Effectiveness of Interventions and Behaviour Change Techniques for Improving Dietary Intake in Young Adults: A Systematic Review and Meta-Analysis of RCTs
Abstract
:1. Introduction
- evaluate the effectiveness of interventions aiming to improve dietary intake among healthy young adults (aged 17–35 years) and
- identify the BCTs used in these interventions and determine which are most effective.
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Types of Participants
2.2.2. Types of Interventions
2.2.3. Types of Comparators
2.2.4. Type of Outcome Measures
2.2.5. Types of studies
2.3. Information Sources and Search
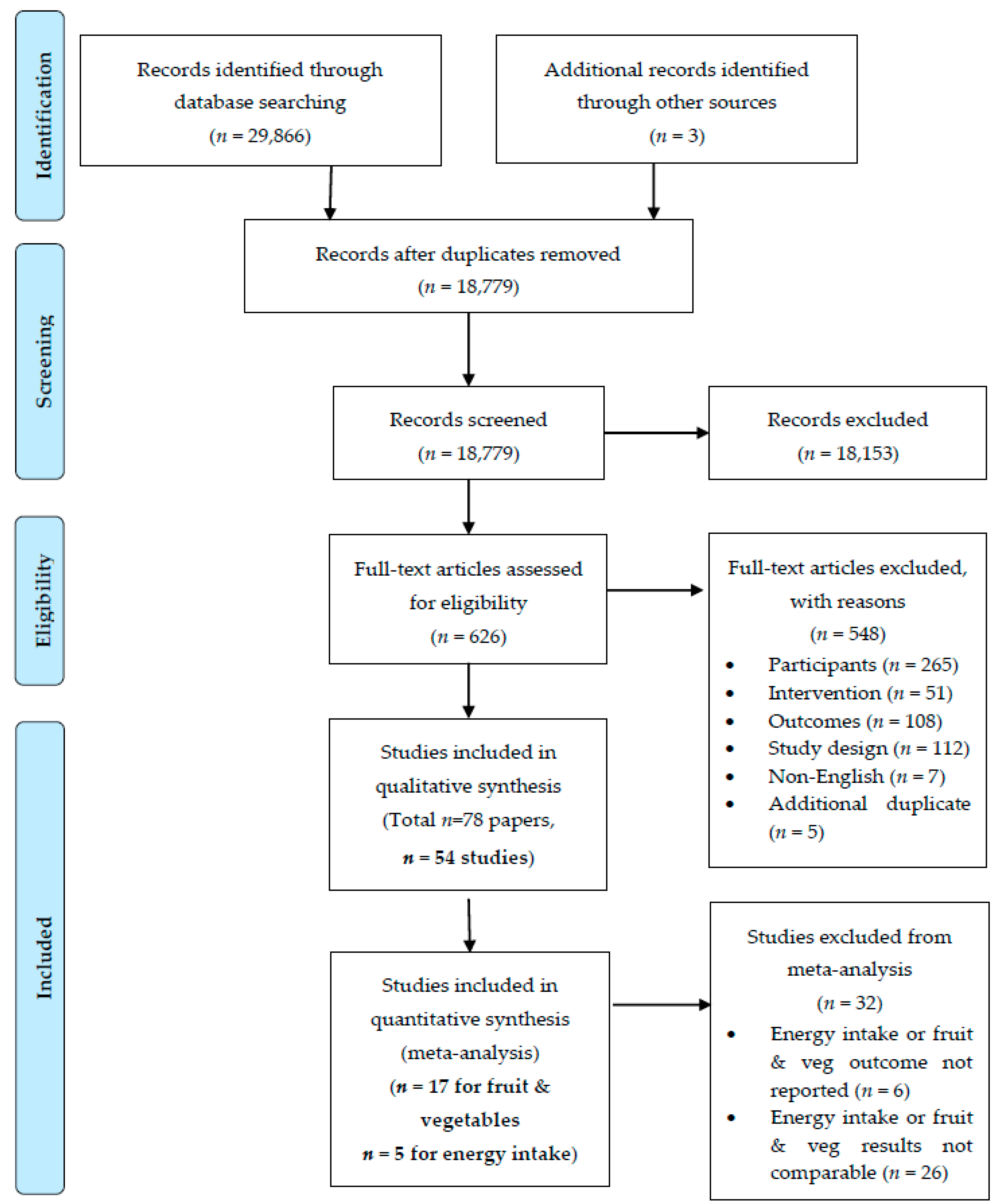
2.4. Study Selection
2.5. Risk of Bias
2.6. Data Extraction
2.7. Coding of BCTs
2.8. Synthesis of Results and Analytic Strategy
2.8.1. Narrative Summary
2.8.2. Meta-Analysis
2.9. Effectiveness of BCTs
3. Results
3.1. Description of Included Studies
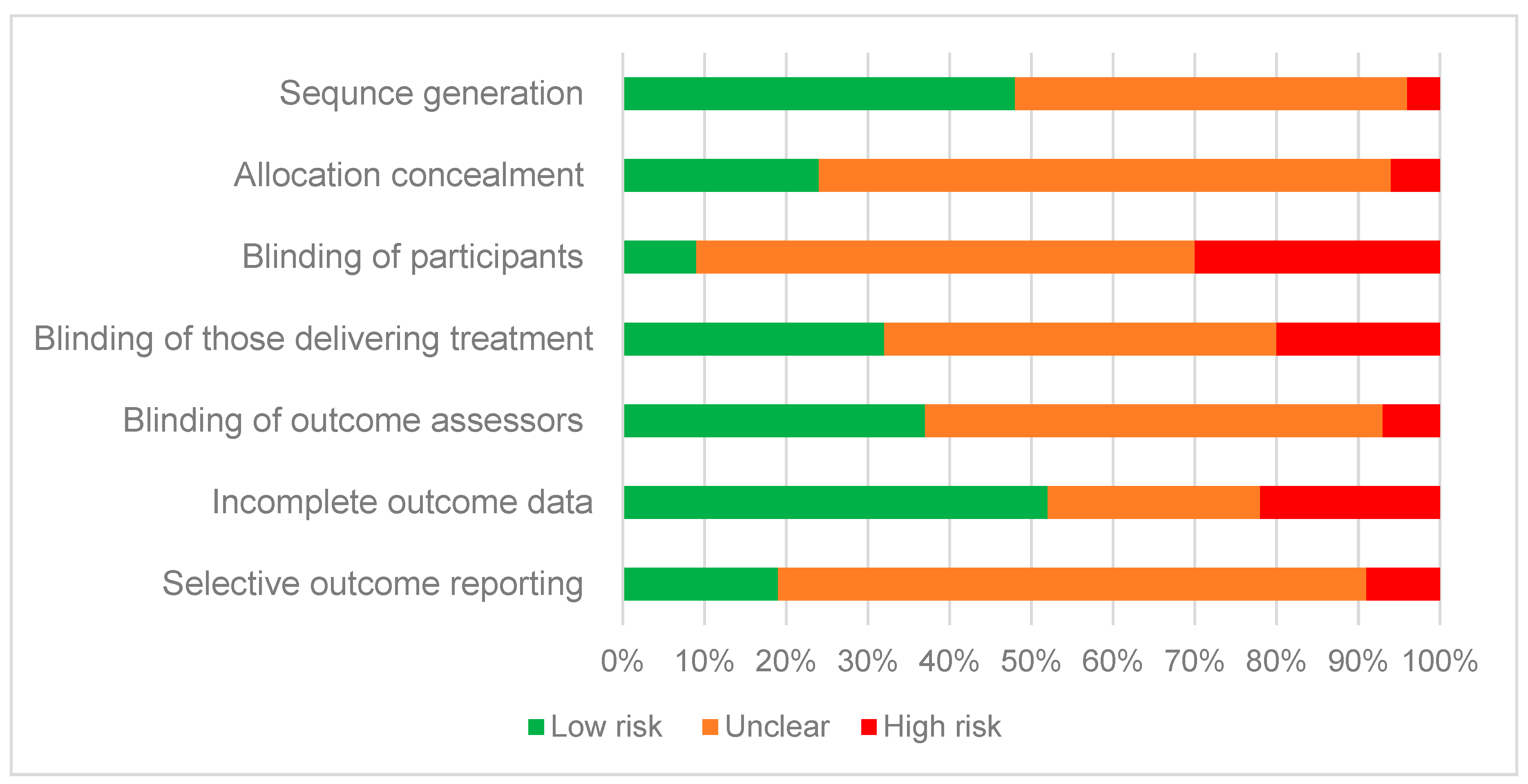
3.2. Risk of Bias
3.3. Dietary Outcome: Total Energy Intake
3.3.1. Total Energy Intake: all Included Studies
3.3.2. Total Energy Intake: Meta-Analysis
3.4. Dietary Outcome: Fruit and Vegetables
3.4.1. Fruit and Vegetable Intake: All Included Studies
3.4.2. Fruit and Vegetable Intake: Meta-Analysis
3.5. Dietary Outcome: Nutrient-Dense Foods (Other Than Vegetables and Fruit)
3.5.1. Diet Quality Score Rating
3.5.2. Individual Food Groups
3.5.3. Percentage of total Daily Energy Intake
3.5.4. Micronutrients & Dietary Fibre
3.6. Dietary Outcome: Energy-Dense Beverages
3.6.1. Sugar Sweetened Beverages (SSBs)
3.6.2. Fruit Juice
3.7. Dietary Outcome: Energy-Dense Nutrient-Poor (Ed-Np) Foods
3.7.1. Percentage of Total Daily Energy Intake
3.7.2. Takeaway/Fast Food
3.7.3. Individual Foods/Food Groups
3.7.4. Salt or Sugar
3.8. Dietary Outcome: Macronutrients
3.8.1. Changes in Intake of Dietary Fats
3.8.2. Changes in Carbohydrate intake
3.8.3. Changes in Protein Intake
3.9. Dietary Outcome: Diet Behaviour
3.10. Behaviour Change Techniques
Description of Behaviour Change Techniques Applied
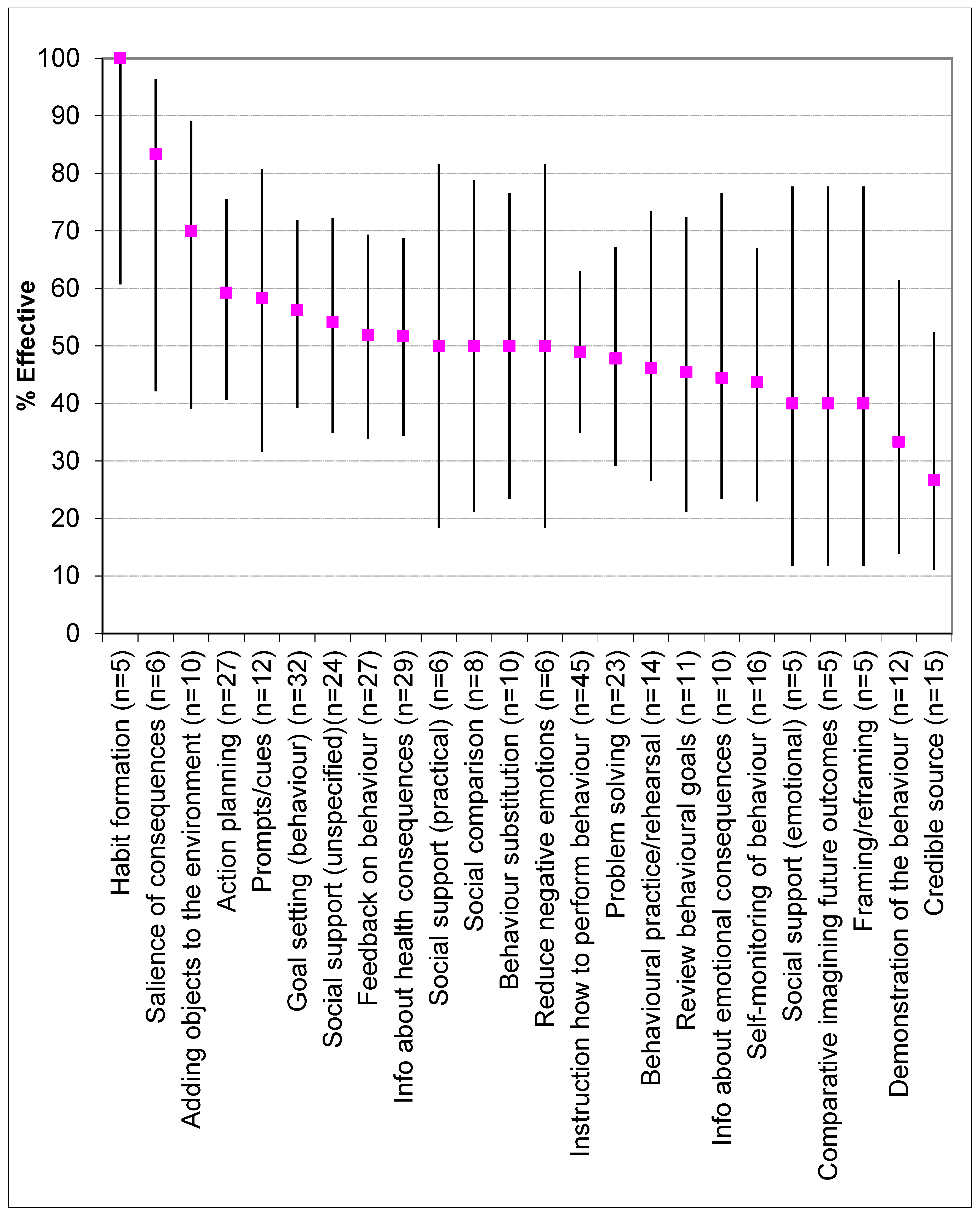
3.11. Effectiveness of Behaviour Change Techniques
4. Discussion
4.1. Effectiveness of Interventions on Dietary Outcomes
4.2. Effectiveness of BCTs
4.3. Strengths and Limitations of Included Studies
4.4. Strengths and Limitations of Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Arnett, J.J.; Žukauskienė, R.; Sugimura, K. The new life stage of emerging adulthood at ages 18–29 years: Implications for mental health. Lancet Psychiatry 2014, 1, 569–576. [Google Scholar] [CrossRef]
- Winpenny, E.M.; Penney, T.L.; Corder, K.; White, M.; van Sluijs, E.M. Change in diet in the period from adolescence to early adulthood: A systematic scoping review of longitudinal studies. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Winpenny, E.M.; van Sluijs, E.M.; White, M.; Klepp, K.-I.; Wold, B.; Lien, N. Changes in diet through adolescence and early adulthood: Longitudinal trajectories and association with key life transitions. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Hayes, A.; Lung, T.; Bauman, A.; Howard, K. Modelling obesity trends in Australia: Unravelling the past and predicting the future. Int. J. Obes. 2017, 41, 178. [Google Scholar] [CrossRef]
- Truesdale, K.P.; Stevens, J.; Lewis, C.E.; Schreiner, P.J.; Loria, C.M.; Cai, J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: The CARDIA study. Int. J. Obes. 2006, 30, 1397. [Google Scholar] [CrossRef] [PubMed]
- Tanamas, S.K.; Magliano, D.J.; Lynch, B.; Sethi, P.; Willenberg, L.; Polkinghorne, K.R.; Chadban, S.; Dunstan, D.; Shaw, J.E. AusDiab 2012—Australian Diabetes, Obesity and Lifestyle Study; Baker IDI Heart and Diabetes Institute: Melbourne, Australia, 2012. [Google Scholar]
- Zheng, Y.; Manson, J.E.; Yuan, C.; Liang, M.H.; Grodstein, F.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017, 318, 255–269. [Google Scholar] [CrossRef]
- Dietz, W.H. Obesity and excessive weight gain in young adults: New targets for prevention. JAMA 2017, 318, 241–242. [Google Scholar] [CrossRef]
- Deliens, T.; Van Crombruggen, R.; Verbruggen, S.; De Bourdeaudhuij, I.; Deforche, B.; Clarys, P. Dietary interventions among university students: A systematic review. Appetite 2016, 105, 14–26. [Google Scholar] [CrossRef]
- Plotnikoff, R.C.; Costigan, S.A.; Williams, R.L.; Hutchesson, M.J.; Kennedy, S.G.; Robards, S.L.; Allen, J.; Collins, C.E.; Callister, R.; Germov, J. Effectiveness of interventions targeting physical activity, nutrition and healthy weight for university and college students: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Willmott, T.J.; Pang, B.; Rundle-Thiele, S.; Badejo, A. Weight Management in Young Adults: Systematic Review of Electronic Health Intervention Components and Outcomes. J. Med. Internet Res. 2019, 21, e10265. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Chen, J.; Allman-Farinelli, M. Efficacy and external validity of electronic and mobile phone-based interventions promoting vegetable intake in young adults: Systematic review and meta-analysis. J. Med. Internet Res. 2016, 18, e58. [Google Scholar] [CrossRef] [PubMed]
- Oosterveen, E.; Tzelepis, F.; Ashton, L.; Hutchesson, M.J. A systematic review of eHealth behavioral interventions targeting smoking, nutrition, alcohol, physical activity and/or obesity for young adults. Prev. Med. 2017, 99, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.; Juan, S.H.; McGeechan, K.; Bauman, A.; Allman-Farinelli, M. Poor quality of external validity reporting limits generalizability of overweight and/or obesity lifestyle prevention interventions in young adults: A systematic review. Obes. Rev. 2015, 16, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Poobalan, A.S.; Aucott, L.S.; Precious, E.; Crombie, I.; Smith, W.C.S. Weight loss interventions in young people (18 to 25 year olds): A systematic review. Obes. Rev. 2010, 11, 580–592. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Cradock, K.A.; ÓLaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A.M. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 18. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Ashton, L.; Collins, C.; Williams, R.; Pezdirc, K.; Whatnall, M.; Hutchesson, M. Systematic Review of Nutrition, Physical Activity and/or Obesity Interventions in Young Adults (aged 17–35 years). Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017075795 (accessed on 10 April 2019).
- National Institutes of Health. Trials Use Technology to Help Young Adults Achieve Healthy Weights; National Institutes of Health: Bethesda, MD, USA, 2010.
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Peters, G.-J.Y.; De Bruin, M.; Crutzen, R. Everything should be as simple as possible, but no simpler: Towards a protocol for accumulating evidence regarding the active content of health behaviour change interventions. Health Psychol. Rev. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef]
- Evans, C.E.; Christian, M.S.; Cleghorn, C.L.; Greenwood, D.C.; Cade, J.E. Systematic review and meta-analysis of school-based interventions to improve daily fruit and vegetable intake in children aged 5 to 12 y–. Am. J. Clin. Nutr. 2012, 96, 889–901. [Google Scholar] [CrossRef]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef]
- Whatnall, M.C.; Patterson, A.J.; Ashton, L.M.; Hutchesson, M.J. Effectiveness of brief nutrition interventions on dietary behaviours in adults: A systematic review. Appetite 2018, 120, 335–347. [Google Scholar] [CrossRef]
- Martin, J.; Chater, A.; Lorencatto, F. Effective behaviour change techniques in the prevention and management of childhood obesity. Int. J. Obes. 2013, 37, 1287. [Google Scholar] [CrossRef]
- Brookie, K.L.; Mainvil, L.A.; Carr, A.C.; Vissers, M.C.; Conner, T.S. The development and effectiveness of an ecological momentary intervention to increase daily fruit and vegetable consumption in low-consuming young adults. Appetite 2017, 108, 32–41. [Google Scholar] [CrossRef]
- Buscemi, J.; Yurasek, A.M.; Dennhardt, A.A.; Martens, M.P.; Murphy, J.G. A randomized trial of a brief intervention for obesity in college students. Clin. Obes. 2011, 1, 131–140. [Google Scholar] [CrossRef]
- Chang, M.W.; Nitzke, S.; Brown, R. Design and outcomes of a Mothers In Motion behavioral intervention pilot study. J. Nutr. Educ. Behav. 2010, 42, S11–S21. [Google Scholar] [CrossRef]
- Chapman, J.; Armitage, C.J.; Norman, P. Comparing implementation intention interventions in relation to young adults’ intake of fruit and vegetables. Psychol. Health 2009, 24, 317–332. [Google Scholar] [CrossRef]
- Franko, D.L.; Jenkins, A.; Rodgers, R.F. Toward Reducing Risk for Eating Disorders and Obesity in Latina College Women. J. Couns. Dev. 2012, 90, 298–307. [Google Scholar] [CrossRef]
- Greene, G.W.; White, A.A.; Hoerr, S.L.; Lohse, B.; Schembre, S.M.; Riebe, D.; Patterson, J.; Kattelmann, K.K.; Shoff, S.; Horacek, T.; et al. Impact of an Online Healthful Eating and Physical Activity Program for College Students. Am. J. Health Promot. 2012, 27, E47–E58. [Google Scholar] [CrossRef]
- Heeren, G.A.; Jemmott, J.B.; Marange, C.S.; Rumosa Gwaze, A.; Batidzirai, J.M.; Ngwane, Z.; Mandeya, A.; Tyler, J.C. Health-Promotion Intervention Increases Self-Reported Physical Activity in Sub-Saharan African University Students: A Randomized Controlled Pilot Study. Behav. Med. 2017, 1–9. [Google Scholar] [CrossRef]
- Kattelmann, K.K.; Bredbenner, C.B.; White, A.A.; Greene, G.W.; Hoerr, S.L.; Kidd, T.; Colby, S.; Horacek, T.M.; Phillips, B.W.; Koenings, M.M.; et al. The effects of Young Adults Eating and Active for Health (YEAH): A theory-based web-delivered intervention. J. Nutr. Educ. Behav. 2014, 46, S27–S41. [Google Scholar] [CrossRef]
- Kothe, E.J.; Mullan, B.A. A randomised controlled trial of a theory of planned behaviour to increase fruit and vegetable consumption. Fresh Facts. Appetite 2014, 78, 68–75. [Google Scholar] [CrossRef]
- Kreausukon, P.; Gellert, P.; Lippke, S.; Schwarzer, R. Planning and self-efficacy can increase fruit and vegetable consumption: A randomized controlled trial. J. Behav. Med. 2012, 35, 443–451. [Google Scholar] [CrossRef]
- Nix, E.; Wengreen, H.J. Social approval bias in self-reported fruit and vegetable intake after presentation of a normative message in college students. Appetite 2017, 116, 552–558. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Shibata, K.; Fukuwatari, T.; Sasaki, Y.; Nakai, K. Randomized controlled trial of educational intervention to increase consumption of vegetables by Japanese university students. Health Educ. 2018, 118, 290–303. [Google Scholar] [CrossRef]
- Pellitteri, K.; Huberty, J.; Ehlers, D.; Bruening, M. Fit Minded College Edition Pilot Study: Can a Magazine-Based Discussion Group Improve Physical Activity in Female College Freshmen? J. Public Health Manag. Pract. 2017, 23, e10–e19. [Google Scholar] [CrossRef]
- Richards, A.; Kattelmann, K.K.; Ren, C. Motivating 18- to 24-year-olds to increase their fruit and vegetable consumption. J. Am. Diet. Assoc. 2006, 106, 1405–1411. [Google Scholar] [CrossRef]
- Schweitzer, A.L.; Ross, J.T.; Klein, C.J.; Lei, K.Y.; Mackey, E.R. An Electronic Wellness Program to Improve Diet and Exercise in College Students: A Pilot Study. JMIR Res. Protoc. 2016, 5. [Google Scholar] [CrossRef]
- Uglem, S.; Stea, T.H.; Kjollesdal, M.K.R.; Frolich, W.; Wandel, M. A nutrition intervention with a main focus on vegetables and bread consumption among young men in the Norwegian National Guard. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef]
- Werch, C.E.; Moore, M.J.; Bian, H.; DiClemente, C.C.; Huang, I.C.; Ames, S.C.; Thombs, D.; Weiler, R.M.; Pokorny, S.B. Are effects from a brief multiple behavior intervention for college students sustained over time? Prev. Med. 2010, 50, 30–34. [Google Scholar] [CrossRef]
- Eiben, G.; Lissner, L. Health Hunters—An intervention to prevent overweight and obesity in young high-risk women. Int. J. Obes. 2006, 30, 691–696. [Google Scholar] [CrossRef]
- Jung, M.E.; Martin Ginis, K.A.; Phillips, S.M.; Lordon, C.D. Increasing calcium intake in young women through gain-framed, targeted messages: A randomised controlled trial. Psychol. Health 2011, 26, 531–547. [Google Scholar] [CrossRef]
- Matvienko, O.; Lewis, D.S.; Schafer, E. A college nutrition science course as an intervention to prevent weight gain in female college freshmen. J. Nutr. Educ. 2001, 33, 95–101. [Google Scholar] [CrossRef]
- Middleton, K.R.; Perri, M.G. A Randomized Trial Investigating the Effect of a Brief Lifestyle Intervention on Freshman-Year Weight Gain. J. Am. Coll. Health 2014, 62, 101–109. [Google Scholar] [CrossRef]
- Share, B.L.; Naughton, G.A.; Obert, P.; Peat, J.K.; Aumand, E.A.; Kemp, J.G. Effects of a Multi-Disciplinary Lifestyle Intervention on Cardiometabolic Risk Factors in Young Women with Abdominal Obesity: A Randomised Controlled Trial. PLoS ONE 2015, 10, e0130270. [Google Scholar] [CrossRef]
- Ashton, L.M.; Morgan, P.J.; Hutchesson, M.J.; Rollo, M.E.; Collins, C.E. Feasibility and preliminary efficacy of the ‘HEYMAN’ healthy lifestyle program for young men: A pilot randomised controlled trial. Nutr. J. 2017, 16, 1–17. [Google Scholar] [CrossRef]
- Do, M.; Kattelmann, K.; Boeckner, L.; Greene, G.; White, A.; Hoerr, S.; Horacek, T.; Lohse, B.; Phillips, B.; Nitzke, S. Low-income young adults report increased variety in fruit and vegetable intake after a stage-tailored intervention. Nutr. Res. 2008, 28, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Morrongiello, B.; Meckling, K. A randomized, controlled trial evaluating the efficacy of an online intervention targeting vitamin D intake, knowledge and status among young adults. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.W.; Trace, S.E.; Mazzeo, S.E. Preventing weight gain in first year college students: An online intervention to prevent the “freshman fifteen”. Eat. Behav. 2010, 11, 33–39. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; Callister, R.; Morgan, P.J.; Pranata, I.; Clarke, E.D.; Skinner, G.; Ashton, L.M.; Whatnall, M.C.; Jones, M.; Oldmeadow, C.; et al. A Targeted and Tailored eHealth Weight Loss Program for Young Women: The Be Positive Be Healthe Randomized Controlled Trial. Healthcare 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Davis, K.K.; Rogers, R.J.; King, W.C.; Marcus, M.D.; Helsel, D.; Rickman, A.D.; Wahed, A.S.; Belle, S.H. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA 2016, 316, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kendzierski, D.; Ritter, R.L.; Stump, T.K.; Anglin, C.L. The effectiveness of an implementation intentions intervention for fruit and vegetable consumption as moderated by self-schema status. Appetite 2015, 95, 228–238. [Google Scholar] [CrossRef] [PubMed]
- LaChausse, R.G. My student body: Effects of an internet-based prevention program to decrease obesity among college students. J. Am. Coll. Health 2012, 60, 324–330. [Google Scholar] [CrossRef]
- Laska, M.N.; Lytle, L.A.; Nanney, M.S.; Moe, S.G.; Linde, J.A.; Hannan, P.J. Results of a 2-year randomized, controlled obesity prevention trial: Effects on diet, activity and sleep behaviors in an at-risk young adult population. Prev. Med. 2016, 89, 230–236. [Google Scholar] [CrossRef]
- Meng, J.; Peng, W.; Shin, S.Y.; Chung, M. Online Self-Tracking Groups to Increase Fruit and Vegetable Intake: A Small-Scale Study on Mechanisms of Group Effect on Behavior Change. J. Med. Internet Res. 2017, 19, e63. [Google Scholar] [CrossRef]
- Sandrick, J.; Tracy, D.; Eliasson, A.; Roth, A.; Bartel, J.; Simko, M.; Bowman, T.; Harouse-Bell, K.; Kashani, M.; Vernalis, M. Effect of a Counseling Session Bolstered by Text Messaging on Self-Selected Health Behaviors in College Students: A Preliminary Randomized Controlled Trial. JMIR Mhealth Uhealth 2017, 5. [Google Scholar] [CrossRef]
- Stice, E.; Rohde, P.; Shaw, H.; Marti, C. Efficacy trial of a selective prevention program targeting both eating disorders and obesity among female college students: 1- and 2-year follow-up effects. J. Consult. Clin. Psychol. 2013, 81, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.R.; Dini-Talatappeh, H.; Rahmati-Najarkolaei, F.; Fesharaki, M.G. Efficacy of HBM-Based Dietary Education Intervention on Knowledge, Attitude, and Behavior in Medical Students. Iran. Red Crescent Med. J. 2016, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Cooke, R. Using a combined motivational and volitional intervention to promote exercise and healthy dietary behaviour among undergraduates. Diabetes Res. Clin. Pract. 2012, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Allman-Farinelli, M.; Partridge, S.R.; McGeechan, K.; Balestracci, K.; Hebden, L.; Wong, A.; Phongsavan, P.; Denney-Wilson, E.; Harris, M.F.; Bauman, A. A Mobile Health Lifestyle Program for Prevention of Weight Gain in Young Adults (TXT2BFiT): Nine-Month Outcomes of a Randomized Controlled Trial. JMIR Mhealth Uhealth 2016, 4, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Annesi, J.J.; Howton, A.; Johnson, P.H.; Porter, K.J. Pilot testing a cognitive-behavioral protocol on psychosocial predictors of exercise, nutrition, weight, and body satisfaction changes in a college-level health-related fitness course. J. Am. Coll. Health 2015, 63, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Hebden, L.; Cook, A.; van der Ploeg, H.P.; King, L.; Bauman, A.; Allman-Farinelli, M. A mobile health intervention for weight management among young adults: A pilot randomised controlled trial. J. Hum. Nutr. Diet. 2014, 27, 322–332. [Google Scholar] [CrossRef]
- Knauper, B.; McCollam, A.; Rosen-Brown, A.; Lacaille, J.; Kelso, E.; Roseman, M. Fruitful plans: Adding targeted mental imagery to implementation intentions increases fruit consumption. Psychol. Health 2011, 26, 601–617. [Google Scholar] [CrossRef]
- Kothe, E.J.; Mullan, B.A.; Butow, P. Promoting fruit and vegetable consumption. Testing an intervention based on the theory of planned behaviour. Appetite 2012, 58, 997–1004. [Google Scholar] [CrossRef]
- Kypri, K.; McAnally, H.M. Randomized controlled trial of a web-based primary care intervention for multiple health risk behaviors. Prev. Med. 2005, 41, 761–766. [Google Scholar] [CrossRef]
- Lhakhang, P.; Godinho, C.; Knoll, N.; Schwarzer, R. A brief intervention increases fruit and vegetable intake. A comparison of two intervention sequences. Appetite 2014, 82, 103–110. [Google Scholar] [CrossRef]
- Park, A.; Nitzke, S.; Kritsch, K.; Kattelmann, K.; White, A.; Boeckner, L.; Lohse, B.; Hoerr, S.; Greene, G.; Zhang, Z. Internet-based interventions have potential to affect short-term mediators and indicators of dietary behavior of young adults. J. Nutr. Educ. Behav. 2008, 40, 288–297. [Google Scholar] [CrossRef]
- Rompotis, C.J.; Grove, J.R.; Byrne, S.M. Benefits of habit-based informational interventions: A randomised controlled trial of fruit and vegetable consumption. Aust. N. Z. J. Public Health 2014, 38, 247–252. [Google Scholar] [CrossRef]
- Kerr, D.A.; Harray, A.J.; Pollard, C.M.; Dhaliwal, S.S.; Delp, E.J.; Howat, P.A.; Pickering, M.R.; Ahmad, Z.; Meng, X.; Pratt, I.S.; et al. The connecting health and technology study: A 6-month randomized controlled trial to improve nutrition behaviours using a mobile food record and text messaging support in young adults. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Rodriguez-Rodriguez, E.; Aparicio, A.; Marin-Arias, L.I.; Lopez-Sobaler, A.M. Responses to two weight-loss programs based on approximating the diet to the ideal: Differences associated with increased cereal or vegetable consumption. Int. J. Vitam. Nutr. Res. 2006, 76, 367–376. [Google Scholar] [CrossRef]
- O’Brien, L.M.; Palfai, T.P. Efficacy of a brief web-based intervention with and without SMS to enhance healthy eating behaviors among university students. Eat. Behav. 2016, 23, 104–109. [Google Scholar] [CrossRef]
- Stephens, J.D.; Yager, A.M.; Allen, J. Smartphone technology and text messaging for weight loss in young adults: A randomized controlled trial. J. Cardiovasc. Nurs. 2017, 32, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Svetkey, L.P.; Batch, B.C.; Lin, P.-H.; Intille, S.S.; Corsino, L.; Tyson, C.C.; Bosworth, H.B.; Grambow, S.C.; Voils, C.; Loria, C.; et al. Cell Phone Intervention for You (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity 2015, 23, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Franko, D.L.; Cousineau, T.M.; Trant, M.; Green, T.C.; Rancourt, D.; Thompson, D.; Ainscough, J.; Mintz, L.B.; Ciccazzo, M. Motivation, self-efficacy, physical activity and nutrition in college students: Randomized controlled trial of an internet-based education program. Prev. Med. 2008, 47, 369–377. [Google Scholar] [CrossRef]
- Williams, D.R.; Lewis, N.M. Effectiveness of nutrition counseling in young adult males. Nutr. Res. 2002, 22, 911–917. [Google Scholar] [CrossRef]
- Hivert, M.; Langlois, M.; Berard, P.; Cuerrier, J.; Carpentier, A. Prevention of weight gain in young adults through a seminar-based intervention program. Int. J. Obes. 2007, 31, 1262–1269. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Fulton, S.L.; McKinley, M.C.; Young, I.S.; Cardwell, C.R.; Woodside, J.V. The effect of increasing fruit and vegetable consumption on overall diet: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2016, 56, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Ravia, J. A systematic review of behavioral interventions to promote intake of fruit and vegetables. J. Am. Diet. Assoc. 2011, 111, 1523–1535. [Google Scholar] [CrossRef]
- Mytton, O.T.; Nnoaham, K.; Eyles, H.; Scarborough, P.; Mhurchu, C.N. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health 2014, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.L. A descriptive study of barriers to enrollment in a collegiate health assessment program. J. Community Health Nurs. 2005, 22, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.G.; Lytle, L.A.; Nanney, M.S.; Linde, J.A.; Laska, M.N. Recruiting and retaining young adults in a weight gain prevention trial: Lessons learned from the CHOICES study. Clin. Trials 2016, 13, 205–213. [Google Scholar] [CrossRef]
- Gardner, B.; de Bruijn, G.-J.; Lally, P. A systematic review and meta-analysis of applications of the self-report habit index to nutrition and physical activity behaviours. Ann. Behav. Med. 2011, 42, 174–187. [Google Scholar] [CrossRef]
- Lally, P.; Van Jaarsveld, C.H.; Potts, H.W.; Wardle, J. How are habits formed: Modelling habit formation in the real world. Eur. J. Soc. Psychol. 2010, 40, 998–1009. [Google Scholar] [CrossRef]
- Lally, P.; Wardle, J.; Gardner, B. Experiences of habit formation: A qualitative study. Psychol. Health Med. 2011, 16, 484–489. [Google Scholar] [CrossRef]
- Orbell, S.; Verplanken, B. The automatic component of habit in health behavior: Habit as cue-contingent automaticity. Health Psychol. 2010, 29, 374. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.T.; Hammond, D.; Hitchman, S.C. The impact of pictures on the effectiveness of tobacco warnings. Bull. World Health Organ. 2009, 640–643. [Google Scholar] [CrossRef]
- Blanton, H.; Snyder, L.B.; Strauts, E.; Larson, J.G. Effect of graphic cigarette warnings on smoking intentions in young adults. PLoS ONE 2014, 9, e96315. [Google Scholar] [CrossRef] [PubMed]
- Ashton, L.M.; Hutchesson, M.J.; Rollo, M.E.; Morgan, P.J.; Thompson, D.I.; Collins, C.E. Young adult males’ motivators and perceived barriers towards eating healthily and being active: A qualitative study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Evans, E.H.; O’Brien, N.; Moynihan, P.J.; Meyer, T.D.; Adamson, A.J.; Errington, L.; Sniehotta, F.F.; White, M.; Mathers, J.C. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: A systematic review and meta-analysis of randomised controlled trials. BMC Med. 2014, 12, 177. [Google Scholar] [CrossRef]
- Evans, J.; Frank, B.; Oliffe, J.L.; Gregory, D. Health, illness, men and masculinities (HIMM): A theoretical framework for understanding men and their health. J. Men’s Health 2011, 8, 7–15. [Google Scholar] [CrossRef]
- Arnett, J.J. Emerging adulthood: What is it, and what is it good for? Child Dev. Perspect. 2007, 1, 68–73. [Google Scholar] [CrossRef]
- World Health Organization. Men, Ageing and Health: Achieving Health Across the Life Span; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Michie, S.; Jochelson, K.; Markham, W.A.; Bridle, C. Low-income groups and behaviour change interventions: A review of intervention content, effectiveness and theoretical frameworks. J. Epidemiol. Community Health 2009, 63, 610–622. [Google Scholar] [CrossRef]



| Total | ||
|---|---|---|
| Publication year | Before 2004 n (%) | 2 (3.7%) |
| 2004–2008 n (%) | 8 (14.8%) | |
| 2009–2013 n (%) | 15 (27.7%) | |
| 2014–October 2018 n (%) | 29 (53.7%) | |
| Country | United States n (%) | 30 (55.5%) |
| Australia n (%) | 9 (16.7%) | |
| Canada n (%) | 3 (5.5%) | |
| UK n (%) | 2 (3.7%) | |
| Other n (%) | 10 (18.5%) | |
| Number of participants | Total | 16,383 |
| Mean | 303.4 | |
| Median | 161.5 | |
| Range | 37 to 2343 | |
| Sex | Female only studies n (%) | 11 (20.4%) |
| Male only studies n (%) | 4 (7.4%) | |
| Studies with both males and females n (%) | 39 (72.2%) | |
| Average proportion of males in gender-neutral programs % | 32% | |
| Age | Mean years | 20.9 |
| 17–≤25 years n (%) | 31 (57.4%) | |
| 17–≤30 years n (%) | 12 (22.2%) | |
| 17–≤35 years n (%) | 11 (20.4%) | |
| Ethnicity | Predominantly white n (%) | 32 (59.3%) |
| Predominantly non-white n (%) | 6 (11.1%) | |
| Not reported n (%) | 16 (29.6%) | |
| Education | Predominantly high school or less n (%) | 1 (1.9%) |
| Current University/ college students’ n (%) | 32 (59.3%) | |
| Not reported or unclear n (%) | 21 (38.9%) | |
| Mode of intervention delivery | eHealth only n (%) | 16 (29.6%) |
| Face-to-face only n (%) | 16 (29.6%) | |
| Print only n (%) | 1 (1.9%) | |
| Multicomponent n (%) | 21 (38.9%) | |
| Primary outcome | Nutrition | 32 (59.3%) |
| PA | 3 (5.6%) | |
| Weight or BMI (Obesity prevention focus) | 11 (20.4%) | |
| Weight, BMI or waist circumference (obesity treatment focus) | 8 (14.8%) | |
| Dietary assessment | FFQ | 25 (46.3%) |
| Specific nutrient/food/diet behaviour questionnaire | 17 (31.5%) | |
| Food record | 5 (9.3%) | |
| Recall (24-hr, 3-day and 7-day) | 5 (9.3%) | |
| Multiple | 2 (3.7%) | |
| Setting | College/University n (%) | 37 (68.5%) |
| Community n (%) | 15 (27.8%) | |
| Military n (%) | 2 (3.7%) | |
| Study arms | Total | 133 |
| 2 arms n (%) | 38 (70.4%) | |
| 3 arms n (%) | 10 (18.5%) | |
| 4 arms n (%) | 4 (7.4%) | |
| 5 arms n (%) | 1 (1.9%) | |
| 6 arms n (%) | 1 (1.9%) | |
| Intervention duration | Mean duration (months) | 4.2 |
| Range | Single session to 24 months | |
| Single session – ≤3-months n (%) | 40 (74.1%) | |
| 4–≤6-months n (%) | 8 (14.8%) | |
| 7–>12-months n (%) | 6 (11.1%) | |
| Length of follow-up from end of intervention | Mean length (months) | 1.9 |
| Range | 0 to 23-months | |
| No follow-up – ≤3-months n (%) | 45 (83.3%) | |
| 4–≤6-months n (%) | 3 (5.6%) | |
| 7–>12-months n (%) | 6 (11.1%) | |
| Retention rate | Post-intervention (mean %) | 77.7% |
| Range | 22% to 98% | |
| At longest follow-up point (mean %) | 66.2% | |
| Range | 11% to 97% |
| Behaviour Change Technique | N | % |
|---|---|---|
| 1.1 Goal setting (behaviour) | 32 | 45.7% |
| 1.2 Problem solving | 23 | 32.9% |
| 1.4 Action planning | 27 | 38.6% |
| 1.5 Review behavioural goals | 11 | 15.7% |
| 1.6 Discrepancy between behaviour and goals | 1 | 1.4% |
| 1.8 Behavioural contract | 2 | 2.9% |
| 1.9 Commitment | 2 | 2.9% |
| 2.1 Monitoring of behaviour by others without feedback | 1 | 1.4% |
| 2.2 Feedback on behaviour | 27 | 38.6% |
| 2.3 Self-monitoring of behaviour | 16 | 22.9% |
| 2.4 Self-monitoring of outcome(s) of behaviour | 4 | 5.7% |
| 2.6 Biofeedback | 2 | 2.9% |
| 2.7 Feedback on outcome(s) of behaviour | 2 | 2.9% |
| 3.1 Social support (unspecified) | 24 | 34.3% |
| 3.2 Social support (practical) | 6 | 8.6% |
| 3.3 Social support (emotional) | 5 | 7.1% |
| 4.1 Instruction on how to perform the behaviour | 45 | 64.3% |
| 4.2 Information about antecedents | 3 | 4.3% |
| 4.4 Behavioural experiments | 1 | 1.4% |
| 5.1 Information about health consequences | 29 | 41.4% |
| 5.2 Salience of consequences | 6 | 8.6% |
| 5.3 Information about social and environmental consequences | 1 | 1.4% |
| 5.6 Information about emotional consequences | 10 | 14.3% |
| 6.1 Demonstration of the behaviour | 12 | 17.1% |
| 6.2 Social comparison | 8 | 11.4% |
| 6.3 Information about others’ approval | 2 | 2.9% |
| 7.1 Prompts/cues | 12 | 17.1% |
| 8.1 Behavioural practice/rehearsal | 14 | 20.0% |
| 8.2 Behaviour substitution | 10 | 14.3% |
| 8.3 Habit formation | 5 | 7.1% |
| 8.4 Habit reversal | 4 | 5.7% |
| 8.7 Graded tasks | 4 | 5.7% |
| 9.1 Credible source | 15 | 21.4% |
| 9.2 Pros and cons | 4 | 5.7% |
| 9.3 Comparative imagining of future outcomes | 5 | 7.1% |
| 10.1 Material incentive (behaviour) | 1 | 1.4% |
| 10.2 Material reward (behaviour) | 1 | 1.4% |
| 10.3 Non-specific reward | 1 | 1.4% |
| 10.9 Self-reward | 1 | 1.4% |
| 10.10 Reward (outcome) | 1 | 1.4% |
| 11.2 Reduce negative emotions | 6 | 8.6% |
| 12.1 Restructuring the physical environment | 4 | 5.7% |
| 12.3 Avoidance/reducing exposure to cues for the behaviour | 4 | 5.7% |
| 12.5 Adding objects to the environment | 10 | 14.3% |
| 12.6 Body changes | 1 | 1.4% |
| 13.2 Framing/reframing | 5 | 7.1% |
| 13.3 Incompatible beliefs | 1 | 1.4% |
| 13.4 Valued self-identify | 2 | 2.9% |
| 13.5 Identity associated with changed behaviour | 3 | 4.3% |
| 15.1 Verbal persuasion about capability | 2 | 2.9% |
| 15.2 Mental rehearsal of successful performance | 2 | 2.9% |
| 15.3 Focus on past success | 3 | 4.3% |
| 15.4 Self-talk | 2 | 2.9% |
| 16.2. Imaginary reward | 2 | 2.9% |
| 16.3 Vicarious consequences | 3 | 4.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashton, L.M.; Sharkey, T.; Whatnall, M.C.; Williams, R.L.; Bezzina, A.; Aguiar, E.J.; Collins, C.E.; Hutchesson, M.J. Effectiveness of Interventions and Behaviour Change Techniques for Improving Dietary Intake in Young Adults: A Systematic Review and Meta-Analysis of RCTs. Nutrients 2019, 11, 825. https://doi.org/10.3390/nu11040825
Ashton LM, Sharkey T, Whatnall MC, Williams RL, Bezzina A, Aguiar EJ, Collins CE, Hutchesson MJ. Effectiveness of Interventions and Behaviour Change Techniques for Improving Dietary Intake in Young Adults: A Systematic Review and Meta-Analysis of RCTs. Nutrients. 2019; 11(4):825. https://doi.org/10.3390/nu11040825
Chicago/Turabian StyleAshton, Lee M., Thomas Sharkey, Megan C. Whatnall, Rebecca L. Williams, Aaron Bezzina, Elroy J. Aguiar, Clare E. Collins, and Melinda J. Hutchesson. 2019. "Effectiveness of Interventions and Behaviour Change Techniques for Improving Dietary Intake in Young Adults: A Systematic Review and Meta-Analysis of RCTs" Nutrients 11, no. 4: 825. https://doi.org/10.3390/nu11040825






