Prophylactic Intra-Uterine β-Cyclodextrin Administration during Intra-Uterine Ureaplasma parvum Infection Partly Prevents Liver Inflammation without Interfering with the Enterohepatic Circulation of the Fetal Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Procedures
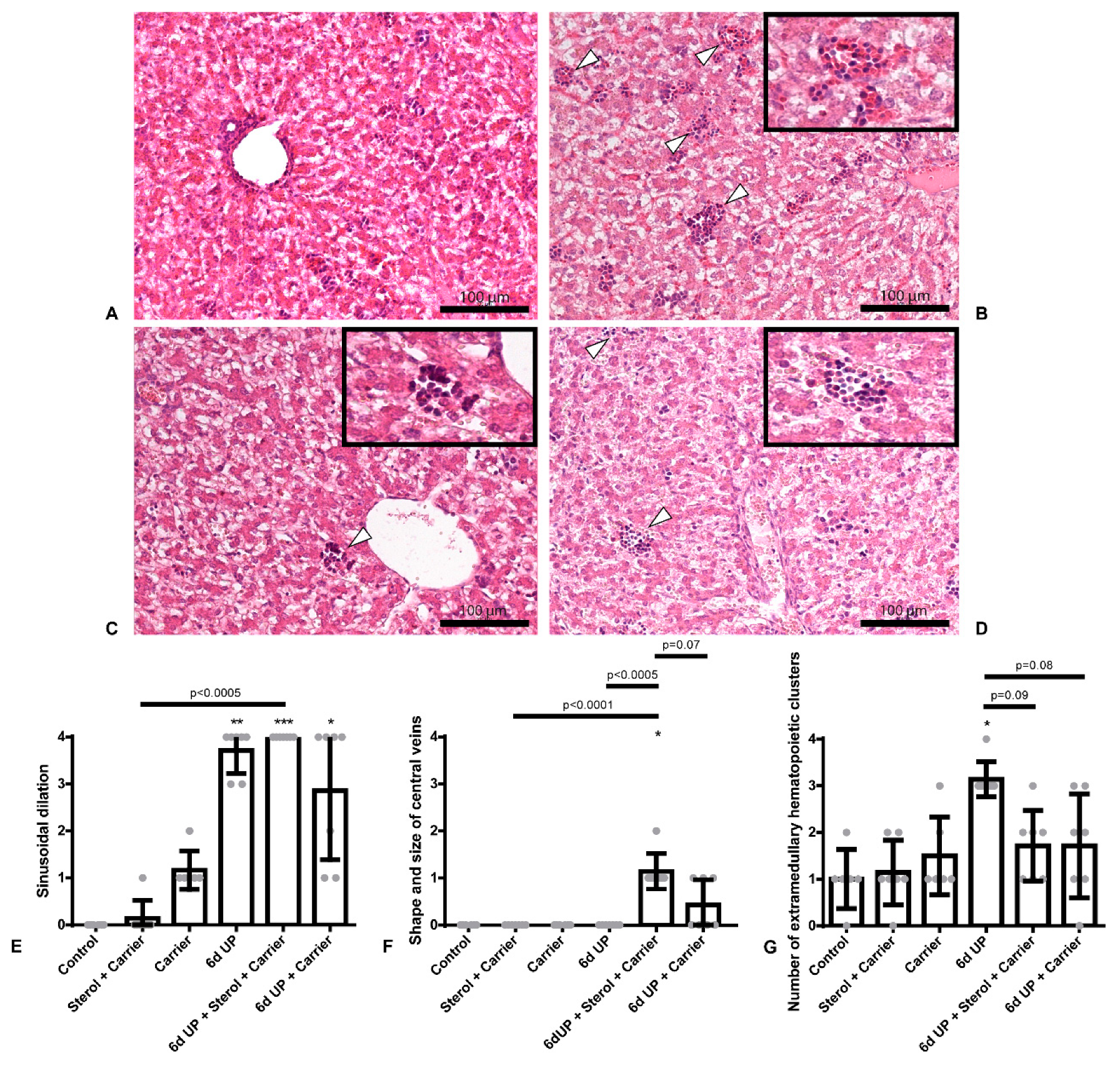
2.2. Qualitative Analysis of Liver Histology
2.3. Total Bile Acid Assay
2.4. RNA Extraction and Real-Time PCR
2.5. Data Analysis
3. Results
3.1. Sterol + Carrier, as Well as Carrier Alone, Partly Decrease Hepatic Inflammation Due to UP-Induced Chorioamnionitis
3.2. Administration of the Carrier Alone Prior to UP Exposure Causes Increased BSEP Expression in the Liver
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASBT | Apical sodium–dependent bile acid transporter |
| AU | Arbitrary unit |
| BAs | Bile acids |
| BSEP | Bile salt export pump |
| CCU | Color-changing units |
| CYP7A1 | Cholesterol 7 alpha-hydroxylase |
| CYP27A1 | Cytochrome P450 Family 27 Subfamily A Member 1 |
| EHC | Enterohepatic circulation |
| FGF19 | Fibroblast growth factor 19 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| IA | Intra-amniotic |
| IBABP | Ileal bile acid-binding protein |
| IQR | Interquartile range |
| NEC | Necrotizing enterocolitis |
| NTCP | Na+-taurocholate cotransporting polypeptide |
| OSTα | Organic solute transporter alpha |
| OSTβ | Organic solute transporter bèta |
| PPIA | Peptidylprolyl isomerase A |
| RPS15 | Ribosomal protein S15 |
| tBAs | Total bile acids |
| UP | Ureaplasma parvum |
| qPCR | Quantitative real-time PCR |
References
- Murphy, S.L.; Mathews, T.J.; Martin, J.A.; Minkovitz, C.S.; Strobino, D.M. Annual summary of vital statistics: 2013–2014. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silwedel, C.; Speer, C.P.; Glaser, K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev. Clin. Immunol. 2017, 13, 1073–1087. [Google Scholar] [CrossRef]
- Been, J.V.; Lievense, S.; Zimmermann, L.J.; Kramer, B.W.; Wolfs, T.G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J. Pediatr. 2013, 162, 236–242e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, W.W.; Goldenberg, R.L.; Faye-Petersen, O.; Cliver, S.; Goepfert, A.R.; Hauth, J.C. The Alabama Preterm Birth study: Polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am. J. Obstet. Gynecol. 2006, 195, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Okogbule-Wonodi, A.C.; Gross, G.W.; Sun, C.C.; Agthe, A.G.; Xiao, L.; Waites, K.B.; Viscardi, R.M. Necrotizing enterocolitis is associated with ureaplasma colonization in preterm infants. Pediatr. Res. 2011, 69, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Onderdonk, A.B.; Delaney, M.L.; DuBois, A.M.; Allred, E.N.; Leviton, A.; Extremely Low Gestational Age Newborns Study, I. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am. J. Obstet. Gynecol. 2008, 198, 110 e1. [Google Scholar] [CrossRef]
- Oh, K.J.; Lee, K.A.; Sohn, Y.K.; Park, C.W.; Hong, J.S.; Romero, R.; Yoon, B.H. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2010, 203, 211 e1. [Google Scholar] [CrossRef]
- Larsen, B.; Hwang, J. Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: A fresh look. Infect. Dis. Obstet. Gynecol. 2010, 2010. [Google Scholar] [CrossRef] [Green Version]
- Hegyi, P.; Maleth, J.; Walters, J.R.; Hofmann, A.F.; Keely, S.J. Guts and Gall: Bile acids in regulation of intestinal epithelial function in health and disease. Physiol. Rev. 2018, 98, 1983–2023. [Google Scholar] [CrossRef] [Green Version]
- Hulzebos, C.V.; van Zoonen, A.G.; Hulscher, J.B.; Schat, T.E.; Kooi, E.M.; Koehorst, M.; Boverhof, R.; Krabbe, P.F.; Groen, A.K.; Verkade, H.J. Fecal Bile Salts and the Development of Necrotizing Enterocolitis in Preterm Infants. PLoS ONE 2017, 12, e0168633. [Google Scholar] [CrossRef]
- Halpern, M.D.; Dvorak, B. Does abnormal bile acid metabolism contribute to NEC? Semin. Perinatol. 2008, 32, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrington, N.J.; Estrada, T.E.; Frisk, H.A.; Canet, M.J.; Hardwick, R.N.; Dvorak, B.; Lux, K.; Halpern, M.D. The hepatic bile acid transporters Ntcp and Mrp2 are downregulated in experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, M.D.; Weitkamp, J.H.; Mount Patrick, S.K.; Dobrenen, H.J.; Khailova, L.; Correa, H.; Dvorak, B. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 623–631. [Google Scholar] [CrossRef]
- Halpern, M.D.; Holubec, H.; Saunders, T.A.; Dvorak, K.; Clark, J.A.; Doelle, S.M.; Ballatori, N.; Dvorak, B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 2006, 130, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, M.D.; Holubec, H.; Dominguez, J.A.; Meza, Y.G.; Williams, C.S.; Ruth, M.C.; McCuskey, R.S.; Dvorak, B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, C.; de Lange, I.H.; Spiller, O.B.; Dewez, F.; Cillero Pastor, B.; Heeren, R.; Kessels, L.; Kloosterboer, N.; van Gemert, W.G.; Beeton, M.L.; et al. Protection of the ovine fetal gut against ureaplasma-induced chorioamnionitis: A potential role for plant sterols. Nutrients 2019, 11, 968. [Google Scholar] [CrossRef] [Green Version]
- Bieghs, V.; Vlassaks, E.; Custers, A.; van Gorp, P.J.; Gijbels, M.J.; Bast, A.; Bekers, O.; Zimmermann, L.J.; Lutjohann, D.; Voncken, J.W.; et al. Chorioamnionitis induced hepatic inflammation and disturbed lipid metabolism in fetal sheep. Pediatr. Res. 2010, 68, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegard, L.; Jessup, W.; Jones, P.J.; Lutjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Bouic, P.J.; Lamprecht, J.H. Plant sterols and sterolins: A review of their immune-modulating properties. Altern. Med. Rev. 1999, 4, 170–177. [Google Scholar]
- Ding, Y.; Nguyen, H.T.; Kim, S.I.; Kim, H.W.; Kim, Y.H. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorg. Med. Chem. Lett. 2009, 19, 3607–3610. [Google Scholar] [CrossRef]
- Valerio, M.; Awad, A.B. beta-Sitosterol down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1 murine macrophages. Int. Immunopharmacol. 2011, 11, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.S.; Minderman, H.; Mace, T.; Awad, A.B. beta-Sitosterol modulates TLR4 receptor expression and intracellular MyD88-dependent pathway activation in J774A.1 murine macrophages. Cell. Immunol. 2013, 285, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Lee, I.A.; Gu, W.; Hyam, S.R.; Kim, D.H. beta-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-kappaB pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Palladino, G.; Distrutti, E.; Fiorucci, S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem. Pharmacol. 2009, 78, 1214–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Velde, A.A.; Brull, F.; Heinsbroek, S.E.; Meijer, S.L.; Lutjohann, D.; Vreugdenhil, A.; Plat, J. Effects of dietary plant sterols and stanol esters with low- and high-fat diets in chronic and acute models for experimental colitis. Nutrients 2015, 7, 8518–8531. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.H.; Kim, J.S.; Kim, J.M.; Kim, N.; Jung, H.C.; Song, I.S. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm. Bowel. Dis. 2006, 12, 1152–1161. [Google Scholar] [CrossRef]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.; Walenbergh, S.M.; van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.; Guichot, Y.D.; et al. Protective role of plant sterol and stanol esters in liver inflammation: Insights from mice and humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef]
- Pfisterer, C.; Faber, R.; Horn, L.C. Chorioamnionitis-induced changes of fetal extramedullar hematopoiesis in the second trimester of gestation. Is diagnosis from fetal autopsy possible? Virchows Arch. 2005, 446, 150–156. [Google Scholar] [CrossRef]
- Brancatelli, G.; Furlan, A.; Calandra, A.; Dioguardi Burgio, M. Hepatic sinusoidal dilatation. Abdom. Radiol. 2018, 43, 2011–2022. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakke, S.S.; Aune, M.H.; Niyonzima, N.; Pilely, K.; Ryan, L.; Skjelland, M.; Garred, P.; Aukrust, P.; Halvorsen, B.; Latz, E.; et al. Cyclodextrin Reduces Cholesterol Crystal-Induced Inflammation by Modulating Complement Activation. J. Immunol. 2017, 199, 2910–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primer | Forward | Reverse |
|---|---|---|
| RPS15 | 5′-CGAGATGGTGGGCAGCAT-3′ | 5′-GCTTGATTTCCACCTGGTTGA-3′ |
| GAPDH | 5′-GGAAGCTCACTGGCATGGC-3′ | 5′-CCTGCTTCACCACCTTCTTG-3′ |
| PPIA | 5′-TTATAAAGGTTCCTGCTTTCACAGAA-3′ | 5′-ATGGACTTGCCACCAGTACCA-3′ |
| CYP7A1 | 5′-GGGCATCACAAGCAAACACC-3′ | 5′-GATGATACTGTCTAGCACGGG-3′ |
| CYP27A1 | 5′- CCCAAGAATACCCAGTTTGTGC-3′ | 5′- GGTGGCAGAAGACTCAGTTCA-3′ |
| NTCP | 5′-TCCTCAAATCCAAACGGCCA-3′ | 5′-GTTTGGATCGTCCATTGAGGC-3′ |
| BSEP | 5′-ACTCAGTAATTCTTCGCAGTGTG-3′ | 5′-ATCGAAACAATCGAAAGAAGCCA-3′ |
| ASBT | 5′-CATGGACCTGAGCGTCAGCAT-3′ | 5′-CACGGAGACGGGAACAACAA-3′ |
| FGF19 | 5′-TTGATGGAGATCAGGGCGGT-3′ | 5′-CGGATCTCCTCCTCGAAAGC-3′ |
| IBABP | 5′-ACAAGAAGTTCAAGGTCACCG-3′ | 5′-TGATACGGCTTTATGGCCCC-3′ |
| OSTα | 5′-ATCCCAGGTACACGGCAGAT-3′ | 5′-ATTGAGGCCAGGACAAGCAA-3′ |
| OSTβ | 5′-CCGAGTAGAGGATGCAACTCC-3′ | 5′-TTTGTTTTTCCGGTGGCAGC-3′ |
| Control | Sterol + Carrier | Carrier | UP | UP + Sterol + Carrier | UP + Carrier | |
|---|---|---|---|---|---|---|
| Hepatic sinusoidal dilation | nc | nc | nc | ↑ | ↑ (Control & Sterol + Carrier | ↑ |
| Shape and size of central veins | nc | nc | nc | nc | ↑ (Control, Sterol + Carrier, UP & UP + Carrier) | nc |
| Number of extramedullary hematopoietic clusters | nc | nc | nc | ↑ | ↓ (UP) | ↓ (UP) |
| NTCP | nc | nc | nc | nc | ↑ (Sterol + Carrier & UP) | nc |
| BSEP | nc | nc | nc | nc | nc | ↑ (Sterol + Carrier, UP & UP + Sterol + Carrier) |
| tBAs plasma | nc | nc | nc | nc | nc | nc |
| tBAs liver | nc | nc | nc | nc | nc | nc |
| CYP7A1 | nc | nc | nc | nc | nc | nc |
| CYP27A1 | nc | nc | nc | nc | nc | nc |
| ASBT | nc | nc | nc | nc | nc | nc |
| OSTα-β | nc | nc | nc | nc | nc | nc |
| FGF19 | nc | nc | nc | nc | nc | nc |
| IBABP | nc | nc | nc | nc | nc | nc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heymans, C.; Heij, L.R.; Lenaerts, K.; den Dulk, M.; Hadfoune, M.; van Heugten, C.; Spiller, O.B.; Beeton, M.L.; Stock, S.J.; Jobe, A.H.; et al. Prophylactic Intra-Uterine β-Cyclodextrin Administration during Intra-Uterine Ureaplasma parvum Infection Partly Prevents Liver Inflammation without Interfering with the Enterohepatic Circulation of the Fetal Sheep. Nutrients 2020, 12, 1312. https://doi.org/10.3390/nu12051312
Heymans C, Heij LR, Lenaerts K, den Dulk M, Hadfoune M, van Heugten C, Spiller OB, Beeton ML, Stock SJ, Jobe AH, et al. Prophylactic Intra-Uterine β-Cyclodextrin Administration during Intra-Uterine Ureaplasma parvum Infection Partly Prevents Liver Inflammation without Interfering with the Enterohepatic Circulation of the Fetal Sheep. Nutrients. 2020; 12(5):1312. https://doi.org/10.3390/nu12051312
Chicago/Turabian StyleHeymans, Cathelijne, Lara R. Heij, Kaatje Lenaerts, Marcel den Dulk, Mhamed Hadfoune, Chantal van Heugten, Owen B. Spiller, Michael L. Beeton, Sarah J. Stock, Alan H. Jobe, and et al. 2020. "Prophylactic Intra-Uterine β-Cyclodextrin Administration during Intra-Uterine Ureaplasma parvum Infection Partly Prevents Liver Inflammation without Interfering with the Enterohepatic Circulation of the Fetal Sheep" Nutrients 12, no. 5: 1312. https://doi.org/10.3390/nu12051312





