Relationship between Nutritional Status and Gastrointestinal Symptoms in Geriatric Patients with End-Stage Renal Disease on Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gastrointestinal Symptoms Assessment
2.2. Nutritional Status
2.3. Statistical Analysis
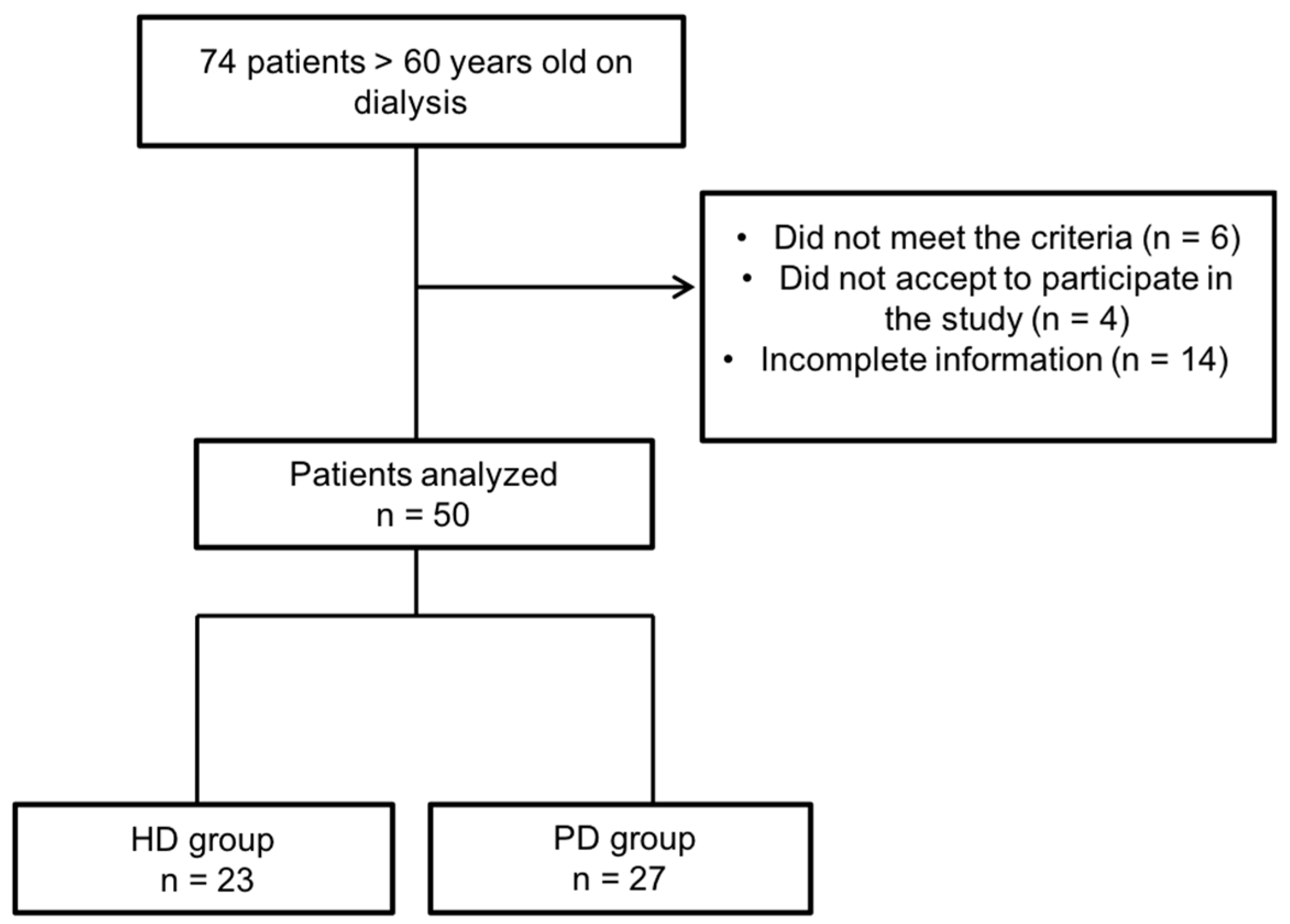
3. Results
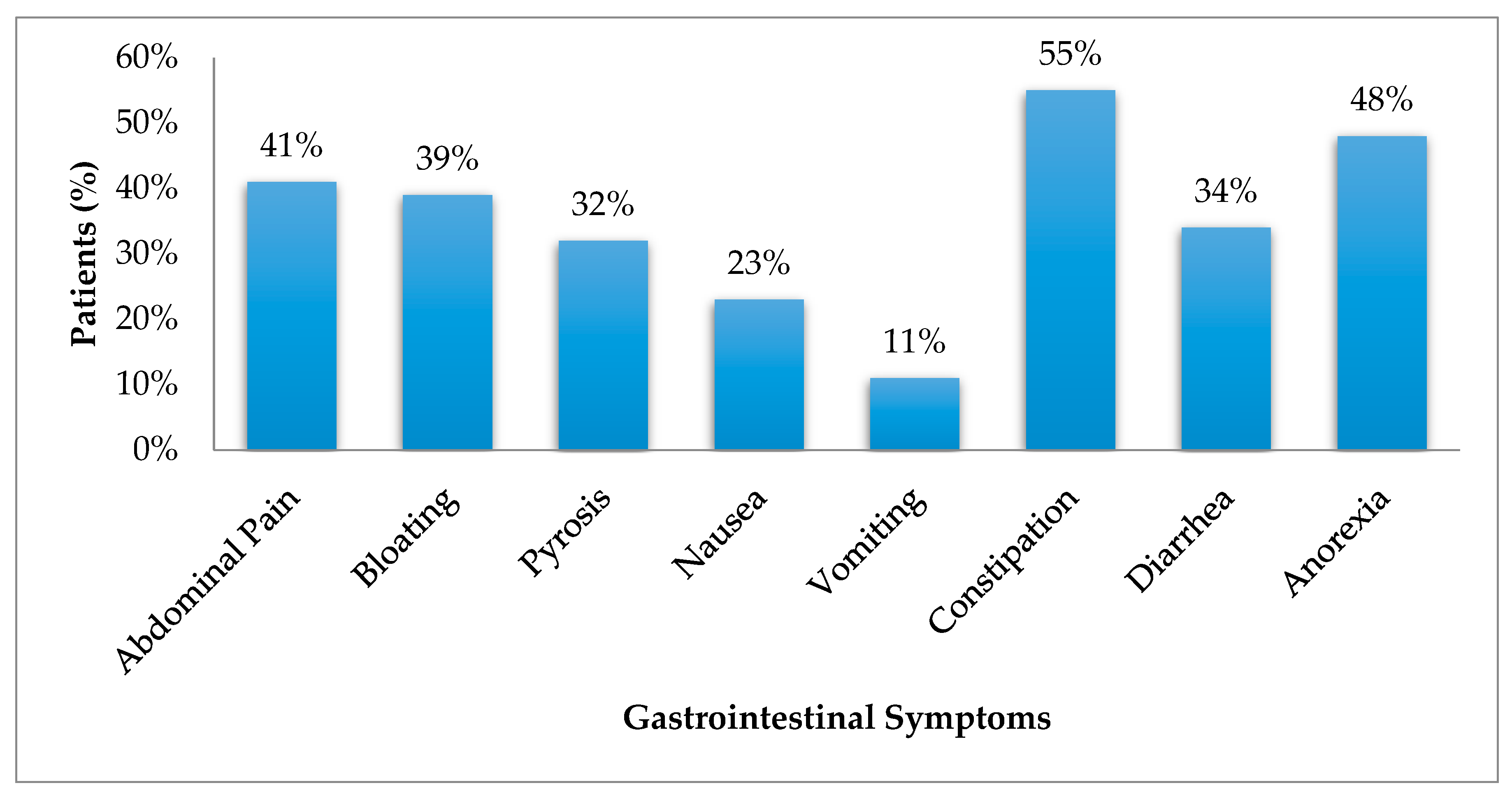
3.1. Gastrointestinal Symptoms
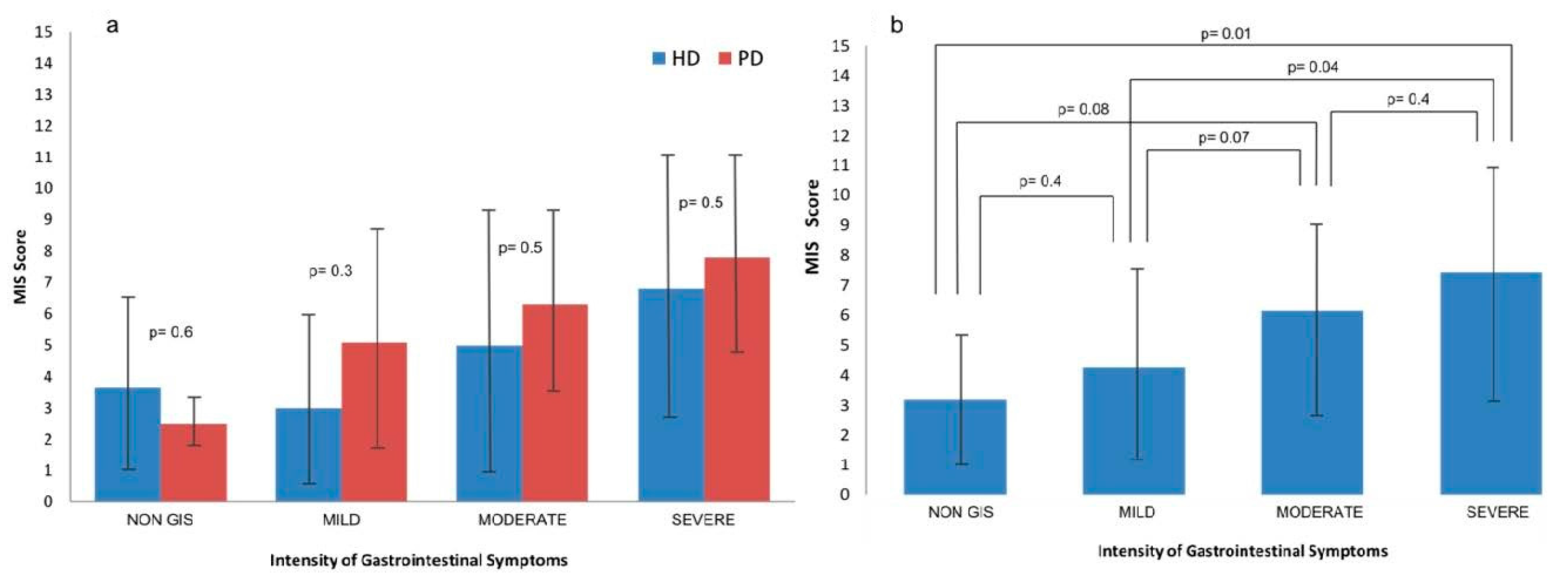
3.2. Nutritional Status
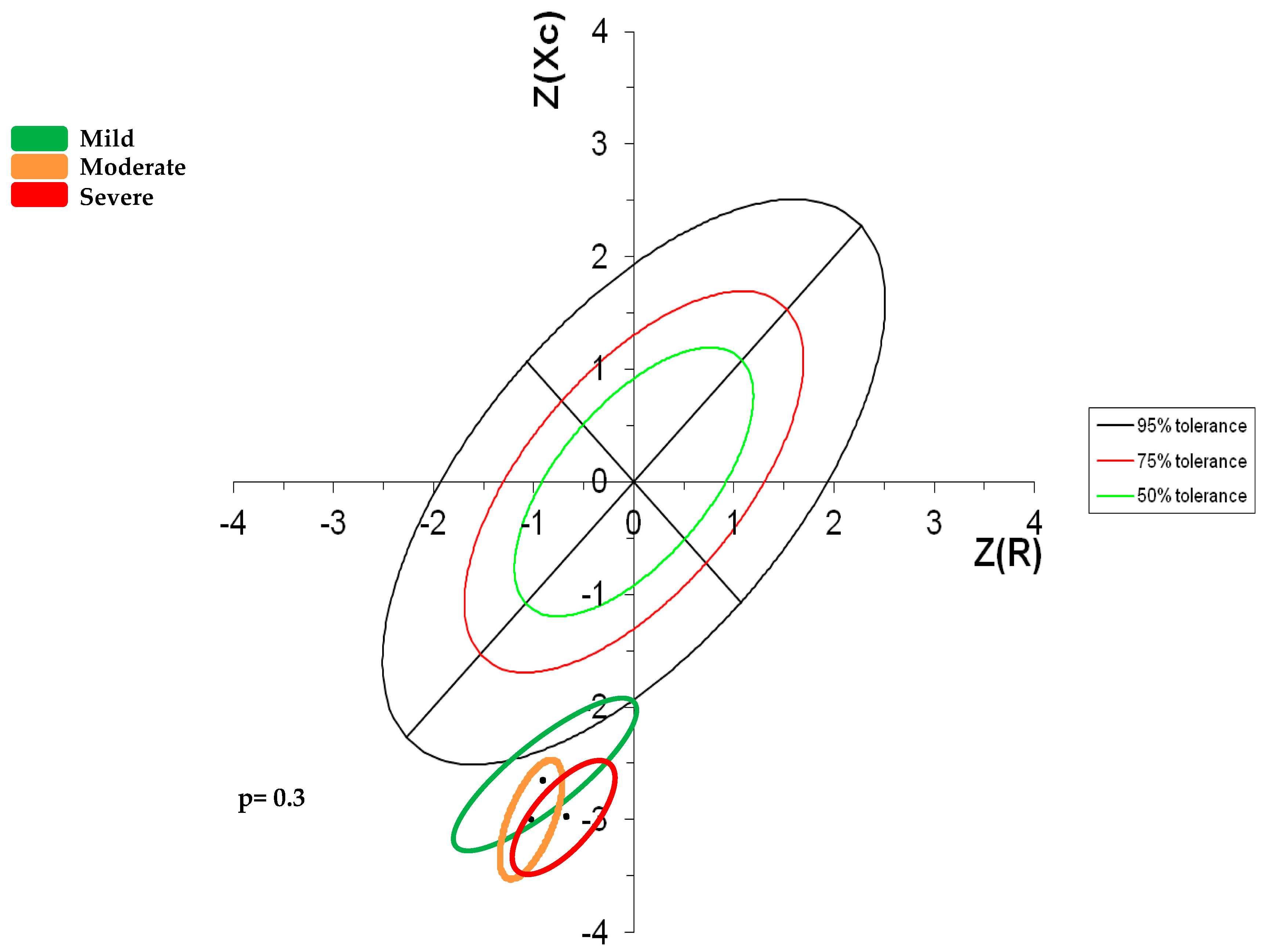
Impedance Vectors
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strid, H.; Simre, M.; Johansson, A.; Svedlund, J.; Samuelsson, O. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol. Dial. Transplant. 2002, 17, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Bajo, M.A.; Espinoza, M.; Olveira, A.; Paiva, A.M.; Codoceo, R.; Garca, P.; Sánchez, S.; Celadilla, O.; Castro, M.J.; et al. Gastrointestinal and Pancreatic Function in Peritoneal Dialysis Patients: Their Relationship With Malnutrition and Peritoneal Membrane Abnormalities. Am. J. Kidney Dis. 2003, 42, 787–796. [Google Scholar] [CrossRef]
- Cano, A.; Neil, A.; Kang, J.-Y.; Barnabas, A.; Douglas, M. Gastrointestinal Symptoms in Patients with End-Stage Renal Disease Undergoing Treatment by Hemodialysis or Peritoneal Dialysis. Am. J. Gastroenterol. 2007, 102, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, J.; Zuo, L.; Division, R. Is subjective global assessment a good index of nutrition in peritoneal. Perit. Dial. Int. 2009, 29, 3–7. [Google Scholar]
- Bossola, M.; Luciani, G.; Rosa, F.; Tazza, L. Appetite and gastrointestinal symptoms in chronic hemodialysis patients. J. Ren. Nutr. 2011, 21, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Salamon, K.; Woods, J.; Paul, E.; Huggins, C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J. Ren. Nutr. 2013, 23, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Kim, S.M.; Lee, Y.M.; Hwang, J.A.; Moon, K.M.; Moon, C.G.; Koo, H.S.; Song, K.H.; Kim, Y.S.; Lee, T.H.; et al. Is there a difference in the prevalence of gastroesophageal reflux disease between peritoneal dialysis and hemodialysis patients? Korean J. Gastroenterol. 2013, 62, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Dong, R. Gastrointestinal symptoms: A comparison between patients undergoing peritoneal dialysis and hemodialysis. World J. Gastroenterol. 2014, 20, 11370–11375. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, Z. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: Multivariate analysis of correlated factors. World J. Gastroenterol. 2010, 16, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bansal, N.; Go, A.S.; Hsu, C. Gastrointestinal symptoms, inflammation and hypoalbuminemia in chronic kidney disease patients: A cross-sectional study. BMC Nephrol. 2015, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Hung, S.Y.; Wang, H.H.; Wang, H.K.; Lin, C.W.; Chang, M.Y.; Ho, L.C.; Chen, Y.T.; Wu, C.F.; Chen, H.C.; et al. Different Risk of Common Gastrointestinal Disease Between Groups Undergoing Hemodialysis or Peritoneal Dialysis or With Non-End Stage Renal Disease A Nationwide Population-Based Cohort Study. Med. J. 2015, 94, 1–9. [Google Scholar]
- Chong, V.H.; Tan, J. Prevalence of gastrointestinal and psychosomatic symptoms among Asian patients undergoing regular hemodialysis. Nephrology 2013, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.R.; Chehter, E.Z. Dyspepsia among patients with chronic kidney disease: A cross sectional study. Int. Arch. Med. 2013, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Durán, A.; Méndez-Bueno, J.; Tapia-Yáñez, T.; Muñoz-Montes, A.A.-S.L. Epidemiología de la insuficiencia renal crónica en México. Diálisis y Transpl. 2010, 31, 7–11. [Google Scholar] [CrossRef]
- Carrero, J.J. Mechanisms of altered regulation of food intake in chronic kidney disease. J. Ren. Nutr. 2011, 21, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.; Chan, T.M. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediat. Inflamm. 2012, 2012, 484167. [Google Scholar] [CrossRef] [PubMed]
- Shirazian, S.; Radhakrishnan, J. Gastrointestinal disorders and renal failure: Exploring the connection. J. Nat. Rev. Nephrol. 2010, 6, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.; Thomson, A.B. Aging and the intestine. World J. Gastroenterol. 2006, 12, 7578–7584. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Petraccia, L.; Mennuni, G.; Fontana, M.; Sarno, A.; Sabetta, S.; Fraioli, A. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr. Hosp. 2011, 26, 659–668. [Google Scholar] [PubMed]
- Alameel, T.; Basheikh, M.; Andrew, M.K. Digestive symptoms in older adults prevalence and associations with institutionalization and mortality. Can. J. Gastroenterol. 2012, 26, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Koh, E.S.; Shin, S.J.; Park, C.W. Malnutrition in patients with chronic kidney disease. Open J. Intern. Med. 2012, 2, 89–99. [Google Scholar] [CrossRef]
- Chung, S.H.; Carrero, J.J.; Lindholm, B. Causes of poor appetite in patients on peritoneal dialysis. J. Ren. Nutr. 2011, 21, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, W.F.; Weenen, H.; Rigby, P.; Hetherington, M.M. Older adults and patients in need of nutritional support: Review of current treatment options and factors influencing nutritional intake. Clin. Nutr. 2010, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Codoceo, R.; Bajo, M.A.; Iglesias, P.; Diéz, J.J.; Barril, G.; Cigarrán, S.; Alvarez, V.; Celadilla, O.; Fernández-Perpén, A.; et al. Eating Behavior Disorders in Uremia: A Question of Balance in Appetite Regulation. Semin. Dial. 2004, 17, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Rosenberg, I. Nutrition and aging: Changes in the regulation of energy metabolism with aging. Physiol. Rev. 2006, 86, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.L.; Morley, J.E. Assessment of protein energy malnutrition in older persons, part II: Laboratory evaluation. Nutrition 2000, 16, 131–140. [Google Scholar] [CrossRef]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J. Am. Soc. Nephrol. 2013, 24, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Viramontes, D.; Márquez, F.; Martín, F.; Vizmanos, B.; Sandoval, A.; Armendáriz, J.; García-Bejarano, H.; Renoirte-López, K.; García-García, G. Effect of a Symbiotic Gel (Lactobacillus acidophilus + Bifidobacterium lactis + Inulin) on Presence and Severity of Gastrointestinal Symptoms in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Nescolarde, L.D.; Rosell, J. Análisis Convencional y Vectorial de Bioimpedancia en la Práctica Clínica. Nefrologia 2002, 22, 228–238. [Google Scholar] [PubMed]
- Espinosa, M.A.; Rivas, L.; González, E.C.; Atilano, X.; Miranda, P.; Correa, R. Vectores de impedancia bioeléctrica para la composición corporal en población mexicana. Rev. Investig. Clín. 2007, 59, 15–24. [Google Scholar]
- Leal, V.O.; Mafra, D.; Fouque, D.; Anjos, L. Use of handgrip strength in the assessment of the muscle function of chronic kidney disease patients on dialysis: A systematic review. Nephrol. Dial. Transplant. 2011, 26, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Bitzer, P.; Talley, N.; Leemon, M. Prevalence of Gastrointestinal Symptoms Associated with Diabetes Mellitus. Arch. Intern. Med. 2001, 161, 1989–1996. [Google Scholar] [CrossRef]
- Fiderkiewicz, B.; Rydzewska-Rosołowska, A.; Myśliwiec, M.; Birecka, M.; Kaczanowska, B.; Rydzewska, G.; Rydzewski, A. Factors associated with irritable bowel syndrome symptoms in hemodialysis patients. World J. Gastroenterol. 2011, 17, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Bovenschen, H.J.; Janssen, M.J.R.; van Oijen, M.G.H.; Laheij, R.J.F.; van Rossum, L.G.M.; Jansen, J.B.M.J. Evaluation of a gastrointestinal symptoms questionnaire. Dig. Dis. Sci. 2006, 51, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-zadeh, K.; Block, G.; Mcallister, C.J.; Humphreys, M.H.; Kopple, J.D.; Al, K.E.T. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Rosa, F.; Dominici, L.; Antocicco, M.; Pazzaglia, C.; Aprile, I.; Tazza, L. Appetite course over time and the risk of death in patients on chronic hemodialysis. Int. Urol. Nephrol. 2013, 45, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Trimingham, C.; McDonald, S.; Dansie, K.; Jesudason, S.; Faull, R.; Clyton, P.; Liew, G.; Le Leu, R.; Meade, A. Bowel health in chronic kidney disease: Patient perceptions differ from clinical definitions. J. Ren. Care 2018. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.J.; Helme, R.D. Age-related differences in pain perception and report. Clin. Geriatr. Med. 2001, 17, 433–456. [Google Scholar] [CrossRef]
| Variable | Total n = 50 | No GIS n = 5 | Mild n = 11 | Moderate n = 14 | Severe n = 20 | p |
|---|---|---|---|---|---|---|
| ₫ Women, n (%) | 22 (44) * | 1 (20) | 8 (73) | 3 (21) | 10 (50) | 0.02 |
| ₫ Men, n (%) | 28 (56) | 4 (80) | 3 (27) | 11 (79) | 10 (50) | 0.06 |
| µ Age (years) | 67 (60–84) | 65 (62–83) | 69 (60–78) | 65 (60–82) | 68 (60–84) | 0.4 |
| µ Time on Dialysis (months) | 20 (3–96) | 13 (3–42) | 14 (3–48) | 20.5 (5–96) | 31.5 (3–84) | 0.4 |
| ₫ DM2, n (%) | 33 (66) | 5 (100) | 5 (45) | 11 (79) | 13 (65) | 0.1 |
| ₫ Use of drugs for GIS, n (%) | 20 (40) | 1(20) | 4 (36) | 6 (43) | 8 (40) | 0.1 |
| Gastrointestinal Symptoms | Intensity of the Symptoms | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | p | |
| n = 11 | n = 14 | n = 20 | ||
| Abdominal pain | 0 (0) | 3 (21) | 12 (60) | 0.0001 μ,‡ |
| Bloating | 3 (27) | 7 (50) | 8 (40) | 0.2 |
| Pyrosis | 3(27) | 5 (36) | 6 (30) | 0.7 |
| Nausea | 0(0) | 2 (14) | 8 (40) | 0.005 ‡ |
| Vomiting | 0(0) | 2 (14) | 3 (15) | 0.2 |
| Constipation | 3(27) | 7 (50) | 14 (70) | 0.03 ‡ |
| Diarrhea | 1(9) | 5 (36) | 8 (40) | 0.1 |
| Anorexia | 3(27) | 5 (36) | 11 (55) | 0.2 |
| Intensity of Gastrointestinal Symptoms | ||||
|---|---|---|---|---|
| Parameters | Mild n = 11 | Moderate n = 14 | Severe n = 20 | p ** |
| Nutritional Parameters | ||||
| Weight (kg) | 65.4 ± 12 | 73.2 ± 10.7 | 65.8 ± 16.7 | 0.4 |
| BMI (kg/m2) | 27 ± 4.6 | 27.8 ± 3.3 | 25.7 ± 4.4 | 0.4 |
| ∞ Triceps skinfold (mm) a | 14 (10–26) | 11 (2–30) | 10 (4–20) | 0.06 |
| Arm circumference (cm) | 28 ± 5 | 28.3 ± 2.2 | 27.2 ± 4.3 | 0.8 |
| Muscle arm circumference (cm) | 23 ± 3.9 | 24.2 ± 2.2 | 23.7 ± 3.8 | 0.8 |
| ∞ Dynamometry (kg) b | 15.5 (10–38) | 17 (12.5–33) | 23.2 (12–40) | 0.1 |
| Laboratory Parameters | ||||
| BUN (mg/dL) | 60 ± 15 | 72 ± 21 | 61.6 ± 26 | 0.3 |
| Creatinine (mg/dL) | 8 ± 4 | 10.9 ± 3.7 | 7.9 ± 2.4 | 0.05 |
| Albumin (g/dL) | 3.3 ± 0.8 | 3.5 ± 0.5 | 3.3 ± 0.4 | 0.6 |
| Hemoglobin (g/dL) | 11.3 ± 0.9 | 11.1 ± 1.8 | 10.7 ± 1.5 | 0.6 |
| TIBC | 241 ± 22 | 249 ± 68 | 266 ± 48 | 0.5 |
| Energy and Nutrient Intake | ||||
| Energy (kcal/day) | 1225.6 ± 372 | 1319 ± 264 | 1564 ± 638 | 0.1 |
| Energy (kcal/kg) | 20 ± 8.6 | 18.6 ± 5 | 25 ± 11 | 0.1 |
| Protein (g/day) | 61 ± 17 | 51.4 ± 14 | 65 ± 24 | 0.1 |
| Protein (g/kg) | 0.98 ± 0.2 | 0.65 ± 0.2 | 1.0 ± 0.4 | 0.03 |
| Lipids (g/day) | 36 ± 18 | 39 ± 18 | 42 ± 24 | 0.7 |
| Carbohydrates (g/day) | 164 ± 46 | 187 ± 44 | 220 ± 100 | 0.09 |
| Bioelectrical Impedance | ||||
| R (Ω) | 526 ± 122 | 445 ± 52 | 514 ± 99 | 0.09 |
| Xc (Ω) | 40 ± 10 | 34 ± 10.7 | 35.6 ± 11.5 | 0.5 |
| R/H (Ω/m) | 342 ± 86 | 275 ± 35 | 324.8 ± 7 | 0.07 |
| Xc/H (Ω/m) | 26 ± 7 | 20.9 ± 6.4 | 22.5 ± 7.6 | 0.3 |
| Phase angle | 4.4 ± 0.7 | 4.3 ± 1 | 4 ± 1 | 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera-Jiménez, D.; Miranda-Alatriste, P.; Atilano-Carsi, X.; Correa-Rotter, R.; Espinosa-Cuevas, Á. Relationship between Nutritional Status and Gastrointestinal Symptoms in Geriatric Patients with End-Stage Renal Disease on Dialysis. Nutrients 2018, 10, 425. https://doi.org/10.3390/nu10040425
Carrera-Jiménez D, Miranda-Alatriste P, Atilano-Carsi X, Correa-Rotter R, Espinosa-Cuevas Á. Relationship between Nutritional Status and Gastrointestinal Symptoms in Geriatric Patients with End-Stage Renal Disease on Dialysis. Nutrients. 2018; 10(4):425. https://doi.org/10.3390/nu10040425
Chicago/Turabian StyleCarrera-Jiménez, Dinorah, Paola Miranda-Alatriste, Ximena Atilano-Carsi, Ricardo Correa-Rotter, and Ángeles Espinosa-Cuevas. 2018. "Relationship between Nutritional Status and Gastrointestinal Symptoms in Geriatric Patients with End-Stage Renal Disease on Dialysis" Nutrients 10, no. 4: 425. https://doi.org/10.3390/nu10040425





