A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting
Abstract
:1. Introduction
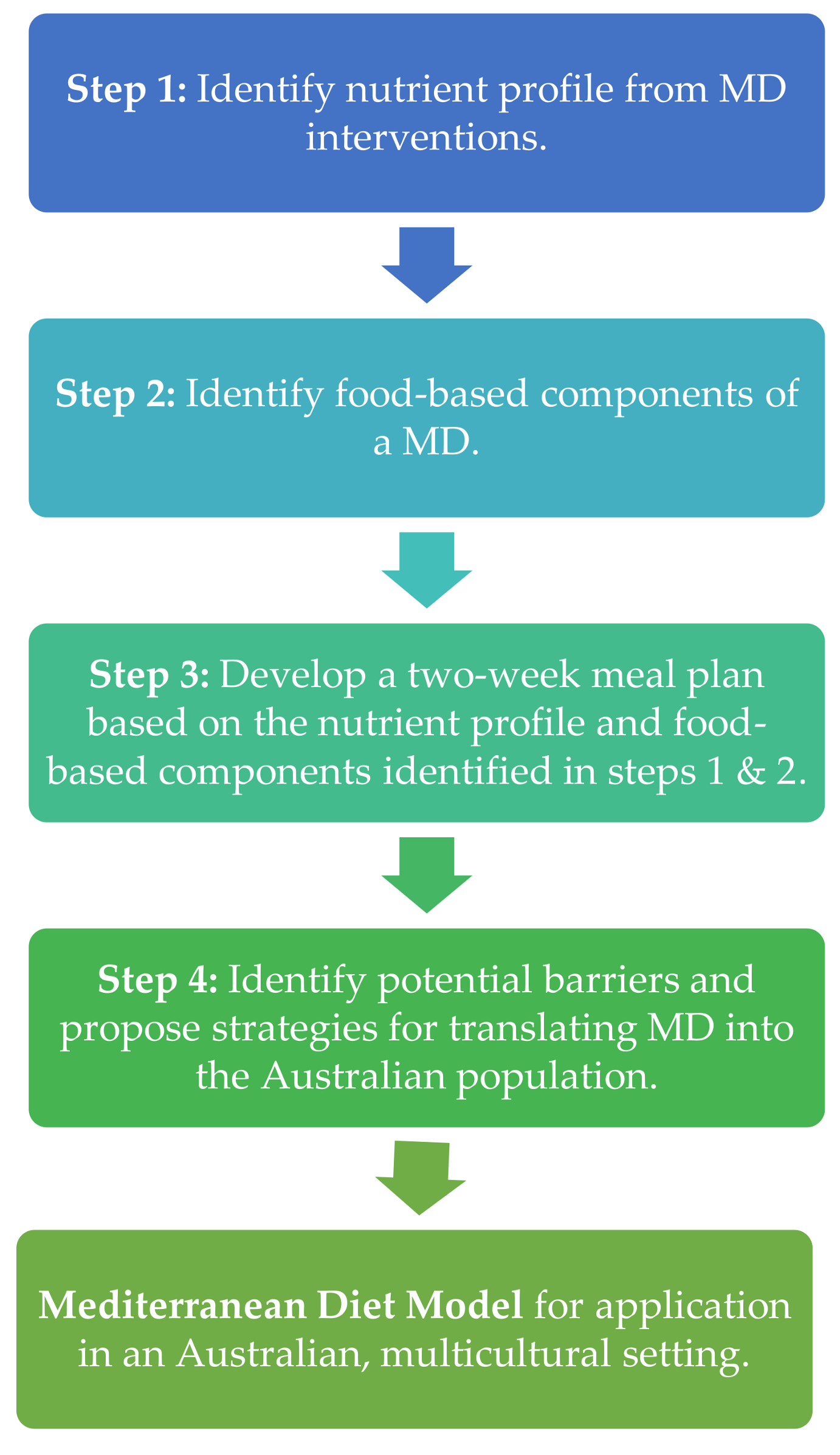
2. Methodological Steps to Address Each Specific Aim
2.1. Step One: Identification of the Nutrient Composition Profile of Previous MD Interventions Delivered to Mediterranean and Australian Populations
2.2. Step Two: Identification of the Key Food-Based Components of a MD with Strong Published Evidence of Health Benefits
2.3. Step Three: Development of a Two-Week Meal Plan Based on the Nutrient Composition Profile and the Key Food Intake Recommendations Identified in the Previous Steps
2.4. Step Four: Identification of Potential Barriers and Proposal of Strategies for Translating MD into the Australian Population
3. Practical Strategies Related to Each Specific Aim
3.1. Nutrient Composition Profiles of Other MD Interventions Delivered to Mediterranean and Australian Populations
3.2. Macronutrient Profile of the MD Model
3.2.1. Fats
Monounsaturated Fatty Acids
Polyunsaturated Fatty Acids
Saturated Fatty Acids
3.2.2. Carbohydrates
3.2.3. Protein
3.2.4. Alcohol
3.3. Identification of the Key Food-Based Components of a MD with Strong Published Evidence of Health Benefits
3.3.1. Food Groups
3.3.2. Cuisine
3.3.3. Herbs and Spices
3.3.4. Cooking
3.3.5. Eating Together
3.3.6. Being Mindful
3.3.7. Biodiversity and Seasonality, Local and Eco Friendly
3.4. A Two-Week Meal Plan Based on the Nutrient Composition Profile and the Key Food Intake Recommendations Identified in the Previous Steps
3.4.1. Breakfast
3.4.2. Lunch/Dinner
3.4.3. Snacks
3.5. Potential Barriers and Proposed Strategies for Translating MD into the Australian Population
4. General Overview and Future Implications
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Hodge, A.; Kaimakamis, M. Can the mediterranean diet prevent prostate cancer? Mol. Nutr. Food Res. 2009, 53, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J.M.; Rowley, K.G.; Cooper, P.; O’Dea, K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac. J. Clin. Nutr. 2005, 14, 131–136. [Google Scholar] [PubMed]
- Australian Bureau of Statistics. Census Reveals a Fast Changing, Culturally Diverse Nation; Australian Government: Canberra, Australia, 2017.
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a Mediterranean dietary intervention for adults with non alcoholic fatty liver disease (MEDINA): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Kucianski, T.; Mayr, H.L.; van Gaal, W.J.; Martinez-Gonzalez, M.; Vally, H. The AUStralian MEDiterranean Diet Heart Trial (AUSMED Heart Trial): A randomised clinical trial in secondary prevention of coronary heart disease in a multi-ethnic Australian population: Study Protocol. Am. Heart J. 2018. under review. [Google Scholar]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.; Dontas, A.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevič, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. The seven countries study: 2,289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Lagiou, A. Mediterranean diet and coronary heart disease: Are antioxidants critical? Nutr. Rev. 1999, 57, 253–255. [Google Scholar] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy traditional mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Brazionis, L.; Kaimakamis, M.; Cameron, M.; Best, J.D.; O’Dea, K.; Rowley, K. Can the mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Baudry, S.; Defoort, C.; Gerber, M.; Bernard, M.C.; Verger, P.; Helal, O.; Portugal, H.; Planells, R.; Grolier, P.; Amiot-Carlin, M.J.; et al. The MEDI-RIVAGE study: Reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am. J. Clin. Nutr. 2005, 82, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A. Dietary prevention of coronary heart disease: The lyon diet heart study. Circulation 1999, 99, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Dietary Guidelines for Adults in Greece. Arch. Hell. Med. 1999, 16, 516–524. [Google Scholar]
- Itsiopoulos, C. The Mediterranean Diet; Macmillan Publishers: Melbourne, Australia, 2013. [Google Scholar]
- National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Available online: https://www.nhmrc.gov.au/health-topics/alcohol-guidelines (accessed on 25 May 2016).
- Meschino, J.P. How components of the Mediterranean diet reduce heart disease and stroke risk. Dyn. Chiropr. 2013, 31, 8. [Google Scholar]
- Ross, S.M. Effects of extra virgin olive oil phenolic compounds and the Mediterranean diet on cardiovascular health. Holist. Nurs. Pract. 2013, 27, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Sidossis, L. What is so special about the traditional diet of Greece. World Rev. Nutr. Diet. 2000, 87, 24–42. [Google Scholar] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.A.; Ball, K.; Crawford, D.; Mishra, G.D. An index of diet and eating patterns is a valid measure of diet quality in an Australian population. J. Nutr. 2008, 138, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Aroney, C.; Aylward, P.; Kelly, A.; Chew, D.; Clune, E. National Heart Foundation of Australia Cardiac Society of Australia and New Zealand guidelines for the management of acute coronary syndromes 2006. Med. J. Aust. 2006, 184, S1–S32. [Google Scholar]
- Wang, C.H.; Wey, K.C.; Mo, L.R.; Chang, K.K.; Lin, R.C.; Kuo, J.J. Current trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinoma. Asian Pac. J Cancer Prev. 2015, 16, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive oil, the Mediterranean diet, and arterial blood pressure: The Greek European prospective investigation into cancer and nutrition (EPIC) study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M.; Gimeno, E.; Fito, M.; Castellote, A.-I.; Covas, M.; de la torre-boronat, M.C.; López-Sabater, M.C. Interaction of olive oil phenol antioxidant components with low-density lipoprotein. Biol. Res. 2004, 37, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Madar, Z. Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr. Rev. 2002, 60, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Lepp, Z.; Chuman, H. Approach to novel functional foods for stress control 1. Toward structure-activity relationship and data mining of food compounds by chemoinformatics. J. Med. Investig. 2005, 52, 240–241. [Google Scholar] [CrossRef]
- Kushi, L.H.; Lenart, E.B.; Willett, W.C. Health implications of mediterranean diets in light of contemporary knowledge. 1. Plant foods and dairy products. Am. J. Clin. Nutr. 1995, 61, 1407S–1415S. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Palencia, A.; López-Sobaler, A.M. Improvement of cholesterol levels and reduction of cardiovascular risk via the consumption of phytosterols. Br. J. Nutr. 2006, 96, S89–S93. [Google Scholar] [CrossRef] [PubMed]
- Serra Majem, L.; García Alvarez, A.; Ngo de la Cruz, J. Dieta Mediterránea: Características y beneficios para la salud. Arch. Latinoam. Nutr. 2004, 54, 44–51. [Google Scholar] [PubMed]
- Kouris-Blazos, A.; Belski, R. Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pac. J. Clin. Nutr. 2016, 25, 1–17. [Google Scholar] [PubMed]
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar] [PubMed]
- Samman, S.; Sivarajah, G.; Man, J.C.; Ahmad, Z.I.; Petocz, P.; Caterson, I.D. A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J. Nutr. 2003, 133, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Díaz-López, A.; Rosique-Esteban, N.; Ros, E.; Buil-Cosiales, P.; Corella, D.; Estruch, R.; Fitó, M.; Serra-Majem, L.; Arós, F.; et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2017. [Google Scholar] [CrossRef]
- Ruxton, C.; Reed, S.C.; Simpson, M.; Millington, K. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.-K. Meat as a component of a healthy diet—Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality: A prospective study of over half a million people. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Ziebland, S.; Yudkin, P.; Roe, L.; Neil, H. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R. Health benefits of nuts: Potential role of antioxidants. Br. J. Nutr. 2006, 96, S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.; Ang, Y. Nuts and health outcomes: New epidemiologic evidence. Am. J. Clin. Nutr. 2009, 89, 1643S–1648S. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S. Fermented dairy products: Starter cultures and potential nutritional benefits. Food Nutr. Sci. 2011, 2, 47–51. [Google Scholar] [CrossRef]
- Ebringer, L.; Ferenčík, M.; Krajčovič, J. Beneficial health effects of milk and fermented dairy products—Review. Folia Microbiol. 2008, 53, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Bartram, H.-P.; Scheppach, W.; Gerlach, S.; Ruckdeschel, G.; Kelber, E.; Kasper, H. Does yogurt enriched with bifidobacterium longum affect colonic microbiology and fecal metabolites in health subjects? Am. J. Clin. Nutr. 1994, 59, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Jacobs, D.; Marquart, L.; Wiemer, K. The role of whole grains in disease prevention. J. Am. Diet. Assoc. 2001, 101, 780–785. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Martini, M.C.; Jacobs, D.R.; Marquart, L. Plausible mechanisms for the protectiveness of whole grains. Am. J. Clin. Nutr. 1999, 70, 459s–463s. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Walker, R.; Allayee, H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Welsh, J.A.; Le, N.-A.; Holzberg, J.; Sharma, P.; Martin, D.R.; Vos, M.B. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 2014, 6, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.J.; Nightingale, Z.D.; Lichtenstein, A.H.; Schaefer, E.J.; Blumberg, J.B. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 1999, 70, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E. Comparison of fatty acid, cholesterol, and vitamin a and e composition in eggs from hens housed in conventional cage and range production facilities. Poult. Sci. 2011, 90, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, C.; Blasina, F.; Arredondo, F.; Ferreira, M.; Abin-Carriquiry, J.A.; Vasquez, L.; Aspillaga, A.A.; Diez, M.S.; Leighton, F.; Dajas, F. Cytoprotection by neutral fraction of tannat red wine against oxidative stress-induced cell death. J. Agric. Food chem. 2004, 52, 7395–7399. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R. Importance of functional foods in the mediterranean diet. Public Health Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.; Marquez, S.; de Mejia, E.G. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W. Carrots, tomatoes and cocoa: Research on dietary antioxidants in dusseldorf. Arch. Biochem. Biophys. 2016, 595, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, J.A.; Bleich, S.N. Is cooking at home associated with better diet quality or weight-loss intention? Public Health Nutr. 2015, 18, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Reardon, R.; McDonald, M.; Vargas-Garcia, E.J. Community interventions to improve cooking skills and their effects on confidence and eating behaviour. Curr. Nutr. Rep. 2016, 5, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Gerber, M. Evaluating and adapting the mediterranean diet for non-mediterranean populations: A critical appraisal. Nutr. Rev. 2013, 71, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radd-Vagenas, S.; Kouris-Blazos, A.; Singh, M.F.; Flood, V.M. Evolution of mediterranean diets and cuisine: Concepts and definitions. Asia Pac. J. Clin. Nutr. 2013, 1–32. [Google Scholar] [CrossRef]
- Jordan, C.H.; Wang, W.; Donatoni, L.; Meier, B.P. Mindful eating: Trait and state mindfulness predict healthier eating behavior. Pers. Indiv. Differ. 2014, 68, 107–111. [Google Scholar] [CrossRef]
- Burlingame, B.; Dernini, S. Sustainable diets: The mediterranean diet as an example. Public Health Nutr. 2011, 14, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; McEvoy, C.; Prior, L.; Lawton, J.; Patterson, C.; Kee, F.; Cupples, M.; Young, I.; Appleton, K.; McKinley, M.; et al. Barriers to adopting a mediterranean diet in northern European adults at high risk of developing cardiovascular disease. J. Hum. Nutr. Diet. 2017. [Google Scholar] [CrossRef] [PubMed]

| Trials | 7 Countries Study | Diabetes Cross Over | PREDIMED | The Medi-RIVAGE | NAFLD Cross Over |
|---|---|---|---|---|---|
| Keys et al. [15] (Cohort) | Itsiopoulos et al. [19] (Intervention) | Estruch et al. [20] (Intervention) | Vincent et al. [21] (Intervention) | Ryan et al. [4] (Intervention) | |
| Dietary data | Prospective cohort | Feeding trial, full provision of diet. Data is recommended diet ^ | Data is from diet consumed * | Data is from recommended diet ^ | Feeding trial, full provision of diet. Data is the recommended diet ^ |
| Population | Mediterranean | Non-Mediterranean (Australia) | Mediterranean (Spain) | Mediterranean (Spain) | Non-Mediterranean (Australia) |
| Nutrients | |||||
| Energy (MJ) | - | 11.9 | 9.2 | - | 11.3 |
| Protein (%E) | 10.5 | 12.0 | 16.3 | 12–15 | 15.8 |
| CHO (%E) | 44.3 | 40.1 | 50 | 33.6 | |
| Total Fat (%E) | 36.1 | 40.2 | 41.3 | 35–38 | 44.3 |
| SFA (%E) | 7.7 | 7.5 | 9.3 | 8–10 | 13.6 |
| MUFA (%E) | 25.8 | 22.9 | 21.5 | 18–20 | 22.8 |
| PUFA (%E) | 2.5 | 5.6 | 6.9 | 8–10 | 7.9 |
| Alcohol (%E) | 4 | 4 | - | ≤5% | 1.5 |
| Fibre (g/d) | - | 46.7 | - | >25 g | 36.4 |
| Linoleic acid n-6 (g) | - | 15.6 | 14.1 | - | 15.1 |
| α-linolenic acid n-3 (g) | - | 1.5 | 1.6 | - | 1.6 |
| EPA (g) | - | 0.44 | - | - | - |
| DHA (g) | - | 0.48 | - | - | - |
| Total LCN3s (mg) | - | - | - | - | 200.3 |
| Key outcome | All-cause mortality CHD | HbA1c | ↓CVD complications | ↓CVD risk | liver fat insulin resistance |
| Nutrients | Australian Mediterranean Diet Composition |
|---|---|
| Energy (MJ) | 9.4 |
| Protein (%E) | 15.8 |
| CHO (%E) | 33.8 |
| Added sugar (%E) | 5.2 |
| Total fat (%E) | 41.8 |
| SFA (%E) | 8.9 |
| MUFA (%E) | 22.3 |
| PUFA (%E) | 10.6 |
| Alcohol (%E) | 2.4 |
| Fibre (g/d) | 41.1 |
| Linoleic acid n-6 (g) | 18.7 |
| α linolenic acid n-3 (g) | 4.9 |
| Total LCN3s (mg) | 932 |
| Recommendation | Practical Dietary Applications | Key Components | Evidence Based Benefits |
|---|---|---|---|
| Use extra virgin olive oil (EVOO) as the main added fat. | Minimum 3–4 tablespoons (60–80 mL) per day | The highest proportions of MUFAs and polyphenols squalene and α-tocopherol are available in extra virgin olive oil. | Prevention of CHD, cancers and modification to immune and inflammatory responses have been attributed to EVOO. The high antioxidant content of EVOOs contributes to health of the vascular system through improved endothelial function [34] and has been shown to inhibit LDL oxidation [35]. EVOO consumption has also been proposed to improve bone mineralisation [36]. |
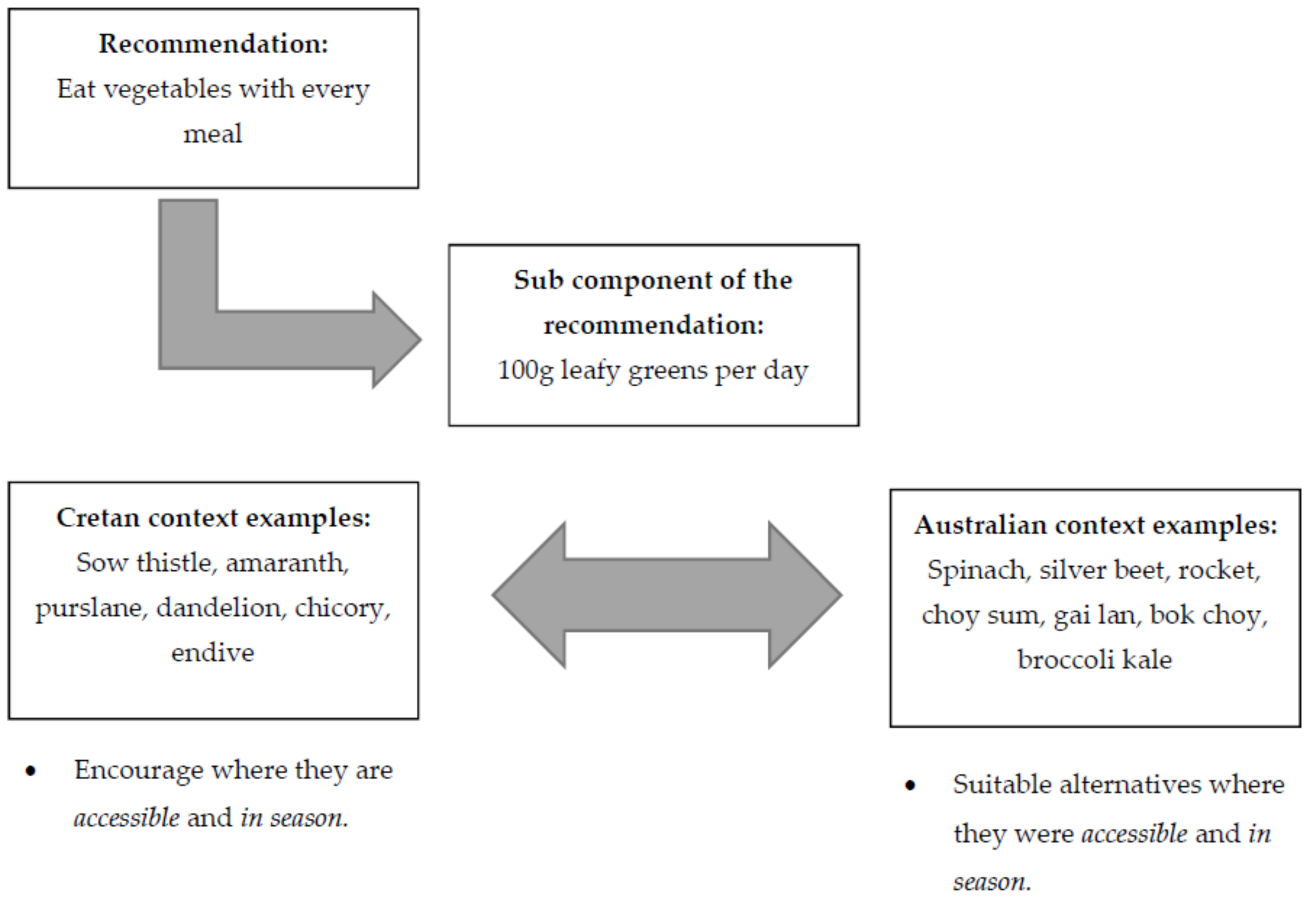
| Eat vegetables with every meal. | Include 100 g leafy greens, and 200 g all other vegetables daily (cauliflower, zucchini, eggplant, capsicum etc.). use onion and garlic daily; include 100 g tomatoes daily; fresh or sofrito (tomato-based sauce). | Vegetables are the most significant source of phenolic compounds. They contain carotenoids, folic acid, fibre and phytosterols. Garlic, onion, herbs and spices also have key benefits. See Section 3.3.2 for importance of combining and/or cooking ingredients. | Flavonoids, are essential bioactive compounds that provide health benefits due to their antioxidant effects and have been associated with improvements in cognitive function and mood [37]. Traditional diets which are predominantly plant-based are associated with lower rates of chronic diseases and increased longevity [38]. Carotenoids, folic acid and fibre play important roles in CHD prevention [38]. Phytosterols contribute to reduced serum cholesterol and CVD risk [39]. Garlic, onions, herbs and spices contain large amounts of flavonoids or allicin which have cardiovascular benefits and also improve cognitive function [40]. Vegetables which are high in potassium, magnesium and calcium tend to reduce arterial blood pressure [34,36]. |
| Include at least two legume meals per week. | Canned or dry legumes are acceptable; this may include tofu (1 serve = 250 g). This should replace meat on days when meat is not consumed. | Legumes are high in fibre, protein, B vitamins, iron, zinc, calcium, magnesium, selenium, phosphorus, copper and potassium [41]. They provide a nutritious, nourishing meat alternative. | Legumes are linked to longevity, and are a strong predictor of survival [42]. Vegetables have been shown to reduce serum homocysteine concentrations and thus coronary events, especially in high risk individuals [43]. An inverse association between the risk of T2DM and CHD and legume intake has been reported [44,45]. |
| Eat at least three servings of fish or shellfish per week. | Fish (1 serve = 100–150 g); shellfish (1 serve = 200 g). Include oily fish at least 1–2 times per week. | Marine long chain omega-3 polyunsaturated fatty acids provide eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). | EPA and DHA effectively regulate haemostatic factors and protect against cardiac arrhythmias, cancer and hypertension and help to maintain neural functions [34,40]. A high intake of fish and seafood has also been shown to reduce systolic blood pressure [34,36]. Immunomodulatory effects may improve inflammatory conditions [46]. |
| Eat red meat less often and choose smaller portions. Choose white meat. | 150–200 g weekly of beef, lamb and, pork. 200–250 g per week of poultry. Choose lean varieties, wild, free range and grass fed varieties are encouraged. | Meat is a bioavailable source of vitamin B12, and iron, selenium, and zinc and are a good source of protein. Excessive amounts of red meat have been linked to adverse health outcomes and excess saturated fatty acids—unfavourable fat ratios and displacement of more nutritious alternatives. Wild, free range and grass fed varieties are preferred due to the improved n-6:n-3 ratio [29]. | Red meat is a good source of protein which assists with satiety [47]. Red and processed meats, especially when consumed in excess, are associated with total CVD and cancer mortality [48]. Excessive SFA intake from meat is also linked to adverse health outcomes [1]. |
| Eat fresh fruit every day. | 300g or 2 serves. | Fruit provides fibre, potassium, vitamins A and C, B vitamins, folate, flavonoids and terpenes providing protection against oxidative processes. | Consumption of fruit has been shown to reduce the risk of CVD and cancers [49]. Fibre, vitamins, minerals, flavonoids and terpenes may provide protection against oxidative processes which drive the onset and exacerbate chronic diseases [40]. Flavonoids have also been associated with improvements in cognitive function and mood [37]. |
| Eat a serve of nuts every day and dried fruit as a snack or dessert. | Nuts-1 serve = ~30 g or 1/4 cup or a small handful daily. Dried fruit—2 tablespoons or 30 g. | Nuts are a good source of monounsaturated fats, fibre, vitamin C and E, selenium, magnesium, providing an abundance of antioxidants including flavonoids, resveratrol, polyphenols and tocopherols [50]. | Monounsaturated fats, phenols, phytosterols, phytic acid and fibre are abundant in nuts and are associated with a reduction in plasma lipids and reduced incidence of CVD [40]. Nut consumption has been associated with the prevention and reversal of oxidative stress [50,51]. |
| Eat dairy every day. | 2 serves per day including milk-1 serve = 250 mL or 1 cup. Yoghurt preferably Greek style yoghurt 1 serve = 150 g or ¾ cup. | Dairy is a good source of calcium, vitamin D, phosphorus, magnesium, zinc, potassium, vitamins A and B12, and lactic acid bacteria confer probiotic effects [52]. Choose mostly fermented dairy which are higher in potent beneficial bioactive compounds from milk such as lactic acid bacteria [53]. | Bioactive milk components have been shown to be protective in several diseases, including hypertension, coronary vascular diseases, obesity, osteoporosis and cancer [53]. Lactic acid bacteria confer probiotic effects, including improvements in gastrointestinal health and immune response. Yogurt, specifically, may induce desirable changes in faecal bacterial flora, potentially reducing the risk of colon cancer. Yoghurt is also likely to regulate mouth-to-caecum transit time [54]. |
| Eat cheese in moderation, about 3 times per week and preferably feta. | 1 serve = 30 g or the size of a matchbox. | ||
| Include wholegrain breads and cereals with meals, such as wholegrain bread, rice, pasta and potato. | 1 serve = 1 slice of bread or; ½ cup or; 50–60 g cooked pasta/rice or; 1 small 100 g potato. | Wholegrains are a good source of fermentable carbohydrates including fibre, resistant starch, and oligosaccharides. They contain phytochemicals, antioxidants including trace minerals, phenolic compounds, lignans and B group vitamins including folate, vitamin E, minerals iron, magnesium, copper and selenium [55]. | Components such as fibre, antioxidants and vitamins and minerals promote health and may be protective against cancer, CVD, T2DM and obesity [56,57]. Production of short chain fatty acids through indigestible carbohydrates promote reduced serum cholesterol levels and decrease cancer risk [58]. |
| Have sweets or sweet drinks in moderate amounts and on special occasions only. | Preferably home made. | Homemade varieties have key ingredients that are encouraged in the MD, such as nuts, EVOO and milk and are less refined and lower in SFA. | Liver fat accumulation may be attributed, at least in part, to excess dietary sugar consumption, especially from fructose, which increases the levels of enzymes involved in hepatic de-novo lipogenesis [59,60]. |
| Consume up to 3 eggs per week. | Free range or omega-3 varieties. | Eggs are a good source of protein, choline, selenium, vitamin B12, riboflavin, phosphorus and fat soluble vitamins A, D and E. They are a bioavailable source of carotenoids; lutein and zeaxanthin, [61]. Free range and omega-3 enriched varieties have higher amounts of omega-3 fatty acids [62]. | The benefits of eggs, including the provision of protein and micronutrients including vitamins, minerals and carotenoids may prevent age related macular degeneration and some cancers [61]. A limit to egg consumption is set to achieve the desired fat ratios in line with other MD guidelines [23]. |
| OPTIONAL Consume wine in moderation. | Choose red wine. Have 0–2 glasses per day, (100 mL per glass) and always with meals. Do not get drunk. | Red wine contains phenolic compounds with high antioxidant properties. For example, red wine has higher amounts of the stilbene polyphenol, resveratrol, compared with white wine [63]. | Red wine provides polyphenols whose antioxidant activity may contribute to the cytoprotective effects. Resveratrol has been found to protect the heart and kidneys from ischaemia-reperfusion injury and has a likely positive effect on endothelial function (vasodilation) with prolonged moderate consumption [64,65]. |
| Cuisine | Greek (Cretan) | Middle Eastern | Indian | Chinese | Western |
|---|---|---|---|---|---|
| Meal | Fasolatha | Mujadara | Dhal | Mapo Tofu | Homemade Baked beans |
| Key ingredients | Legumes, onions, garlic, tomato, herbs, EVOO | Lentils, rice, onions, spices, EVOO | Lentils onion, garlic, tomatoes, ghee | Tofu, garlic scallions, peppers, ginger, soy sauce +/− pork, peanut +/− sesame oils | Legumes, onion, garlic, tomato, vegetable oil |
| Fat Modifications | - | - | Replace part or all added fat with EVOO | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, E.S.; Kucianski, T.; Mayr, H.L.; Moschonis, G.; Tierney, A.C.; Itsiopoulos, C. A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting. Nutrients 2018, 10, 465. https://doi.org/10.3390/nu10040465
George ES, Kucianski T, Mayr HL, Moschonis G, Tierney AC, Itsiopoulos C. A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting. Nutrients. 2018; 10(4):465. https://doi.org/10.3390/nu10040465
Chicago/Turabian StyleGeorge, Elena S., Teagan Kucianski, Hannah L. Mayr, George Moschonis, Audrey C. Tierney, and Catherine Itsiopoulos. 2018. "A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting" Nutrients 10, no. 4: 465. https://doi.org/10.3390/nu10040465
APA StyleGeorge, E. S., Kucianski, T., Mayr, H. L., Moschonis, G., Tierney, A. C., & Itsiopoulos, C. (2018). A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting. Nutrients, 10(4), 465. https://doi.org/10.3390/nu10040465







