The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Characteristics
2.2. Study Design
- -
- Symptoms of restless legs syndrome (RLS);
- -
- Symptoms of the chronic fatigue syndrome (CFS);
- -
- Sleeping behaviour;
- -
- Subjective physical, psychological health and quality of life (QOL);
- -
- Trophic changes in fingernails or hair;
- -
- Symptoms of pica.
2.3. Statistical Analysis
3. Results
3.1. Clinical Outcomes
3.1.1. Restless Legs Syndrome
3.1.2. Fatigue
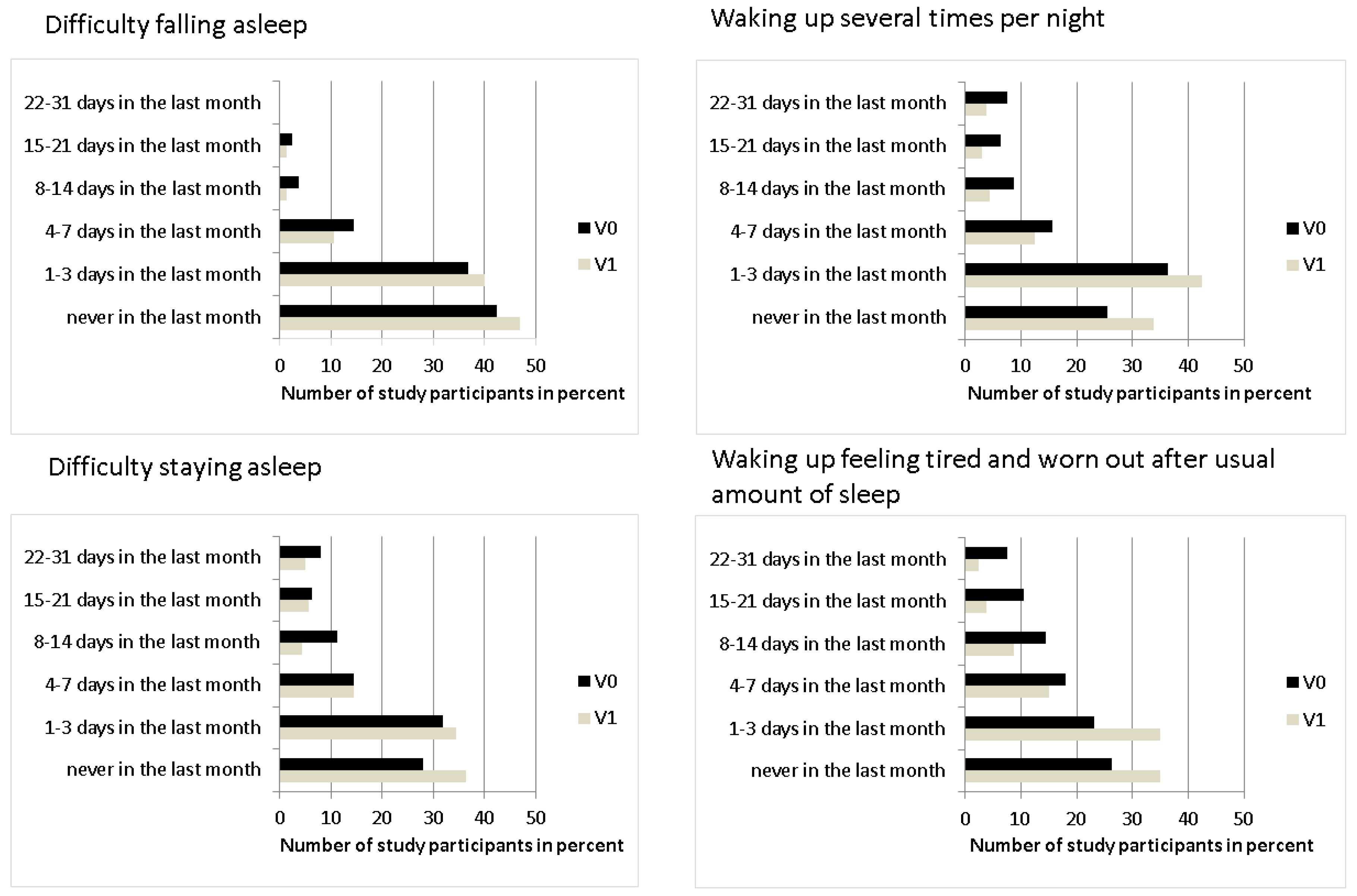
3.1.3. Quality of Sleep
3.1.4. Different Symptoms
3.1.5. Further Results–Type of Iron Treatment and Ferritin Levels at Visit 1
4. Discussion
4.1. Restless Legs Syndrome (RLS)
4.2. Fatigue
4.3. Quality of Sleep
4.4. Other Symptoms
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diaz, J.R.; de las Cagigas, A.; Rodriguez, R. Micronutrient deficiencies in developing and affluent countries. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 1), S70–S72. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Earley, C.J.; Heckler, D.; Allen, R.P. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004, 5, 231–235. [Google Scholar] [CrossRef]
- Kryger, M.H.; Otake, K.; Foerster, J. Low body stores of iron and restless legs syndrome: A correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002, 3, 127–132. [Google Scholar] [CrossRef]
- Sun, E.R.; Chen, C.A.; Ho, G.; Earley, C.J.; Allen, R.P. Iron and the restless legs syndrome. Sleep 1998, 21, 371–377. [Google Scholar] [CrossRef]
- Koo, B.B.; Bagai, K.; Walters, A.S. Restless legs syndrome: Current concepts about disease pathophysiology. Tremor Other Hyperkinetic Mov. 2016, 6, 401. [Google Scholar]
- Afari, N.; Buchwald, D. Chronic fatigue syndrome: A review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef]
- Verdon, F.; Burnand, B.; Stubi, C.L.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; De Vevey, M.; Studer, J.P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: A systematic review of randomised controlled trials. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef]
- Krayenbuehl, P.A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E. Iron deficiency and cognitive function. Annu. Rev. Nutr. 1993, 13, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Jacobson, J.L.; Burden, M.J.; Armony-Sivan, R.; Dodge, N.C.; Angelilli, M.L.; Lozoff, B.; Jacobson, S.W. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010, 126, e427–e434. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Preziosi, P.; Galan, P. Iron deficiency in Europe. Public Health Nutr. 2001, 4, 537–545. [Google Scholar] [CrossRef]
- Ghaffari, S.; Pourafkari, L. Koilonychia in iron-deficiency anemia. New Engl. J. Med. 2018, 379, e13. [Google Scholar] [CrossRef]
- Rushton, D.H. Nutritional factors and hair loss. Clin. Exp. Dermatol. 2002, 27, 396–404. [Google Scholar] [CrossRef]
- Germain, M.; Delage, G.; Robillard, P.; Katz, L.M.; Gregoire, Y. The association between frequency of blood donation and the occurrence of low birthweight, preterm delivery, and stillbirth: A retrospective cohort study. Transfusion 2016, 56, 2760–2767. [Google Scholar] [CrossRef]
- Conrad, M.E.; Crosby, W.H.; Jacobs, A.; Kaltwasser, J.P.; Nusbacher, J. The Hippocratian principle of ‘primum nil nocere’ demands that the metabolic state of a donor should be normalized prior to a subsequent donation of blood or plasma. How much blood, relative to his body weight, can a donor give over a certain period, without a continuous deviation of iron metabolism in the direction of iron deficiency? Vox Sang. 1981, 41, 336–343. [Google Scholar]
- Page, E.A.; Coppock, J.E.; Harrison, J.F. Study of iron stores in regular plateletpheresis donors. Transfus. Med. 2010, 20, 22–29. [Google Scholar] [CrossRef]
- Spencer, B.R.; Kleinman, S.; Wright, D.J.; Glynn, S.A.; Rye, D.B.; Kiss, J.E.; Mast, A.E.; Cable, R.G.; REDS-II RISE Analysis Group. Restless legs syndrome, pica, and iron status in blood donors. Transfusion 2013, 53, 1645–1652. [Google Scholar] [CrossRef]
- Bryant, B.J.; Yau, Y.Y.; Arceo, S.M.; Hopkins, J.A.; Leitman, S.F. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion 2013, 53, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Semmelrock, M.J.; Raggam, R.B.; Amrein, K.; Avian, A.; Schallmoser, K.; Lanzer, G.; Semmelrock, H.J.; Prueller, F.; Berghold, A.; Rohde, E. Reticulocyte hemoglobin content allows early and reliable detection of functional iron deficiency in blood donors. Clin. Chim. Acta 2012, 413, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.L. Iron, iron everywhere but not enough to donate. Transfusion 2002, 42, 664. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.L.; Garry, P.J.; Hooper, E.M. Iron stores in blood donors. JAMA 1981, 245, 2038–2043. [Google Scholar] [CrossRef]
- Brittenham, G.M. Iron deficiency in whole blood donors. Transfusion 2011, 51, 458–461. [Google Scholar] [CrossRef]
- Cable, R.G.; Glynn, S.A.; Kiss, J.E.; Mast, A.E.; Steele, W.R.; Murphy, E.L.; Wright, D.J.; Sacher, R.A.; Gottschall, J.L.; Vij, V.; et al. Iron deficiency in blood donors: Analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2011, 51, 511–522. [Google Scholar] [CrossRef]
- Finch, C.A.; Cook, J.D.; Labbe, R.F.; Culala, M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood 1977, 50, 441–447. [Google Scholar] [CrossRef]
- Baart, A.M.; van Noord, P.A.; Vergouwe, Y.; Moons, K.G.; Swinkels, D.W.; Wiegerinck, E.T.; de Kort, W.L.; Atsma, F. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion 2012, 53, 1670–1677. [Google Scholar] [CrossRef]
- Pedrazzini, B.; Waldvogel, S.; Vaucher, P.; Cornuz, J.; Heinzer, R.; Tissot, J.D.; Favrat, B. Prevalence of restless legs syndrome in female blood donors 1 week after blood donation. Vox Sang. 2014, 107, 44–49. [Google Scholar] [CrossRef]
- Birgegard, G.; Schneider, K.; Ulfberg, J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: Intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010, 99, 354–361. [Google Scholar] [CrossRef]
- Silber, M.H.; Richardson, J.W. Multiple blood donations associated with iron deficiency in patients with restless legs syndrome. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2003; Volume 78, pp. 52–54. [Google Scholar]
- Drexler, C.; Macher, S.; Lindenau, I.; Holter, M.; Moritz, M.; Stojakovic, T.; Pieber, T.R.; Schlenke, P.; Amrein, K. High-dose intravenous versus oral iron in blood donors with iron deficiency: The IronWoMan randomized, controlled clinical trial. Clin. Nutr. 2020, 39, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Iron Deficiency is a Common Disorder in General Population and Independently Predicts All-Cause Mortality: Results from the Gutenberg Health Study. Available online: https://link.springer.com/article/10.1007/s00392-020-01631-y (accessed on 25 March 2020).
- Macher, S.; Drexler, C.; Lindenau, I.; Sareban, N.; Schlenke, P.; Amrein, K. High-dose intravenously administered iron versus orally administered iron in blood donors with iron deficiency: Study protocol for a randomised, controlled trial. Trials 2016, 17, 527. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.; Hening, W.A.; Trenkwalder, C.; Walters, A.S.; Montplaisi, J. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003, 4, 101–119. [Google Scholar] [CrossRef]
- Stein, K.D.; Jacobsen, P.B.; Blanchard, C.M.; Thors, C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manag. 2004, 27, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Vahtera, J.; Pentti, J.; Helenius, H.; Kivimaki, M. Sleep disturbances as a predictor of long-term increase in sickness absence among employees after family death or illness. Sleep 2006, 29, 673–682. [Google Scholar] [CrossRef]
- Jenkins, C.D.; Stanton, B.A.; Niemcryk, S.J.; Rose, R.M. A scale for the estimation of sleep problems in clinical research. J. Clin. Epidemiol. 1988, 41, 313–321. [Google Scholar] [CrossRef]
- Chalmers, R.P. mirt: A multidimensional item response theory package for the R environment. J. Stat. Softw. 2012, 48, 1–29. [Google Scholar] [CrossRef]
- Pratt, J.J.; Khan, K.S. Non-anaemic iron deficiency—A disease looking for recognition of diagnosis: A systematic review. Eur. J. Haematol. 2016, 96, 618–628. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017, 31, 225–233. [Google Scholar] [CrossRef]
- Allen, R.P.; Stillman, P.; Myers, A.J. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: Prevalence and characteristics. Sleep Med. 2010, 11, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Godau, J.; Klose, U.; Di Santo, A.; Schweitzer, K.; Berg, D. Multiregional brain iron deficiency in restless legs syndrome. Mov. Disord. 2008, 23, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Ponnuru, P.; Wang, X.S.; Patton, S.M.; Allen, R.P.; Earley, C.J. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011, 134 Pt 4, 959–968. [Google Scholar] [CrossRef]
- Wang, J.; O’Reilly, B.; Venkataraman, R.; Mysliwiec, V.; Mysliwiec, A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009, 10, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Silber, M.H.; Becker, P.M.; Earley, C.; Garcia-Borreguero, D.; Ondo, W.G.; Medical Advisory Board of the Willis; Ekbom Disease Foundation. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2013; Volume 88, pp. 977–986. [Google Scholar]
- Winkelman, J.W.; Armstrong, M.J.; Allen, R.P.; Chaudhuri, K.R.; Ondo, W.; Trenkwalder, C.; Zee, P.C.; Gronseth, G.S.; Gloss, D.; Zesiewicz, T. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2016, 87, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Auerbach, M.; Cho, Y.W.; Connor, J.R.; Earley, C.J.; Garcia-Borreguero, D.; Kotagal, S.; Manconi, M.; Ondo, W.; et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med. 2018, 41, 27–44. [Google Scholar] [CrossRef]
- Allen, R.P.; Walters, A.S.; Montplaisir, J.; Hening, W.; Myers, A.; Bell, T.J.; Ferini-Strambi, L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005, 165, 1286–1292. [Google Scholar] [CrossRef]
- Hogl, B.; Kiechl, S.; Willeit, J.; Saletu, M.; Frauscher, B.; Seppi, K.; Müller, J.; Rungger, G.; Gasperi, A.; Wenning, G.; et al. Restless legs syndrome: A community-based study of prevalence, severity, and risk factors. Neurology 2005, 64, 1920–1924. [Google Scholar] [CrossRef]
- Barriere, G.; Cazalets, J.R.; Bioulac, B.; Tison, F.; Ghorayeb, I. The restless legs syndrome. Prog. Neurobiol. 2005, 77, 139–165. [Google Scholar] [CrossRef]
- Ulfberg, J.; Nystrom, B. Restless legs syndrome in blood donors. Sleep Med. 2004, 5, 115–118. [Google Scholar] [CrossRef]
- Burchell, B.J.; Allen, R.P.; Miller, J.K.; Hening, W.A.; Earley, C.J. RLS and blood donation. Sleep Med. 2009, 10, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, S.; Pedrazzini, B.; Vaucher, P.; Bize, R.; Cornuz, J.; Tissot, J.D.; Favrat, B. Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: A randomized controlled trial. BMC Med. 2012, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Fontana, S.; Jüni, P.; Niederhauser, C.; Keller, P. Lack of effectiveness of intravenous iron infusion in healthy blood donors with low ferritin: A double-blind randomized controlled trial: P-134. Vox Sang. 2014, 107, 98. [Google Scholar]
- Yokoi, K.; Konomi, A. Iron deficiency without anaemia is a potential cause of fatigue: Meta-analyses of randomised controlled trials and cross-sectional studies. Br. J. Nutr. 2017, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Hlavata, K.; Sedivy, P.; Dezortova, M.; Borlaug, B.A.; Petrak, J.; Kautzner, J.; Hajek, M. Skeletal muscle abnormalities and iron deficiency in chronic heart failurean exercise (31)P magnetic resonance spectroscopy study of calf muscle. Circ. Heart Fail. 2018, 11, e004800. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Allen, R.P.; Earley, C.J. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep Med. 2016, 25, 16–23. [Google Scholar] [CrossRef]
- Rushton, D.H. Management of hair loss in women. Dermatol. Clin. 1993, 11, 47–53. [Google Scholar] [CrossRef]
- Kantor, J.; Kessler, L.J.; Brooks, D.G.; Cotsarelis, G. Decreased serum ferritin is associated with alopecia in women. J. Investig. Dermatol. 2003, 121, 985–988. [Google Scholar] [CrossRef]
- Simpson, E.; Mull, J.D.; Longley, E.; East, J. Pica during pregnancy in low-income women born in Mexico. West. J. Med. 2000, 173, 20–24; discussion 5. [Google Scholar] [CrossRef]
- Rector, W.G., Jr. Pica: Its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J. Gen. Intern. Med. 1989, 4, 512–513. [Google Scholar] [CrossRef]
- Kiss, J.E.; Brambilla, D.; Glynn, S.A.; Mast, A.E.; Spencer, B.R.; Stone, M.; Kleinman, S.H.; Cable, R.G. Oral iron supplementation after blood donation: A randomized clinical trial. JAMA 2015, 313, 575–583. [Google Scholar] [CrossRef] [PubMed]





| Type of Donor | Women | Men | Total |
|---|---|---|---|
| Whole blood | 126 | 31 | 157 |
| Platelet (apheresis) | 12 | 7 | 19 |
| Study Group | |||
| IV | 69 | 17 | 86 |
| PO | 69 | 21 | 90 |
| Sum | 138 | 38 | 176 |
| Donor Characteristics | |||
| Age, mean (SD), years | 36 (13) | 46 (13) | |
| Weight, mean (SD), kg | 69 (11) | 82 (16) | |
| Body mass index, mean (SD) | 24 (4) | 26 (4) | |
| Total calculated blood volume, mean (SD), liters | 4.0 (0.3) | 5.3 (0.6) |
| N | V0 | V1 | P | ||
|---|---|---|---|---|---|
| Restless Legs Syndrome | Diagnosis | 134 | 31 (23.1%) | 23 (17.2%) | - |
| Severity | 4 (3–6) | 2 (1–5) | <0.001 | ||
| Fatigue | General | 169 | 6 (3–10) | 3 (0–6) | <0.001 |
| Physical | 2 (0–4) | 1 (0–2) | <0.001 | ||
| Emotional | 4 (1–7) | 3 (1–5) | <0.001 | ||
| Mental | 3 (1–6) | 3 (1–5) | <0.001 | ||
| Vigor | 15 (13–18) | 17 (14–19) | <0.001 | ||
| Overall | 0 (−10–13) | −7 (−14–3) | <0.001 | ||
| Quality of Sleep | Difficulty falling asleep | 160 | 5 (5–6) | 5 (5–6) | 0.004 |
| Waking up several times | 5 (4–5) | 5 (5–6) | <0.001 | ||
| Difficulty staying asleep | 5 (3–6) | 5 (4–6) | 0.002 | ||
| Waking up tired | 4 (3–6) | 5 (4–6) | <0.001 | ||
| Different Symptoms | Brittle Nails | 162 | 63 (38.9%) | 60 (37%) | 0.678 |
| Hair loss | 40 (24.7 %) | 32 (19.8%) | 0.096 | ||
| Headache | 53 (32.5%) | 38 (23.3%) | 0.02 | ||
| Dyspnea | 58 (36.0%) | 40 (24.8%) | 0.005 | ||
| Dizziness | 57 (35.4%) | 34 (21.1%) | <0.001 | ||
| Painful or plain tongue | 4 (2.4%) | 2 (1.2%) | 0.687 | ||
| Pica | 11 (6.8%) | 5 (3.1%) | 0.109 | ||
| Quality of Life | 171 | 4 (4–5) | 4 (4–5) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macher, S.; Herster, C.; Holter, M.; Moritz, M.; Matzhold, E.M.; Stojakovic, T.; Pieber, T.R.; Schlenke, P.; Drexler, C.; Amrein, K. The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT. Nutrients 2020, 12, 1313. https://doi.org/10.3390/nu12051313
Macher S, Herster C, Holter M, Moritz M, Matzhold EM, Stojakovic T, Pieber TR, Schlenke P, Drexler C, Amrein K. The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT. Nutrients. 2020; 12(5):1313. https://doi.org/10.3390/nu12051313
Chicago/Turabian StyleMacher, Susanne, Cornelia Herster, Magdalena Holter, Martina Moritz, Eva Maria Matzhold, Tatjana Stojakovic, Thomas R. Pieber, Peter Schlenke, Camilla Drexler, and Karin Amrein. 2020. "The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT" Nutrients 12, no. 5: 1313. https://doi.org/10.3390/nu12051313
APA StyleMacher, S., Herster, C., Holter, M., Moritz, M., Matzhold, E. M., Stojakovic, T., Pieber, T. R., Schlenke, P., Drexler, C., & Amrein, K. (2020). The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT. Nutrients, 12(5), 1313. https://doi.org/10.3390/nu12051313







