin. Nutr. 1989, 50, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Novakova, K.; Kummer, O.; Bouitbir, J.; Stoffel, S.D.; Hoerler-Koerner, U.; Bodmer, M.; Roberts, P.; Urwyler, A.; Ehrsam, R.; Krahenbuhl, S. Effect of l-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur. J. Nutr. 2016, 55, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J.; Chenard, C.A. Metabolic fate of dietary carnitine in human adults: Identification and quantification of urinary and fecal metabolites. J. Nutr. 1991, 121, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Carnitine function and requirements during the life cycle. FASEB J. 1992, 6, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.; Elwin, C.E.; Cederblad, G. Pharmacokinetics of bolus intravenous and oral doses of l-carnitine in healthy subjects. Eur. J. Clin. Pharmacol. 1988, 35, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Jameson, E.; Doxey, A.C.; Airs, R.; Purdy, K.J.; Murrell, J.C.; Chen, Y. Metagenomic data-mining reveals contrasting microbial populations responsible for trimethylamine formation in human gut and marine ecosystems. Microb. Genom. 2016, 2, e000080. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.L.; Jeejeebhoy, K.N.; Sole, M.J. The management of conditioned nutritional requirements in heart failure. Heart Fail Rev. 2006, 11, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Merli, E.; Cicchitelli, G.; Mele, D.; Fucili, A.; Ceconi, C. Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: A review. Ann. N. Y. Acad. Sci. 2004, 1033, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R. Carnitine and peripheral arterial disease. Ann. N. Y. Acad. Sci. 2004, 1033, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Borghi-Silva, A.; Baldissera, V.; Sampaio, L.M.; Pires-DiLorenzo, V.A.; Jamami, M.; Demonte, A.; Marchini, J.S.; Costa, D. l-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz. J. Med. Biol. Res. 2006, 39, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.N.; Khan, A.A.; Gupta, A.; Vajifdar, B.U.; Lokhandwala, Y.Y. l-carnitine moderately improves the exercise tolerance in chronic stable angina. J. Assoc. Phys. India 2000, 48, 1050–1052. [Google Scholar]
- Loster, H.; Miehe, K.; Punzel, M.; Stiller, O.; Pankau, H.; Schauer, J. Prolonged oral l-carnitine substitution increases bicycle ergometer performance in patients with severe, ischemically induced cardiac insufficiency. Cardiovasc. Drugs Ther. 1999, 13, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ajisaka, R.; Masuoka, T.; Yamanouchi, T.; Saitou, T.; Toyama, M.; Takeyasu, N.; Sakamoto, K.; Sugishita, Y. Effects of l- and dl-carnitine on patients with impaired exercise tolerance. Jpn. Heart J. 1995, 36, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Ricoy, J.R.; Encinas, A.R.; Pola, P.; D’Iddio, S.; Zeviani, M.; Didonato, S.; Corsi, M. Carnitine in muscle, serum, and urine of nonprofessional athletes: Effects of physical exercise, training, and l-carnitine administration. Muscle Nerve 1991, 14, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.; Vogt, M.; Kreis, R.; Boesch, C.; Bigler, P.; Hoppeler, H.; Krahenbuhl, S. Long-term administration of l-carnitine to humans: Effect on skeletal muscle carnitine content and physical performance. Clin. Chim. Acta 2002, 318, 51–61. [Google Scholar] [CrossRef]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D. Carbohydrate, protein, and fat metabolism during exercise after oral carnitine supplementation in humans. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D. Effects of exercise intensity and altered substrate availability on cardiovascular and metabolic responses to exercise after oral carnitine supplementation in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Dragan, G.I.; Wagner, W.; Ploesteanu, E. Studies concerning the ergogenic value of protein supply and l-carnitine in elite junior cyclists. Physiologie 1988, 25, 129–132. [Google Scholar] [PubMed]
- Dragan, I.G.; Vasiliu, A.; Georgescu, E.; Eremia, N. Studies concerning chronic and acute effects of l-carnitina in elite athletes. Physiologie 1989, 26, 111–129. [Google Scholar] [PubMed]
- Huertas, R.; Campos, Y.; Diaz, E.; Esteban, J.; Vechietti, L.; Montanari, G.; D’Iddio, S.; Corsi, M.; Arenas, J. Respiratory chain enzymes in muscle of endurance athletes: Effect of l-carnitine. Biochem. Biophys. Res. Commun. 1992, 188, 102–107. [Google Scholar] [CrossRef]
- Orer, G.E.; Guzel, N.A. The effects of acute l-carnitine supplementation on endurance performance of athletes. J. Strength Cond. Res. 2014, 28, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Siliprandi, N.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Maccari, F.; Menabo, R.; Giamberardino, M.A.; Vecchiet, L. Metabolic changes induced by maximal exercise in human subjects following l-carnitine administration. Biochim. Biophys. Acta 1990, 1034, 17–21. [Google Scholar] [CrossRef]
- Vecchiet, L.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Menabo, R.; Giamberardino, M.A.; Siliprandi, N. Influence of l-carnitine administration on maximal physical exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Broad, E.M.; Maughan, R.J.; Galloway, S.D. Effects of four weeks l-carnitine l-tartrate ingestion on substrate utilization during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Colombani, P.; Wenk, C.; Kunz, I.; Krahenbuhl, S.; Kuhnt, M.; Arnold, M.; Frey-Rindova, P.; Frey, W.; Langhans, W. Effects of l-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: A double-blind crossover field study. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Decombaz, J.; Deriaz, O.; Acheson, K.; Gmuender, B.; Jequier, E. Effect of l-carnitine on submaximal exercise metabolism after depletion of muscle glycogen. Med. Sci. Sports Exerc. 1993, 25, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.W.; Costill, D.L.; Goodpaster, B.; Vukovich, M.D.; Fink, W.J. The effects of l-carnitine supplementation on performance during interval swimming. Int. J. Sports Med. 1994, 15, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Vukovich, M.D.; Costill, D.L.; Fink, W.J. Carnitine supplementation: Effect on muscle carnitine and glycogen content during exercise. Med. Sci. Sports Exerc. 1994, 26, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.B.; Wall, B.T.; Marimuthu, K.; Shannon, C.E.; Constantin-Teodosiu, D.; Macdonald, I.A.; Greenhaff, P.L. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J. Physiol. 2013, 591, 4655–4666. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.; Costill, D.L.; Vukovich, M.D.; Cole, K.J.; Goodpaster, B.H.; Trappe, S.W.; Fink, W.J. Effect of l-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int. J. Sport Nutr. 1994, 4, 280–288. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Hart, S.; Marshall, T.; Joseph, E.; Reau, A.; Swain, C.B.; Diehl, J. Enhanced aerobic exercise performance in women by a combination of three mineral chelates plus two conditionally essential nutrients. J. Int. Soc. Sports Nutr. 2017, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Cammalleri, L.; Gargante, M.P.; Vacante, M.; Colonna, V.; Motta, M. l-carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: A randomized and controlled clinical trial. Am. J. Clin. Nutr. 2007, 86, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Pistone, G.; Marino, A.; Leotta, C.; Dell’Arte, S.; Finocchiaro, G.; Malaguarnera, M. Levocarnitine administration in elderly subjects with rapid muscle fatigue: Effect on body composition, lipid profile and fatigue. Drugs Aging 2003, 20, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Cristaldi, E.; Colonna, V.; Messano, M.; Koverech, A.; Neri, S.; Vacante, M.; Cammalleri, L.; Motta, M. Acetyl l-carnitine (alc) treatment in elderly patients with fatigue. Arch. Gerontol. Geriatr. 2008, 46, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.Q.; Jit, H.; Maxwell, C.V.; Nelssen, J.L.; Goodband, R.D.; Tokach, M.D.; Tremblay, G.C.; Koo, S.I. Dietary l-carnitine suppresses mitochondrial branched-chain keto acid dehydrogenase activity and enhances protein accretion and carcass characteristics of swine. J. Anim. Sci. 2001, 79, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Varney, J.L.; Fowler, J.W.; Gilbert, W.C.; Coon, C.N. Utilisation of supplemented l-carnitine for fuel efficiency, as an antioxidant, and for muscle recovery in Labrador retrievers. J. Nutr. Sci. 2017, 6, e8. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of l-carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. (Lond.) 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Hatfield, D.L.; Vingren, J.L.; Fragala, M.S.; Ho, J.Y.; Thomas, G.A.; Hakkinen, K.; Volek, J.S. Effects of l-carnitine l-tartrate supplementation on muscle oxygenation responses to resistance exercise. J. Strength Cond. Res. 2008, 22, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Spiering, B.A.; Volek, J.S.; Ratamess, N.A.; Sharman, M.J.; Rubin, M.R.; French, D.N.; Silvestre, R.; Hatfield, D.L.; Van Heest, J.L.; et al. Androgenic responses to resistance exercise: Effects of feeding and l-carnitine. Med. Sci. Sports Exerc. 2006, 38, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, R.; Rossetto, M.; Martina, B. Plasma and urine carnitine concentrations in well-trained athletes at rest and after exercise. Influence of l-carnitine intake. Drugs Exp. Clin. Res. 1999, 25, 167–171. [Google Scholar] [PubMed]
- Giamberardino, M.A.; Dragani, L.; Valente, R.; Di Lisa, F.; Saggini, R.; Vecchiet, L. Effects of prolonged l-carnitine administration on delayed muscle pain and ck release after eccentric effort. Int. J. Sports Med. 1996, 17, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Volek, J.S.; French, D.N.; Rubin, M.R.; Sharman, M.J.; Gomez, A.L.; Ratamess, N.A.; Newton, R.U.; Jemiolo, B.; Craig, B.W.; et al. The effects of l-carnitine l-tartrate supplementation on hormonal responses to resistance exercise and recovery. J. Strength Cond. Res. 2003, 17, 455–462. [Google Scholar] [PubMed]
- Maggini, S.; Bänziger, K.R.; Walter, P. l-carnitine supplementation results in improved recovery after strenuous exercise—A preliminary study. Ann. Nutr. Metab. 2000, 44, 86–88. [Google Scholar]
- Stuessi, C.; Hofer, P.; Meier, C.; Boutellier, U. l-carnitine and the recovery from exhaustive endurance exercise: A randomised, double-blind, placebo-controlled trial. Eur. J. Appl. Physiol. 2005, 95, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Kraemer, W.J.; Rubin, M.R.; Gomez, A.L.; Ratamess, N.A.; Gaynor, P. l-carnitine l-tartrate supplementation favorably affects markers of recovery from exercise stress. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Vingren, J.L.; Hatfield, D.L.; Fragala, M.S.; Ho, J.Y.; Maresh, C.M.; Anderson, J.M.; Volek, J.S. Responses of criterion variables to different supplemental doses of l-carnitine l-tartrate. J. Strength Cond. Res. 2007, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Judelson, D.A.; Silvestre, R.; Yamamoto, L.M.; Spiering, B.A.; Hatfield, D.L.; Vingren, J.L.; Quann, E.E.; Anderson, J.M.; Maresh, C.M.; et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am. J. Cardiol. 2008, 102, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Parandak, K.; Arazi, H.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The effect of two-week l-carnitine supplementation on exercise -induced oxidative stress and muscle damage. Asian J. Sports Med. 2014, 5, 123–128. [Google Scholar] [PubMed]
- Ho, J.Y.; Kraemer, W.J.; Volek, J.S.; Fragala, M.S.; Thomas, G.A.; Dunn-Lewis, C.; Coday, M.; Hakkinen, K.; Maresh, C.M. l-carnitine l-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and women. Metabolism 2010, 59, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Oyono-Enguelle, S.; Freund, H.; Ott, C.; Gartner, M.; Heitz, A.; Marbach, J.; Maccari, F.; Frey, A.; Bigot, H.; Bach, A.C. Prolonged submaximal exercise and l-carnitine in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Volek, J.S.; Spiering, B.A.; Vingren, J.L. l-carnitine supplementation: A new paradigm for its role in exercise. Monatshefte Chem. 2005, 136, 1383–1390. [Google Scholar] [CrossRef]
- Dubelaar, M.L.; Lucas, C.M.; Hulsmann, W.C. The effect of l-carnitine on force development of the latissimus dorsi muscle in dogs. J. Card. Surg. 1991, 6, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hulsmann, W.C.; Dubelaar, M.L. Carnitine requirement of vascular endothelial and smooth muscle cells in imminent ischemia. Mol. Cell. Biochem. 1992, 116, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Schwane, J.A.; Johnson, S.R.; Vandenakker, C.B.; Armstrong, R.B. Delayed-onset muscular soreness and plasma cpk and ldh activities after downhill running. Med. Sci. Sports Exerc. 1983, 15, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Newham, D.J.; McPhail, G.; Mills, K.R.; Edwards, R.H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J. Neurol. Sci. 1983, 61, 109–122. [Google Scholar] [CrossRef]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J. Orthop. Sports Phys. Ther. 2002, 32, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cleak, M.J.; Eston, R.G. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br. J. Sports Med. 1992, 26, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Parthimos, T.; Schulpis, K.H.; Angelogianni, P.; Tsopanakis, C.; Parthimos, N.; Tsakiris, S. The in vivo and in vitro effects of l-carnitine supplementation on the erythrocyte membrane acetylcholinesterase, Na+, K+-atpase and Mg2+-atpase activities in basketball players. Clin. Chem. Lab. Med. 2008, 46, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Balasubramaniam, M.; Suri, P.; Mackintosh, S.G.; Tackett, A.J.; Sullivan, D.H.; Shmookler Reis, R.J.; Dennis, R.A. Proteins that accumulate with age in human skeletal-muscle aggregates contribute to declines in muscle mass and function in caenorhabditis elegans. Aging (Albany, NY) 2016, 8, 3486–3497. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010, 24, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; Van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type ii muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J. Nutr. 2006, 136, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L.; Tapsell, L.C.; Owen, A.; Gutteridge, I. The influence of red meat intake upon the response to a resistance exercise-training programm in older Australians. Aisa Pac. J. Clin. Nutr. 2003, 12, 17. [Google Scholar]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Messier, V.; Suppere, C.; Briand, P.; Rabasa-Lhoret, R. Effect of cysteine-rich whey protein (immunocal(r)) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J. Nutr. Health Aging 2015, 19, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; Van der Zwaluw, N.; Verdijk, L.B.; Van de Rest, O.; De Groot, L.C.; Van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Van de Rest, O.; Dirks, M.L.; Van der Zwaluw, N.; Mensink, M.; Van Loon, L.J.; De Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.K.; Quinn, M.A.; Saunders, D.H.; Greig, C.A. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2016, 17, 959.e1–959.e9. [Google Scholar] [CrossRef] [PubMed]
- Costell, M.; O’Connor, J.E.; Grisolia, S. Age-dependent decrease of carnitine content in muscle of mice and humans. Biochem. Biophys. Res. Commun. 1989, 161, 1135–1143. [Google Scholar] [CrossRef]
- Karlic, H.; Lohninger, A.; Laschan, C.; Lapin, A.; Bohmer, F.; Huemer, M.; Guthann, E.; Rappold, E.; Pfeilstocker, M. Downregulation of carnitine acyltransferases and organic cation transporter octn2 in mononuclear cells in healthy elderly and patients with myelodysplastic syndromes. J. Mol. Med. (Berl.) 2003, 81, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Badrasawi, M.; Shahar, S.; Zahara, A.M.; Nor Fadilah, R.; Singh, D.K. Efficacy of l-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: A double-blind, randomized, placebo-controlled clinical trial. Clin. Interv. Aging 2016, 11, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.G.; Gannon, J.; Self, M.; Rich, P.A. l-carnitine supplementation combined with aerobic training does not promote weight loss in moderately obese women. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Pooyandjoo, M.; Nouhi, M.; Shab-Bidar, S.; Djafarian, K.; Olyaeemanesh, A. The effect of (l-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Ringseis, R.; Koc, A.; Lukas, I.; Kluge, H.; Eder, K. Supplementation with l-carnitine downregulates genes of the ubiquitin proteasome system in the skeletal muscle and liver of piglets. Animal 2012, 6, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the igf-1/pi3k/akt signalling pathway and down regulates the e3 ligase murf1 in skeletal muscle of rats. Nutr. Metab. (Lond.) 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical involvement in aging. Pathophysiology and therapeutic implications. Drugs Aging 1993, 3, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qu, H.J.; Li, P.; Wang, C.B.; Wang, L.X.; Han, Z.W. Single dose administration of l-carnitine improves antioxidant activities in healthy subjects. Tohoku J. Exp. Med. 2011, 224, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Mitochondrial decay in aging. Biochim. Biophys. Acta 1995, 1271, 165–170. [Google Scholar] [CrossRef]
- Nicassio, L.; Fracasso, F.; Sirago, G.; Musicco, C.; Picca, A.; Marzetti, E.; Calvani, R.; Cantatore, P.; Gadaleta, M.N.; Pesce, V. Dietary supplementation with acetyl-l-carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats. Exp. Gerontol. 2017, 98, 99–109. [Google Scholar] [CrossRef] [PubMed]
| Authors/Title | # Subject | Age (Mean or Range) | Dose Duration | Outcome |
|---|---|---|---|---|
| Athletes/Well Trained (Professionals, Age 16–36) | ||||
| [29] Carnitine in muscle, serum, and urine of nonprofessional athletes: effects of physical exercise, training, and l-carnitine administration. | 24 athletes | 19–27 | 1 g BID for 6 mo of training | Prevention of training decreased total and free. Carnitine, positive effect on recovery. |
| [33] Studies concerning the ergogenic value of protein supply and l-carnitine in elite junior cyclists. | 7 junior athletes | na | 1 g/d for 6 wk and 2 g/d for 10 d (before competition) | Supplemented group showed better stress-induced efforts and obtained higher performances. |
| [34] Studies concerning chronic and acute effects of l-carnitine in elite athletes. | 110 athletes (in 6 studies) | 16–33 | 4 g oral of 1 g iv (single dose) 3 g/d for 3 wk or placebo | Single dose: beneficial effects on physical output, lipid metabolism, muscular function (contraction), lactate accumulation after exercise and urine mucoproteins. 3 week treatment: Beneficial effects on the lipid metabolism, evoked muscular potential, VO2max, behavior and the biological output. |
| [35] Respiratory chain enzymes in muscle of endurance athletes: effect of l-carnitine. | 14 athletes | na | 2 g BID for 4 wk of training | Increase in respiratory-chain enzyme activities in the muscle. |
| [36] The effects of acute l-carnitine supplementation on endurance performance of athletes. | 26 athletes | 18.42 ± 0.5 | 12 received 3 g 14 received 4 g | Compared to placebo, l-carnitine supplemented groups showed lower lactate levels and lower heart rate. |
| [40] Effects of l-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: a double-blind crossover field study. | 7 athletes | 36 ± 3 | 2 g before start and after 20 km run | Significant increase in l-carnitine plasma concentration. No effect on performance or metabolism. |
| [54] Effects of l-carnitine l-tartrate supplementation on muscle oxygenation responses to resistance exercise. | 9 healthy, previously resistance trained men | 25.2 ± 6 | 2 g/d for 23 d or placebo | Enhanced oxygen consumption => hypoxic stress is attenuated with carnitine supplementation. |
| [55] Androgenic responses to resistance exercise: effects of feeding and l-carnitine. | 10 resistance-trained men | 22 ± 1 | 2 g/d for 21 d or placebo | Increased androgen receptor content and enhanced luteinizing hormone. |
| [56] Plasma and urine carnitine concentrations in well-trained athletes at rest and after exercise. Influence of l-carnitine intake. | 9 athletes | na | 1 g before and after treadmill ergometer or placebo | No decrease in serum carnitine levels after exercise in the supplementation group. No effect on maximal exercise. No effect on maximal exercise. |
| [32] Effects of exercise intensity and altered substrate availability on cardiovascular and metabolic responses to exercise after oral carnitine supplementation in athletes. | 15 athletes | Pl: 31 ± 8 LC: 34 ± 10 | 3 g/d for 15 d or placebo | No significant difference between whole-body rates of CHO and fat oxidation at any workload. At day 15, heart rate and blood glucose concentration were lower during exercise in the l-carnitine group compared to Placebo. |
| [31] Carbohydrate, protein, and fat metabolism during exercise after oral carnitine supplementation in humans. | 20 active male athletes | Pl: 32 ± 9 LC: 34 ± 10 | 2 g/d for 2 wks or placebo | After 2 wk of l-carnitine supplementation, plasma ammonia response to exercise tended to be suppressed. No effects on fat, carbohydrate, or protein contribution to metabolism during prolonged moderate-intensity cycling exercise |
| Healthy (recreationally active, age 18–50) | ||||
| [37] Metabolic changes induced by maximal exercise in human subjects following l-carnitine administration. | 10 moderately trained men | 18.42 ± 0.50 | 2 g before high-intensity exercise | Stimulation of PDH activity, and decrease in plasma lactate and pyruvate. |
| [38] Influence of l-carnitine administration on maximal physical exercise. | 10 moderately trained men | 22–30 | 2 g before high-intensity exercise | Increased VO2max. |
| [39] Effects of four weeks l-carnitine l-tartrate ingestion on substrate utilization during prolonged exercise. | 15 trained males | 20–46 | 3 g for 4 wk or placebo | No effect on substrate utilization or endurance performance. |
| [57] Effects of prolonged l-carnitine administration on delayed muscle pain and CK release after eccentric effort. | 6 untrained subjects | 26 ± 3.8 | 3 g/d for 3 wk | Protective effect against pain and damage from eccentric effort. |
| [58] The effects of l-carnitine l-tartrate supplementation on hormonal responses to resistance exercise and recovery. | 10 healthy, recreationally weight-trained men | 23.7 ± 2.3 | 2 g/d for 3 wk | Increased IGFBP-3 concentrations prior to and at 30, 120, and 180 min after acute exercise => protection from muscle damage. |
| [41] Effect of l-carnitine on submaximal exercise metabolism after depletion of muscle glycogen. | 9 healthy males | 24.9 ± 1.0 | 3 g/d for 7 d | No effects on fat oxidation, RQ, perceived exertion, lactate, heart rate during exercise after glycogen depletion. |
| [42] The effects of l-carnitine supplementation on performance during interval swimming. | 20 (swimmers) | 20.1 ± 0.6 | 2 g BID for 7 d or placebo | Elevation in serum l-carnitine and carnitine fractions. No differences in performance times between trials or groups was observed; similar response related to blood pH, LA and BE in both groups during each trial was revealed. |
| [43] Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise. | 8 | 26.8 ± 2.31 | 4 g/d for 14 d | Increase in serum carnitine. No effect on muscle carnitine content, lipid oxidation and lactate accumulation. |
| [59] l-carnitine supplementation results in improved recovery after strenuous exercise—a preliminary study. | 12 (trained/untrained) | 25.7 ± 4 | 2 g/d for 5 d | Improved recovery in 9 of 12 subjects. |
| [60] l-carnitine and the recovery from exhaustive endurance exercise: a randomised, double-blind, placebo-controlled trial. | 12 | 25 ± 3 | 2 g/d for 14 d or placebo | 2 g of l-carnitine taken 2 h before a first of 2 constant-load exercise tests had no influence on the second tests performed 3h after the first test compared with placebo. |
| [61] l-Carnitine l-tartrate supplementation favorably affects markers of recovery from exercise stress. | 30 healthy subjects | 30 ± 8 | 2 g for 3 wk or placebo | Improvement in postprandial vascular functions after a high-fat meal. |
| [62] Responses of criterion variables to different supplemental doses of l-carnitine l-tartrate. | 8 healthy men | 22 ± 3 | 0, 1 g, 2 g for 3 wk | Increase in serum carnitine concentrations. Reduction in post-exercise serum hypoxanthine, serum xanthine oxidase, serum myoglobin, and perceived muscle soreness. Reduced metabolic stress, less muscle damage. |
| [63] Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. | 30 healthy men and women | 30 ± 8 | 2 g/d for 3 wk or placebo | Improvement in postprandial flow-mediated dilatation after a high-fat meal. |
| [64] The effect of two-week l-carnitine supplementation on exercise-induced oxidative stress and muscle damage. | 21 active healthy men | About 22 | 2 g/d for 14 d or placebo | Increase in total antioxidant capacity after 14d and 24h post exercise. Lower malondialdehyde-TBARS, creatine kinase and lactate dehydrogenase 24 h post exercise. |
| [65] l-Carnitine l-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and women. | 18 healthy men and women | m: 45.4 ± 5.3 f: 51.9 ± 5.0 | 2 g/d for 24 d | Positive effects on purine metabolism, free radical formation, muscle tissue disruption, muscle soreness. No effect on physical performance. |
| [30] Long-term administration of l-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. | 8 healthy male adults | 23–25 | 2 × 2 g/d for 3 months | No significant differences between VO2max, RERmax, and Pmax between the three time points investigated: pre/post at baseline and post exercise after 3 months. No pre/post difference in muscle carnitine content at baseline and post-exercise at 3 months. Activities of citrate synthase and cytochrome oxidase, as well as the skeletal muscle fiber composition remained unaffected. |
| [66] Prolonged submaximal exercise and l-carnitine in humans. | 10 young males | na | 2 g/d for 4 wks; followed by 0 g/d for 6–8 wks | Twenty-five percent increase in free and total l-carnitine plasma levels during supplementation. These levels returned to normal 6–8 weeks after the supplementation stopped. There were no changes in endogenous lipids for fuel supply, indicating the possibility that this population has sufficient levels of l-carnitine |
| Elderly (age 55–106) | ||||
| [48] l-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. | 66 centenarians | 100–106 | 2 g/d or placebo for 6 mo | Reduction of total fat mass, increases total muscular mass, and facilitates an increased capacity for physical and cognitive activity by reducing fatigue and improving cognitive functions. |
| [49] Levocarnitine administration in elderly subjects with rapid muscle fatigue: effect on body composition, lipid profile and fatigue. | 84 elderly subjects | 81.5 ± 6.7 | 2 g BID for 30 day or placebo | Improvements in the following parameters: total fat mass, total muscle mass, total cholesterol, LDL-C, HDL-C, triglycerides, apoAl, and apoB. Decreased physical and fatigue. |
| [50] Acetyl l-carnitine (ALC) treatment in elderly patients with fatigue. | 96 aged subjects | 71–88 | 2 g BID for 180 d or placebo | Reduction in both physical and mental fatigue and improvement of both the cognitive status and physical functions. |
| [53] Efficacy of a novel formulation of l-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study. | 42 healthy older adults | 55–70 | 1.5 g carnitine or carnitine combination or placebo for 8 wk | l-Carnitine combined with creatine, l-leucine, and Vitamin D significantly improved muscle mass and strength compared to placebo; increase in mTOR protein level. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. https://doi.org/10.3390/nu10030349
Fielding R, Riede L, Lugo JP, Bellamine A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients. 2018; 10(3):349. https://doi.org/10.3390/nu10030349
Chicago/Turabian StyleFielding, Roger, Linda Riede, James P. Lugo, and Aouatef Bellamine. 2018. "l-Carnitine Supplementation in Recovery after Exercise" Nutrients 10, no. 3: 349. https://doi.org/10.3390/nu10030349
APA StyleFielding, R., Riede, L., Lugo, J. P., & Bellamine, A. (2018). l-Carnitine Supplementation in Recovery after Exercise. Nutrients, 10(3), 349. https://doi.org/10.3390/nu10030349




