The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily
Abstract
:1. Introduction
2. Materials and Methods
2.1. CyC Preparation
2.2. Animals
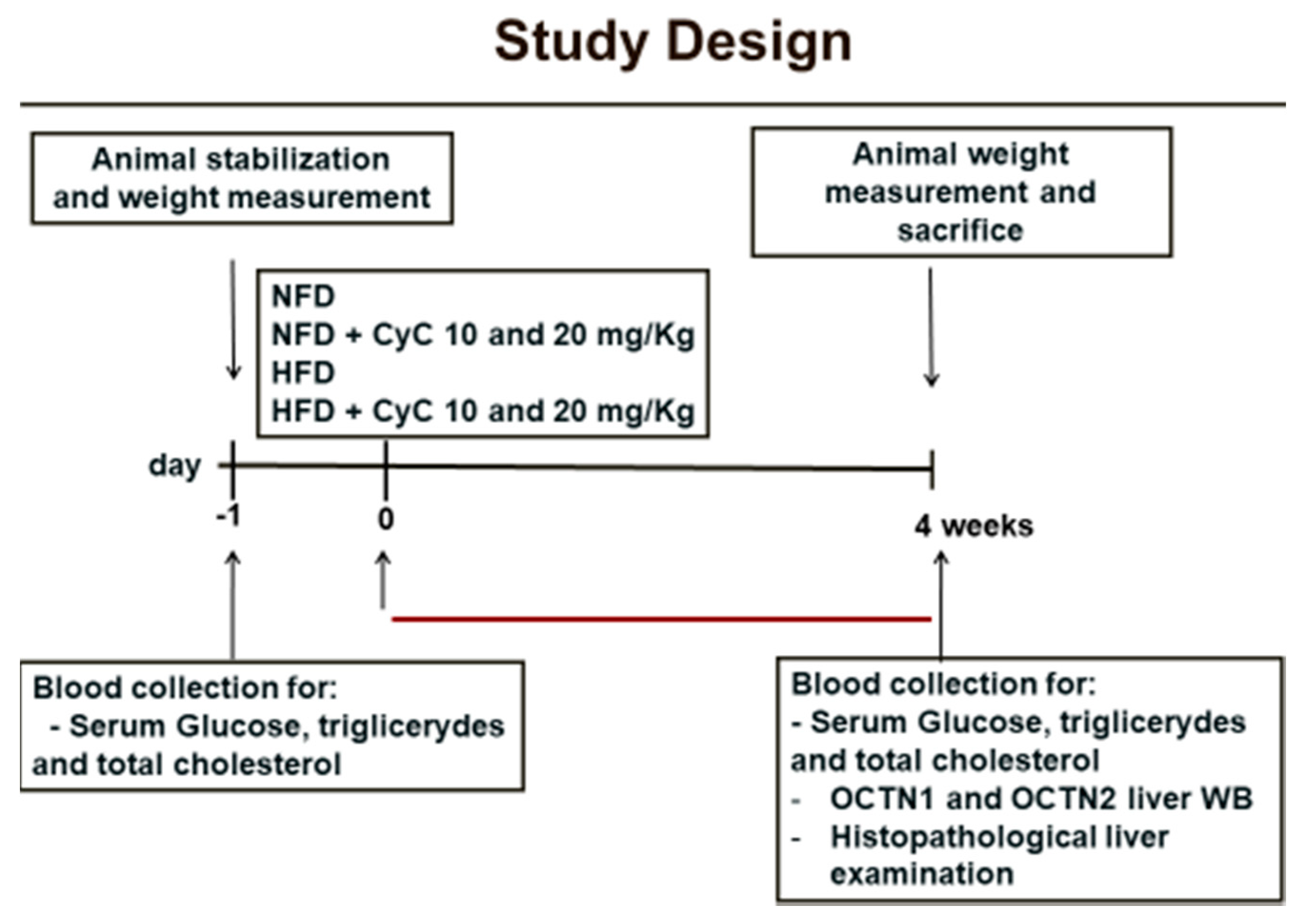
2.3. Study Design
2.4. Blood Biochemical Analysis
2.5. Morphological Analysis of Liver
2.6. Western Blotting Analysis
2.7. Statistical Analysis
3. Results
3.1. The Effect of CyC on Serum Glucose, Cholesterol, Triglyceride Levels and Body Weight in NFD and HFD Rats
3.2. CyC Supplementation Improves HFD-Related Liver Steatosis in Rats
3.3. CyC Supplementation Counteracts OCTN1 and OCTN2 Transporter Reduction in Liver Tissue of Rats Fed HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, M.A.; Fletcher, J.A.; Meers, G.M.; Thyfault, J.P.; Laughlin, M.H.; Rector, R.S. A return to ad libitum feeding following caloric restriction promotes hepatic steatosis in hyperphagic OLETF rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G387–G395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongiovanni, P.; Valenti, L. A Nutrigenomic Approach to Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2017, 18, 1534. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; Colombo, M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2016, 1, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.K.S.E.; Gomes, F.O.D.S.; Santos Silva, B.D.; Ribeiro, E.L.; Oliveira, A.C.; Araújo, S.M.D.R.; de Lima, I.T.; Oliveira, A.G.V.; Rudnicki, M.; Abdalla, D.S.P.; et al. Chronic LPSF/GQ-02 treatment attenuates inflammation and atherosclerosis development in LDLr-/- mice. Eur. J. Pharmacol. 2016, 791, 622–631. [Google Scholar] [CrossRef]
- Banini, B.A.; Sanyal, A.J. Nonalcoholic Fatty Liver Disease: Epidemiology, Pathogenesis, Natural History, Diagnosis, and Current Treatment Options. Clin. Med. Insights Ther. 2016, 8, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Lee, C.H.; Lee, K.Y.; Jung, S.H.; Lee, B.H. Increased hepatic Fatty Acid uptake and esterification contribute to tetracycline-induced steatosis in mice. Toxicol. Sci. 2015, 145, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar]
- Fontes, A.; Alemany-Pagès, M.; Oliveira, P.J.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Antioxidant Versus Pro-Apoptotic Effects of Mushroom-Enriched Diets on Mitochondria in Liver Disease. Int. J. Mol. Sci. 2019, 20, 3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimini, F.A.; Barchetta, I.; Carotti, S.; Bertoccini, L.; Baroni, M.G.; Vespasiani-Gentilucci, U.; Cavallo, M.G.; Morini, S. Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J. Gastroenterol. 2016, 22, 4079–4090. [Google Scholar] [CrossRef]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front. Endocrinol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, X.; Wang, Q.; Li, J.Z. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell 2018, 9, 164–177. [Google Scholar] [CrossRef]
- Aguirre, L.; Portillo, M.P.; Hijona, E.; Bujanda, L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J. Gastroenterol. 2014, 20, 7366–7380. [Google Scholar] [CrossRef]
- McNelis, J.C.; Olefsky, J.M. Macrophages, immunity, and metabolic disease. Immunity 2014, 41, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, M.; Schober, A. Macrophage MicroRNAs as Therapeutic Targets for Atherosclerosis, Metabolic Syndrome, and Cancer. Int. J. Mol. Sci. 2018, 19, 1756. [Google Scholar] [CrossRef] [Green Version]
- Scalise, M.; Galluccio, M.; Pochini, L.; Console, L.; Barile, M.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Studying Interactions of Drugs with Cell Membrane Nutrient Transporters: New Frontiers of Proteoliposome Nanotechnology. Curr. Pharm. Des. 2017, 23, 3871–3883. [Google Scholar] [CrossRef]
- Indiveri, C.; Pochini, L.; Oppedisano, F.; Tonazzi, A. The carnitine transporter network: Interactions with drugs. Curr. Chem. Biol. 2010, 4, 108–123. [Google Scholar]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. OCTN cation transporters in health and disease: Role as drug targets and assay development. J. Biomol. Screen. 2013, 18, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Indiveri, C.; Galluccio, M.; Scalise, M.; Pochini, L. Strategies of bacterial over expression of membrane transporters relevant in human health: The successful case of the three members of OCTN subfamily. Mol. Biotechnol. 2013, 54, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Nakamichi, N.; Hosotani, H.; Masuo, Y.; Sugiura, T.; Kato, Y. Organic cation transporter-mediated ergothioneine uptake in mouse neural progenitor cells suppresses proliferation and promotes differentiation into neurons. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Rondanelli, F.; Monteferrario, S.; Perna, M.A.; Faliva, A.; Opizzi, A. Health-promoting properties of artichoke in preventing cardiovascular disease by its lipidic and glycemicreducing action. Monaldi Arch. Chest Dis. 2013, 80, 17–26. [Google Scholar]
- Rondanelli, M.; Giacosa, A.; Opizzi, A. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia:a double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef]
- Mollace, V.; Bombardelli, E. Phytochemical characterization of active ingredients found in Cynara cardunculus wild type from Calabrian Region. J. Trad. Comp. Med. 2020, in press. [Google Scholar]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol. Vitro 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Qiang, Z.; Lee, S.; Ye, Z.; Wu, X.; Hendrich, S. Artichoke Extract Lowered Plasma Cholesterol and Increased Fecal Bile Acids in Golden Syrian Hamster. Phytother. Res. 2012, 26, 1048–1052. [Google Scholar]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 25–30. [Google Scholar] [CrossRef]
- Musolino, V.; Mollace, V. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Trad. Compl. Med 2020, in press. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Naveau, S.; Thabut, D.; Charlotte, F.; Messous, D.; Capron, D.; Abella, A.; Massard, J.; Ngo, Y.; et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp. Hepatol. 2005, 4, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Preziosi, P.; Loscalzo, B. Pharmacological properties of 1,4-dicaffeylquinicacid, the active principle of Cynara scolymus. Arch. Int. Pharmacodyn 1958, 117, 63–80. [Google Scholar]
- Preziosi, P.; Loscalzo, B.; Marmo, E.; Miele, E. Effects of single or repeated treatment with several anti-cholesterolemiccompounds on biliary excretion of cholesterol. Biochem. Pharmacol. 1960, 5, 251–262. [Google Scholar] [CrossRef]
- Englisch, W.; Beckers, C.; Unkauf, M.; Ruepp, M.; Zinserling, V. Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine 2008, 15, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Roscini, A.R.; Trinca, F.; Gemelli, F. Artichoke juice improves endothelial function in hyperlipidemia. Life Sci. 2004, 76, 775–782. [Google Scholar] [CrossRef]
- Pittler, M.H.; Thompson, C.O.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2002, 3, 33–37. [Google Scholar]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.J.; Hemmerle, H. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef]
- Matsui, T.; Ogunwande, I.A.; Abesundara, K.J.; Matsumoto, K. Anti-hyperglycemic potential of natural products. Mini Rev. Med. Chem. 2006, 2006. 6, 349–356. [Google Scholar] [CrossRef]
- Maros, T.; Racz, G.; Katonai, B.; Kovacs, V.V. Effects of Cynara scolymus extracts on the regeneration of rat liver. Arzneimittelforschung 1966, 16, 127–129. [Google Scholar]
- Adzet, T.; Camarasa, J.; Hernandez, J.S.; Laguna, J.C. Action of an artichoke extract against CCl4-induced hepatotoxicity in rats. Acta Pharm. Jugosl. 1987, 37, 183–187. [Google Scholar]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef]
- Matuschowski, P. Testing of Cynara scolymus in the isolated perfused rat liver. 43rd Ann. Congr. Soc. Med. Plant Res. 1996, 6, 3–7. [Google Scholar]
- Saénz, R.T.; García, G.D.; De la Puerta, V.R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002, 9, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, R.; Beckers, C.; Kirchhoff, G.M.; Trinczek- Gartner, H.; Petrowicz, O.; Reimann, H.J. Increase in choleresis by means of artichoke extract. Phytomedicine 1994, 1, 107–115. [Google Scholar] [CrossRef]
- Fintelmann, V. Therapeutic profile and mechanism of action of artichoke leaf extract: Hypolipemic, antioxidant, hepatoprotective and choleretic properties. Phytomedicine 1996, 36, 50–56. [Google Scholar]
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of CyC, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407. [Google Scholar] [CrossRef]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Kim, S.Y.; Choi, M.S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients 2018, 10, 979. [Google Scholar] [CrossRef] [Green Version]
- Shao, T.; Yu, Q.; Zhu, T.; Liu, A.; Gao, X.; Long, X.; Liu, Z. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 2020, 123, 308–318. [Google Scholar] [CrossRef] [Green Version]



| Parametres | NFD (n = 6) | HFD (n = 6) | NFD + CyC (10 mg/Kg) (n = 6) | NFD + CyC (20 mg/Kg) (n = 6) | HFD + CyC (10 mg/Kg) (n = 6) | HFD + CyC (20 mg/Kg) (n = 6) | |
|---|---|---|---|---|---|---|---|
| Serum Glucose | |||||||
| Basal | 62 ± 2 | 65 ± 3 | 68 ± 3 | 64 ± 4 | 66 ± 5 | 66 ± 5 | |
| 4 weeks | 2 ± 1 | 22 ± 4 * | 3 ± 1 | 2 ± 0,5 | 15 ± 5 § | 4 ± 2 § | |
| Total Cholesterol | |||||||
| Basal | 135 ± 5 | 138 ± 4 | 138 ± 5 | 141 ± 6 | 136 ± 6 | 138 ± 5 | |
| 4 weeks | 4 ± 1 | 38 ± 3 * | 2 ± 1 | 2 ± 0.5 | 18 ± 3 § | 6 ± 2 § | |
| Triglycerides | |||||||
| Basal | 145 ± 5 | 143 ± 4 | 146 ± 4 | 144 ± 4 | 144 ± 5 | 147 ± 4 | |
| 4 weeks | 4 ± 1 | 48 ± 3 * | 4 ± 1 | 2 ± 0,5 | 27 ± 4 § | 8 ± 2 § | |
| SteatoTest | |||||||
| Basal | 0.40 ± 0.05 | 0.42 ± 0.04 | 0.41 ± 0.04 | 0.43 ± 0.03 | 0.41 ± 0.05 | 0.40 ± 0.04 | |
| 4 weeks | 0.12 ± 0.02 | 0.42 ± 0.03 * | 0.14 ± 0.021 | 0.16 ± 0.03 | 0.22 ± 0.04 § | 0.36 ± 0.02 § | |
| MDA | |||||||
| Basal | 1.1 ± 0.05 | 1.2 ± 0.04 | 1 ± 0.04 | 1.2 ± 0.03 | 1.2 ± 0.05 | 1 ± 0.05 | |
| 4 weeks | 0.1 ± 0.05 | 0.7 ± 0.03 * | 0.2 ± 0.05 | 0.1 ± 0.05 | 0.4 ± 0.04 § | 0.6 ± 0.03 § |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. https://doi.org/10.3390/nu12051435
Oppedisano F, Muscoli C, Musolino V, Carresi C, Macrì R, Giancotta C, Bosco F, Maiuolo J, Scarano F, Paone S, et al. The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients. 2020; 12(5):1435. https://doi.org/10.3390/nu12051435
Chicago/Turabian StyleOppedisano, Francesca, Carolina Muscoli, Vincenzo Musolino, Cristina Carresi, Roberta Macrì, Caterina Giancotta, Francesca Bosco, Jessica Maiuolo, Federica Scarano, Sara Paone, and et al. 2020. "The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily" Nutrients 12, no. 5: 1435. https://doi.org/10.3390/nu12051435







