“Benifuuki” Extract Reduces Serum Levels of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Ligands Containing Apolipoprotein B: A Double-Blind Placebo-Controlled Randomized Trial
Abstract
:1. Introduction
2. Materials and Methods
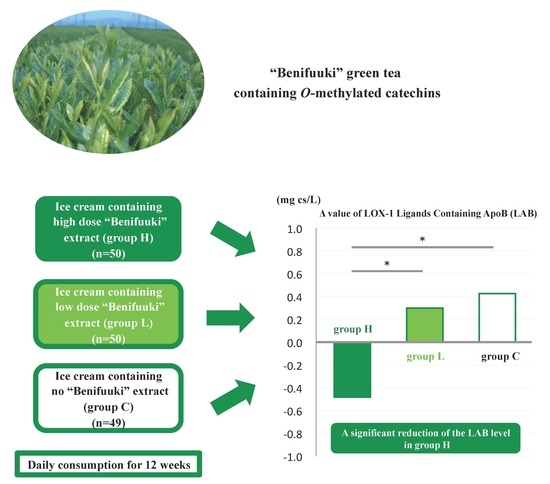
2.1. Participants and Enrolment
2.2. Randomization
2.3. Sample Size
2.4. Restrictions during the Study Period
2.5. Outcomes and Measures
2.6. Ice Cream and Intervention
2.7. Statistical Methods
3. Results
3.1. Participants and Follow-Up
3.2. Baseline Data and Outcomes
3.3. Ancillary Analysis
3.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braitman, L.E.; Adlin, E.V.; Stanton, J.L., Jr. Obesity and caloric intake: The national health and nutrition examination survey of 1971–1975 (HANES I). J. Chronic Dis. 1985, 38, 727–732. [Google Scholar] [CrossRef]
- Pedersen, T.R.; Kjekshus, J.; Berg, K.; Haghfelt, T.; Faergeman, O.; Faergeman, G.; Pyorala, K.; Miettinen, T.; Wilhelmsen, L.; Olsson, A.G.; et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4S). 1994. Atheroscler. Suppl. 2004, 5, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Lee, D.H.; Steffes, M.; Gross, M.; Jacobs, D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 2008, 299, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Katagiri, H.; Gao, J.; Yamada, T.; Imai, J.; Uno, K.; Hasegawa, Y.; Kaneko, K.; Ogihara, T.; Ishihara, H.; et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation 2008, 118, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Watanabe, J.; Tomita, T.; Sano, M.; Tomita, I. The inhibitory effects of tea polyphenols (flavan-3-ol derivatives) on Cu2+ mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 1994, 17, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Watanabe, J.; Sano, M.; Tomita, T.; Osawa, T.; Hara, Y.; Tomita, I. Effects of various natural antioxidants on the Cu(2+)-mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 1995, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Imasato, Y.; Sasaki, E.; Nakayama, M.; Nagao, H.; Takeo, T.; Yayabe, F.; Sugano, M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta 1992, 1127, 141–146. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Ema, K.; Shibuichi, I. In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology 2007, 55, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Imbe, H.; Sano, H.; Miyawaki, M.; Fujisawa, R.; Miyasato, M.; Nakatsuji, F.; Haseda, F.; Tanimoto, K.; Terasaki, J.; Maeda-Yamamoto, M.; et al. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: A double-blind, placebo-controlled randomized trial. J. Funct. Foods 2016, 25, 25–37. [Google Scholar] [CrossRef]
- Hirano-Ohmori, R.; Takahashi, R.; Momiyama, Y.; Taniguchi, H.; Yonemura, A.; Tamai, S.; Umegaki, K.; Nakamura, H.; Kondo, K.; Ohsuzu, F. Green tea consumption and serum malondialdehyde-modified LDL concentrations in healthy subjects. J. Am. Coll. Nutr. 2005, 24, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Inami, S.; Takano, M.; Yamamoto, M.; Murakami, D.; Tajika, K.; Yodogawa, K.; Yokoyama, S.; Ohno, N.; Ohba, T.; Sano, J.; et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int. Heart J. 2007, 48, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007, 15, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Ema, K.; Monobe, M.; Tokuda, Y.; Tachibana, H. Epicatechin-3-O-(3″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J. Agric. Food Chem. 2012, 60, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.N.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I.; Low Dog, T. Safety of green tea extracts: A systematic review by the us pharmacopeia. Drug Saf. 2008, 31, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nishimichi, N.; Nakano, A.; Takikawa, K.; Inoue, N.; Matsuda, H.; Sawamura, T. Determination of LOX-1-ligand activity in mouse plasma with a chicken monoclonal antibody for ApoB. Atherosclerosis 2008, 200, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell. Physiol. Biochem. 2016, 38, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Moriwaki, H.; Murase, T.; Sawamura, T.; Masaki, T.; Hashimoto, N.; Kita, T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999, 99, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Miura, K.; Sawamura, T.; Kadota, A.; Hisamatsu, T.; Fujiyoshi, A.; Miyamatsu, N.; Takashima, N.; Miyagawa, N.; Kadowaki, T.; et al. Serum level of LOX-1 ligand containing ApoB is associated with increased carotid intima-media thickness in Japanese community-dwelling men, especially those with hypercholesterolemia LOX-1 ligand and IMT in Japanese. J. Clin. Lipidol. 2016, 10, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Sekikawa, A.; Sawamura, T.; Kadowaki, T.; Barinas-Mitchell, E.; Mackey, R.H.; Kadota, A.; Evans, R.W.; Edmundowicz, D.; Higashiyama, A.; et al. LOX-1 ligands containing apolipoprotein B and carotid intima-media thickness in middle-aged community-dwelling US Caucasian and Japanese men. Atherosclerosis 2013, 229, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, K.; Suehiro, A.; Nakanishi, M.; Sawamura, T.; Wakabayashi, I. Associations of atherosclerotic risk factors with oxidized low-density lipoprotein evaluated by LOX-1 ligand activity in healthy men. Clin. Chim. Acta 2011, 412, 1643–1647. [Google Scholar] [CrossRef] [PubMed]



| Placebo | Benifuuki Extract Low-Dose | Benfuuki Extract High-Dose | |
|---|---|---|---|
| calories (kcal) | 138 | 138 | 138 |
| protein (g) | 3.3 | 3.3 | 3.3 |
| fat (g) | 6.9 | 6.9 | 6.9 |
| carbohydrate (g) | 21.1 | 20.1 | 18.8 |
| Sodium choloride (mg) | 58 | 56 | 56 |
| total catechins (mg) | 0 | 322 | 676 |
| total O-methyltated catechins ((−)-epigallocatechin-3-O-(3-O-methyl) gallate + (−)-gallocatechin-3-O-(3-O-methyl) gallate) (mg) | 0 | 32 | 66 |
| (−)-epigallocatechin-3-O-gallate + (−)-gallocatechin-3-O-gallate (mg) | 0 | 138 | 314 |
| caffeine | 0 | 33 | 66 |
| Study Group | 0 Week | 12 Week | Δ Value at 12 Weeks a | Paired t Test b | Kruskal-Wallis Test c | Steel–Dwass Test d | Steel Test e | |
|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | |||||
| Baseline characteristics | ||||||||
| Sex | H group | 16/34 | ||||||
| (male/female) | L group | 18/32 | ||||||
| C group | 19/30 | |||||||
| Age | H group | 52.1 ± 10.9 | ||||||
| (year) | L group | 54.4 ± 9.7 | ||||||
| C group | 53.8 ± 11.2 | |||||||
| Diet survey | ||||||||
| Total daily intake | H group | 2109.0 ± 1055.6 | 2160.0 ± 1178 | 51.0 ± 835.1 | 0.6676 | 0.4977 | §: 0.7108 | §: 0.6455 |
| (kcal/day) | L group | 2009.2 ± 539.7 | 2128.6 ± 946.1 | 119.3 ± 802.3 | 0.8101 | §§: 0.7322 | §§: 0.6696 | |
| C group | 2215.1 ± 903.9 | 2175.5 ± 902.6 | −39.5 ± 1145.1 | 0.2980 | §§§: 0.9759 | |||
| Anthropometric values | ||||||||
| Body weight | H group | 65.5 ± 10.6 | 66.2 ± 10.7 | 0.7 ± 1.3 | 0.0003 *** | 0.8074 | §: 0.6455 | §: 0.9777 |
| (kg) | L group | 60.9 ± 8.5 | 61.5 ± 8.6 | 0.6 ± 1.1 | <0.0001 **** | §§: 0.6696 | §§: 0.6695 | |
| C group | 62.0 ± 8.9 | 62.8 ± 9.0 | 0.8 ± 1.1 | <0.0001 **** | §§§: 0.6696 | |||
| Body Mass Index | H group | 24.9 ± 3.6 | 25.1 ± 3.7 | 0.3 ± 0.5 | 0.0002 *** | 0.8112 | §: 0.9618 | §: 0.9494 |
| (kg/m2) | L group | 23.5 ± 2.5 | 23.7 ± 2.5 | 0.2 ± 0.4 | <0.0001 **** | §§: 0.7615 | §§: 0.7032 | |
| C group | 23.5 ± 2.6 | 23.9 ± 2.5 | 0.3 ± 0.4 | <0.0001 **** | §§§: 0.9666 | |||
| Waist circumference | H group | 90.0 ± 8.5 | 89.2 ± 9.1 | −0.8 ± 2.1 | 0.0082 ** | 0.9879 | §: 0.9989 | §: 0.9985 |
| (cm) | L group | 85.9 ± 6.8 | 85.2 ± 6.9 | −0.7 ± 2.2 | 0.0328 * | §§: 0.9882 | §§: 0.9842 | |
| C group | 87.1 ± 7.2 | 86.4 ± 7.1 | −0.7 ± 2.2 | 0.0353 * | §§§: 0.9929 | |||
| SBP | H group | 122.0 ± 15.6 | 122.6 ± 13.8 | 0.7 ± 11.2 | 0.6687 | 0.4400 | §: 0.6998 | §: 0.6333 |
| (mmHg) | L group | 127.5 ± 19.6 | 126.9 ± 18.5 | −0.7 ± 11.5 | 0.6901 | §§: 0.8209 | §§: 0.7731 | |
| C group | 120.6 ± 14.0 | 119.2 ± 15.7 | −1.3 ± 13.4 | 0.4991 | §§§: 0.4532 | |||
| DBP | H group | 75.5 ± 10.1 | 76.9 ± 9.1 | 1.4 ± 6.3 | 0.1312 | 0.5862 | §: 0.5870 | §: 0.5118 |
| (mmHg) | L group | 78.4 ± 10.6 | 79.2 ± 10.0 | 0.8 ± 5.0 | 0.2731 | §§: 0.9996 | §§: 0.9994 | |
| C group | 75.1 ± 9.1 | 75.4 ± 8.6 | 0.4 ± 5.4 | 0.6465 | §§§: 0.7092 | |||
| Pulse | H group | 70.1 ± 12.0 | 71.8 ± 11.6 | 1.8 ± 8.1 | 0.1311 | 0.2087 | §: 0.3121 | §: 0.2484 |
| (b.p.m.) | L group | 72.0 ± 8.9 | 70.8 ± 8.9 | −1.9 ± 9.6 | 0.3122 | §§: 0.9908 | §§: 0.9877 | |
| C group | 72.1 ± 11.8 | 70.2 ± 10.3 | −1.2 ± 8.4 | 0.1796 | §§§: 0.2516 | |||
| Lipid parameters | ||||||||
| Total cholesterol | H group | 236.2 ± 26.5 | 246.5 ± 26.3 | 10.3 ± 36.2 | 0.0493 * | 0.3176 | §: 0.8395 | §: 0.7955 |
| (mg/dL) | L group | 247.1 ± 30.0 | 248.6 ± 27.7 | 1.5 ± 42.0 | 0.7992 | §§: 0.6007 | §§: 0.5261 | |
| C group | 236.7 ± 24.2 | 245.2 ± 27.7 | 8.6 ± 32.7 | 0.0734 | §§§: 0.3061 | |||
| Triglyceride | H group | 112.9 ± 51.2 | 103.0 ± 48.2 | −9.9 ± 41.6 | 0.0981 | 0.3753 | §: 0.8152 | §: 0.7663 |
| (mg/dL) | L group | 116.1 ± 60.6 | 112.3 ± 48.7 | −3.8 ± 42.8 | 0.5289 | §§: 0.8711 | §§: 0.8342 | |
| C group | 105.8 ± 51.4 | 103.1 ± 54.3 | −2.7 ± 33.9 | 0.5886 | §§§: 0.2781 | |||
| HDL cholesterol | H group | 58.0 ± 13.5 | 62.6 ± 15.3 | 4.6 ± 6.8 | <0.0001 **** | 0.1637 | §: 0.6373 | §: 0.5649 |
| (mg/dL) | L group | 61.8 ± 13.5 | 66.5 ± 14.3 | 4.7 ± 5.6 | <0.0001 **** | §§: 0.1486 | §§: 0.1113 | |
| C group | 59.7 ± 12.8 | 65.7 ± 13.9 | 6.0 ± 6.0 | <0.0001 **** | §§§: 0.5472 | |||
| LDL choresterol | H group | 155.6 ± 25.9 | 163.3 ± 29.3 | 7.7 ± 36.1 | 0.1399 | 0.5772 | §: 0.6604 | §: 0.6503 |
| (mg/dL) | L group | 162.1 ± 26.2 | 159.7 ± 30.7 | −2.4 ± 42.4 | 0.6870 | §§: 0.6183 | §§: 0.6960 | |
| C group | 155.8 ± 20.2 | 158.9 ± 28.6 | 3.1 ± 32.2 | 0.5030 | §§§: 0.9862 | |||
| LOX index-associated parameters | ||||||||
| sLOX-1 | H group | 271.8 ± 135.8 | 232.5 ± 101.4 | −39.3 ± 110.1 | 0.0150 * | 0.4188 | §: 0.4656 | §: 0.3901 |
| (ng/L) | L group | 297.3 ± 137.7 | 231.3 ± 94.8 | −65.8 ± 103.7 | <0.0001 **** | §§: 0.9839 | §§: 0.9786 | |
| C group | 285.2 ± 181.3 | 207.6 ± 76.6 | −77.5 ± 161.2 | 0.0015 ** | §§§: 0.5272 | |||
| LAB | H group | 6.2 ± 2,2 | 5.7 ± 2.5 | −0.5 ± 1.5 | 0.0296 * | 0.0120 * | §: 0.0337 * | §: 0.0238 * |
| (mg cs/L) | L group | 6.1 ± 2.7 | 6.4 ± 3.4 | 0.3 ± 1.4 | 0.1421 | §§: 0.9983 | §§: 0.9977 | |
| C group | 6.3 ± 2.7 | 6.7 ± 3.3 | 0.4 ± 1.8 | 0.1042 | §§§: 0.0228 * | |||
| LOX index | H group | 1682.3 ± 994.5 | 1312.3 ± 764.8 | −370.0 ± 624.9 | 0.0001 *** | 0.7130 | §: 0.7778 | §: 0.7222 |
| L group | 1807.1 ± 1122.9 | 1455.9 ± 924.4 | −351.2 ± 537.2 | <0.0001 **** | §§: 0.7511 | §§: 0.6913 | ||
| C group | 1785.6 ± 1454.1 | 1388.2 ± 797.9 | −397.3 ± 1025.6 | 0.0093 ** | §§§: 0.9710 | |||
| Glycometabolism-associated parameters | ||||||||
| HbA1c | H group | 5.28 ± 0.26 | 5.32 ± 0.25 | 0.04 ± 0.03 | 0.0701 | 0.6052 | §: 0.7259 | §: 0.6625 |
| (%) | L group | 5.24 ± 0.25 | 5.31 ± 0.29 | 0.07 ± 0.24 | 0.0529 | §§: 0.9890 | §§: 0.9853 | |
| C group | 5.22 ± 0.29 | 5.30 ± 0.32 | 0.08 ± 0.21 | 0.0115 * | §§§: 0.6140 | |||
| GA | H group | 14.15 ± 1.10 | 13.77 ± 1.13 | −0.38 ± 0.33 | <0.0001 **** | 0.0022 * | §:0.0191 * | §:0.0133 * |
| (%) | L group | 14.01 ± 0.85 | 13.82 ± 0.82 | −0.19 ± 0.32 | <0.0001 **** | §§: 0.7859 | §§: 0.7316 | |
| C group | 14.13 ± 1.08 | 13.91 ± 1.11 | −0.22 ± 0.31 | <0.0001 **** | §§§: 0.0035 ** | |||
| Blood glucose | H group | 89.9 ± 6.4 | 90.6 ± 9.0 | 0.7 ± 6.7 | 0.4394 | 0.9474 | §: 0.9790 | §: 0.9721 |
| (mg/dL) | L group | 89.6 ± 7.5 | 90.0 ± 7.2 | 0.4 ± 5.4 | 0.6405 | §§: 0.9357 | §§: 0.9157 | |
| C group | 89.2 ± 8.7 | 89.3 ± 6.9 | 0.4 ± 7.1 | 0.7037 | §§§: 0.9987 | |||
| IRI | H group | 6.5 ± 4.2 | 6.9 ± 4.2 | 0.4 ± 2.3 | 0.2674 | 0.2453 | §: 0.5301 | §: 0.4536 |
| (μU/mL) | L group | 5.1 ± 2.6 | 5.8 ± 3.6 | 0.7 ± 2.5 | 0.0589 | §§: 0.2505 | §§: 0.1951 | |
| C group | 6.3 ± 6.9 | 5.5 ± 2.4 | −0.8 ± 6.6 | 0.4081 | §§§: 0.7493 | |||
| HOMA-IR | H group | 1.4 ± 0.9 | 1.6 ± 1.0 | 0.1 ± 0.6 | 0.1602 | 0.2848 | §: 0.5213 | §: 0.4449 |
| L group | 1.1 ± 0.6 | 1.3 ± 0.8 | 0.1 ± 0.6 | 0.0714 | §§: 0.2708 | §§: 0.2124 | ||
| C group | 1.5 ± 2.1 | 1.1 ± 0.6 | −0.2 ± 2.0 | 0.4049 | §§§: 0.9057 | |||
| Cytokines | ||||||||
| Adiponectin | H group | 3.6 ± 2.3 | 3.9 ± 2.5 | 0.3 ± 0.7 | 0.0025 ** | 0.8585 | §: 0.8859 | §: 0.8525 |
| (μg/mL) | L group | 4.0 ± 2.3 | 3.8 ± 2.0 | 0.3 ± 0.5 | 0.0016 ** | §§: 0.8875 | §§: 0.8545 | |
| C group | 3.5 ± 1.9 | 4.3 ± 2.5 | 0.3 ± 0.6 | 0.0017 ** | §§§: 0.9890 | |||
| A biomarker for advances glycation end products | ||||||||
| Pentosidine | H group | 0.037 ± 0.010 | 0.045 ± 0.013 | 0.008 ± 0.016 | 0.0018 ** | 0.6181 | §: 0.9736 | §: 0.9649 |
| (μg/mL) | L group | 0.037 ± 0.009 | 0.046 ± 0.013 | 0.009 ± 0.013 | <0.0001 **** | §§: 0.5763 | §§: 0.5008 | |
| C group | 0.038 ± 1.000 | 0.044 ± 0.013 | 0.007 ± 0.014 | 0.0022 ** | §§§: 0.8089 | |||
| Complete blood count | ||||||||
| Hb | H group | 14.2 ± 1.0 | 14.5 ± 1.2 | 0.3 ± 0.6 | 0.0038 * | 0.9913 | §: 0.9913 | §: 0.9883 |
| (g/dL) | L group | 14.0 ± 1.3 | 14.3 ± 1.5 | 0.3 ± 0.6 | 0.0019 * | §§: 0.9983 | §§: 0.9977 | |
| C group | 14.1 ± 1.1 | 14.4 ± 1.2 | 0.3 ± 0.6 | 0.0015 * | §§§: 0.9971 | |||
| Hct | H group | 42.5 ± 3.2 | 43.5 ± 3.5 | 0.9 ± 1.9 | 0.0015 * | 0.8793 | §: 0.9103 | §: 0.8832 |
| (%) | L group | 41.9 ± 3.7 | 42.9 ± 4.2 | 1.0 ± 1.8 | 0.0002 * | §§: 0.9985 | §§: 0.9980 | |
| C group | 43.1 ± 3.2 | 43.1 ± 3.6 | 1.0 ± 2.0 | 0.0008 * | §§§: 0.8936 | |||
| RBC | H group | 459.9 ± 35.4 | 466.2 ± 33.5 | 6.3 ± 20.8 | 0.0364 * | 0.8523 | §: 0.9913 | §: 0.9884 |
| (×104/μL) | L group | 453.4 ± 33.7 | 460.3 ± 38.4 | 6.9 ± 18.7 | 0.0122 * | §§: 0.9479 | §§: 0.9314 | |
| C group | 454.7 ± 37.6 | 462.8 ± 42.1 | 8.2 ± 26.0 | 0.0328 * | §§§: 0.8182 | |||
| Plt | H group | 24.9 ± 5.0 | 27.9 ± 13.8 | 3.1 ± 12.7 | 0.0940 | 0.4660 | §: 0.9931 | §: 0.9908 |
| (×104/μL) | L group | 25.6 ± 5.9 | 25.9 ± 5.9 | 0.3 ± 2.5 | 0.3404 | §§: 0.4383 | §§: 0.3640 | |
| C group | 25.5 ± 5.3 | 26.1 ± 4.6 | 0.6 ± 2.6 | 0.0921 | §§§: 0.6535 | |||
| WBC | H group | 5298.0 ± 1307.0 | 5616.0 ± 1553.8 | 318.0 ± 1422.8 | 0.1204 | 0.2471 | §: 0.3171 | §: 0.2528 |
| (/μL) | L group | 5068.0 ± 1299.1 | 5200 ± 1149.1 | 132.0 ± 1080.9 | 0.3921 | §§: 0.3268 | §§: 0.2615 | |
| C group | 5187.8 ± 1426.5 | 5183.7 ± 1339.4 | −4.1 ± 938.5 | 0.9758 | §§§: 0.9656 | |||
| Liver functions | ||||||||
| AST | H group | 22.5 ± 6.7 | 21.7 ± 5.4 | −0.9 ± 6.3 | 0.3378 | 0.8213 | §: 0.9651 | §: 0.9538 |
| (U/L) | L group | 24.9 ± 10.6 | 24.7 ± 9.5 | −0.0 ± 3.2 | 0.8932 | §§: 0.9044 | §§: 0.8758 | |
| C group | 22.0 ± 5.3 | 22.0 ± 5.3 | −0.1 ± 7.3 | 0.9298 | §§§: 0.8312 | |||
| ALT | H group | 25.4 ± 15.8 | 25.3 ± 13.0 | −0.1 ± 11.8 | 0.9618 | 0.2705 | §: 0.2441 | §: 0.1897 |
| (U/L) | L group | 22.7 ± 14.8 | 23.1 ± 13.6 | 0.5 ± 10.6 | 0.7597 | §§: 0.4827 | §§: 0.4067 | |
| C group | 21.7 ± 11.5 | 22.0 ± 10.7 | 0.3 ± 4.7 | 0.6749 | §§§: 0.9817 | |||
| γGTP | H group | 30.1 ± 22.3 | 31.7 ± 27.2 | 1.6 ± 8.1 | 0.1653 | 0.4453 | §: 0.3913 | §: 0.3199 |
| (U/L) | L group | 41.0 ± 35.6 | 42.2 ± 38.3 | 1.2 ± 13.8 | 0.5484 | §§: 0.6982 | §§: 0.6314 | |
| C group | 23.8 ± 14.7 | 23.9 ± 14.9 | 0.1 ± 4.6 | 0.8516 | §§§: 0.9617 | |||
| Iron metabolism | ||||||||
| Fe | H group | 19.3 ± 6.0 | 18.8 ± 7.1 | −0.5 ± 7.3 | 0.6542 | 0.3897 | §: 0.6716 | §: 0.6021 |
| (μmol/L) | L group | 18.6 ± 6.7 | 19.2 ± 6.5 | 0.6 ± 7.2 | 0.5488 | §§: 0.3785 | §§: 0.3081 | |
| C group | 19.1 ± 5.4 | 17.6 ± 5.7 | −1.6 ± 6.2 | 0.0772 | §§§: 0.8403 | |||
| Kruskal–Wallis Test a | ANCOVA Test b (Adjusted for Age) | ANCOVA Test b (Adjusted for Sex) | |
|---|---|---|---|
| p-Value | p-Value | p-Value | |
| the Δ value of sLOX-1 (ng/L) | 0.4188 | 0.3432 | 0.2901 |
| the Δ value of LAB (mg cs/L) | 0.0120 * | 0.0149 * | 0.0122 * |
| the Δ value of LOX index | 0.7130 | 0.9543 | 0.9378 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyawaki, M.; Sano, H.; Imbe, H.; Fujisawa, R.; Tanimoto, K.; Terasaki, J.; Maeda-Yamamoto, M.; Tachibana, H.; Hanafusa, T.; Imagawa, A. “Benifuuki” Extract Reduces Serum Levels of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Ligands Containing Apolipoprotein B: A Double-Blind Placebo-Controlled Randomized Trial. Nutrients 2018, 10, 924. https://doi.org/10.3390/nu10070924
Miyawaki M, Sano H, Imbe H, Fujisawa R, Tanimoto K, Terasaki J, Maeda-Yamamoto M, Tachibana H, Hanafusa T, Imagawa A. “Benifuuki” Extract Reduces Serum Levels of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Ligands Containing Apolipoprotein B: A Double-Blind Placebo-Controlled Randomized Trial. Nutrients. 2018; 10(7):924. https://doi.org/10.3390/nu10070924
Chicago/Turabian StyleMiyawaki, Masahiro, Hiroyuki Sano, Hisashi Imbe, Reiko Fujisawa, Keiji Tanimoto, Jungo Terasaki, Mari Maeda-Yamamoto, Hirofumi Tachibana, Toshiaki Hanafusa, and Akihisa Imagawa. 2018. "“Benifuuki” Extract Reduces Serum Levels of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Ligands Containing Apolipoprotein B: A Double-Blind Placebo-Controlled Randomized Trial" Nutrients 10, no. 7: 924. https://doi.org/10.3390/nu10070924