Fecal Microbiota and Hair Glucocorticoid Concentration Show Associations with Growth during Early Life in a Pig Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Diet
2.2. Sample Collection
2.3. Cortisol and Cortisone Hair Concentration Analysis
2.4. Short-Chain Fatty Acid Analysis
2.5. 16S rRNA Gene Sequencing
2.6. Statistical Analysis
3. Results
3.1. Cortisol and Cortisone Concentrations in Hair
3.2. Microbiota Structure and Biodiversity
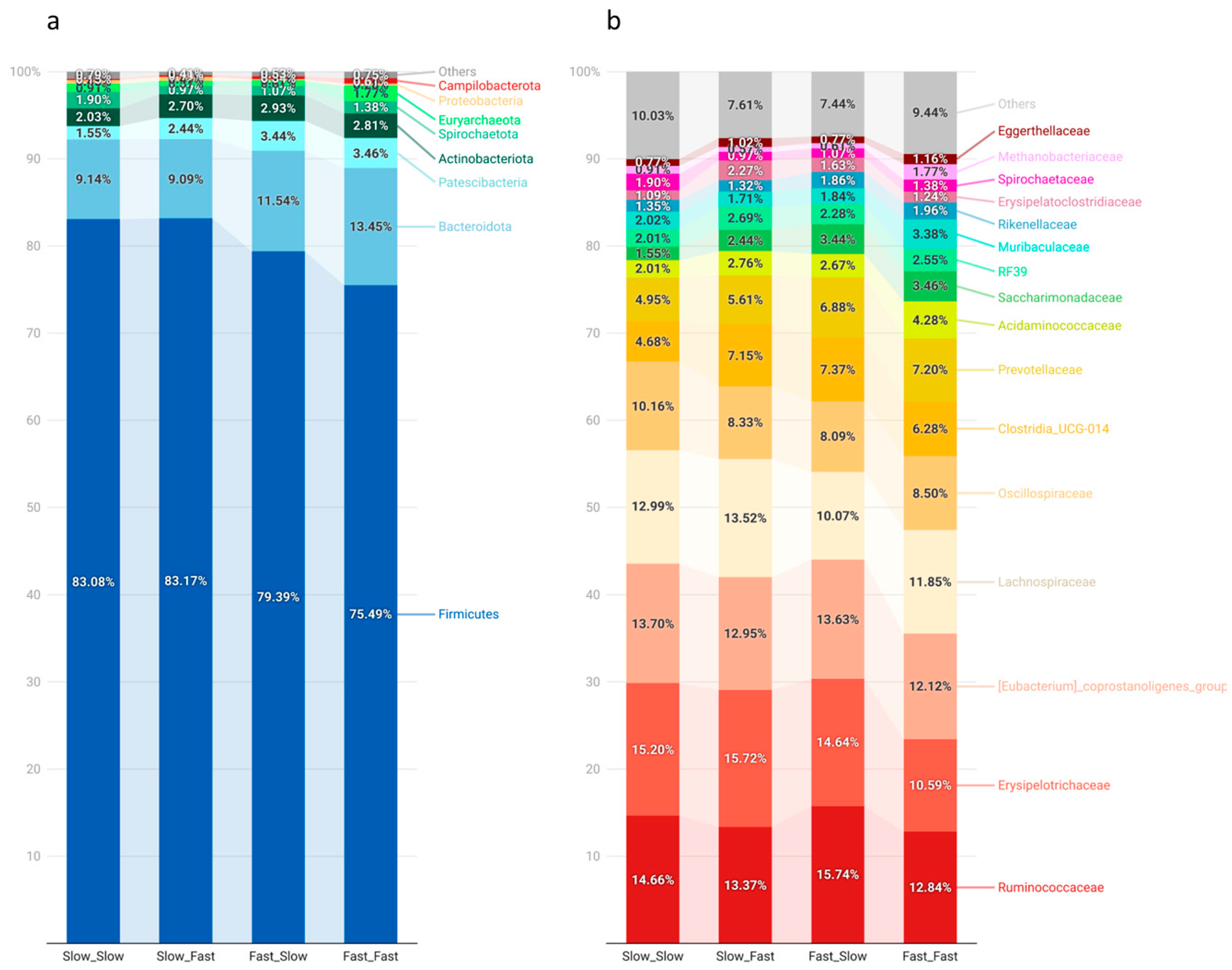
3.3. Fecal Microbiota Composition
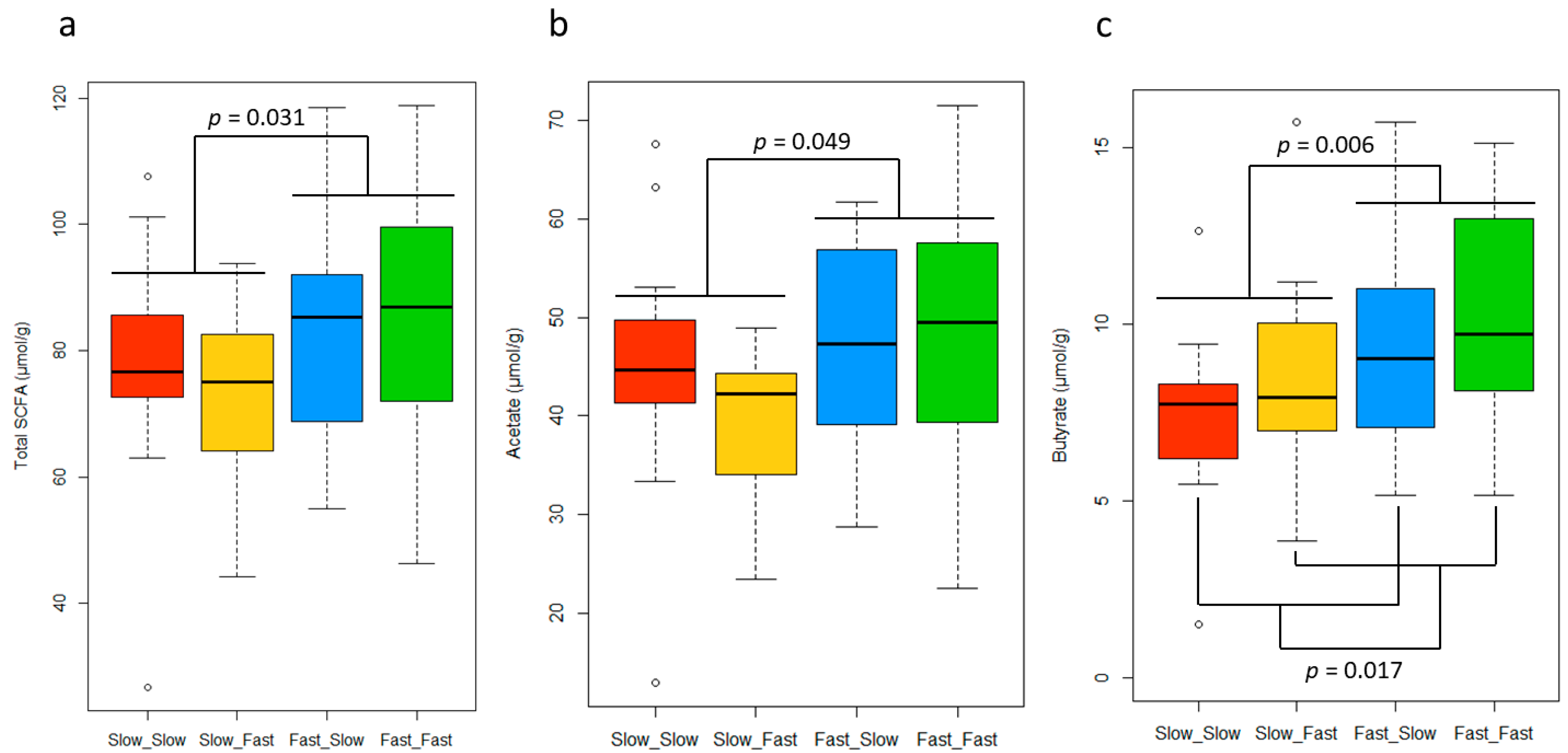
3.4. Short-Chain Fatty Acid Concentrations in Feces
4. Discussion
4.1. Cortisol and Cortisone in Hair
4.2. Fecal Microbiota Composition
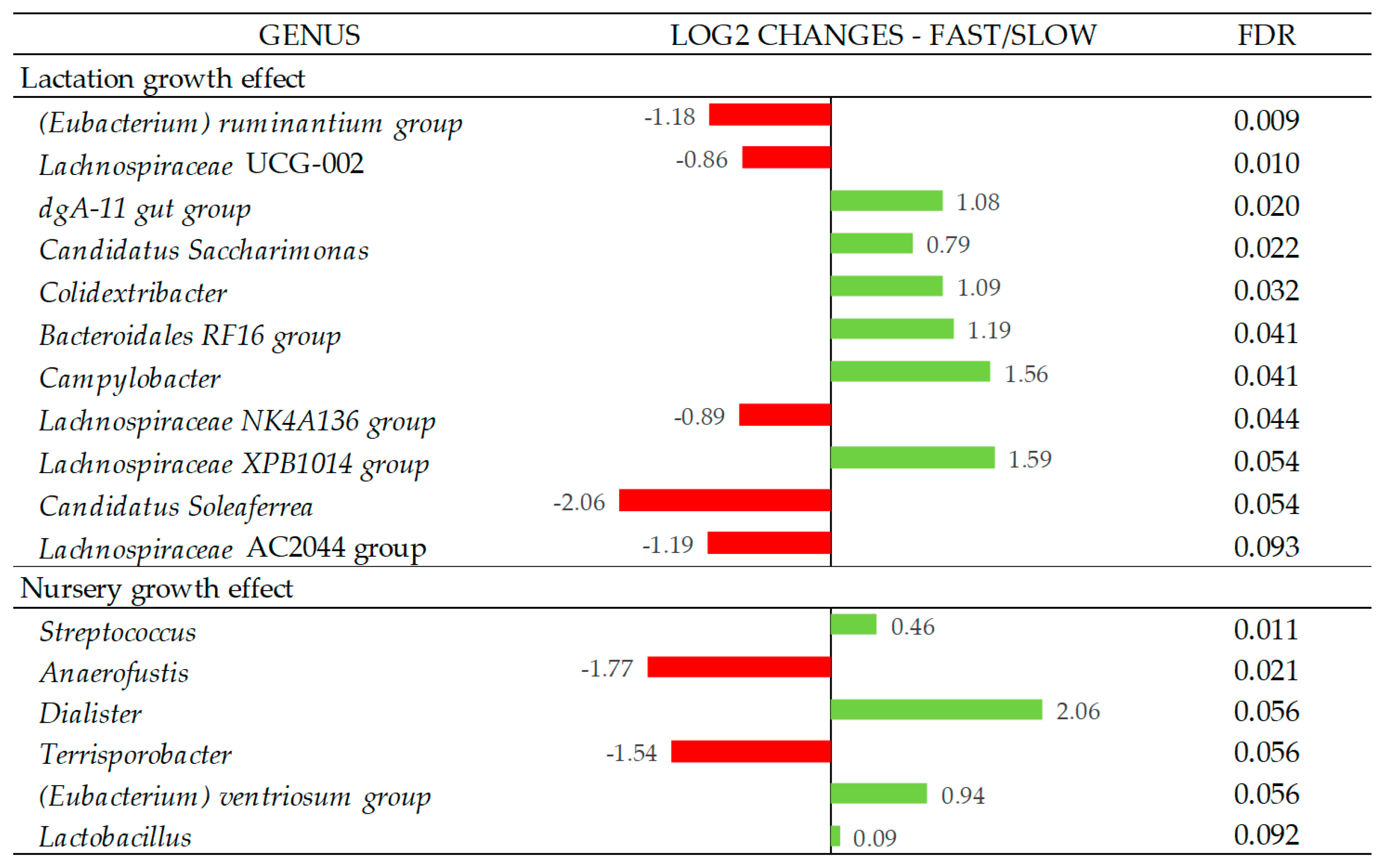
4.2.1. Lactation Growth Effect on Fecal Microbiota and SCFA Concentrations
4.2.2. Nursery Growth Effect on Fecal Microbiota and SCFA Concentrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francino, M.P. Early Development of the Gut Microbiota and Immune Health. Pathogens 2014, 4, 769–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogelman, N.; Canli, T. Early life stress, physiology, and genetics: A review. Front. Psychol. 2019, 10, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef]
- Abrantes, M.A.; Valencia, A.M.; Bany-Mohammed, F.; Aranda, J.V.; Beharry, K.D. Combined antenatal and postnatal steroid effects on fetal and postnatal growth, and neurological outcomes in neonatal rats. Am. J. Transl. Res. 2019, 11, 1697. [Google Scholar]
- Mukherjee, D.; Bhopal, S.; Bhavnani, S.; Sharma, K.K.; Roy, R.; Divan, G.; Mandal, S.; Soremekun, S.; Kirkwood, B.; Patel, V. The effect of cumulative early life adversities, and their differential mediation through hair cortisol levels, on childhood growth and cognition: Three-year follow-up of a birth cohort in rural India. Wellcome Open Res. 2022, 7, 74. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [Green Version]
- López-Vergé, S.; Gasa, J.; Farré, M.; Coma, J.; Bonet, J.; Solà-Oriol, D. Potential risk factors related to pig body weight variability from birth to slaughter in commercial conditions. Transl. Anim. Sci. 2018, 2, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Milligan, B.N.; Fraser, D.; Kramer, D.L. Within-litter birth weight variation in the domestic pig and its relation to pre-weaning survival, weight gain, and variation in weaning weights. Livest. Prod. Sci. 2002, 76, 181–191. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Huting, A.M.S.; Middelkoop, A.; Guan, X.; Molist, F. Using Nutritional Strategies to Shape the Gastro-Intestinal Tracts of Suckling and Weaned Piglets. Animals 2021, 11, 402. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Niekamp, S.R.; Rodriguez-Zas, S.L.; Salak-Johnson, J.L. Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J. Anim. Sci. 2006, 84, 588–596. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- López-Arjona, M.; Tecles, F.; Mateo, S.V.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Cerón, J.J.; Martínez-Subiela, S. Measurement of cortisol, cortisone and 11β-hydroxysteroid dehydrogenase type 2 activity in hair of sows during different phases of the reproductive cycle. Vet. J. 2020, 259, 105458. [Google Scholar] [CrossRef]
- Richardson, A.J.; Calder, A.G.; Stewart, C.S.; Smith, A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 1989, 9, 5–8. [Google Scholar] [CrossRef]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. 1995, 61, 293–304. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Solé, F.; Solà-Oriol, D.; Ramayo-Caldas, Y.; Rodriguez-Prado, M.; González Ortiz, G.; Bedford, M.R.; Pérez, J.F. Supplementation of xylo-oligosaccharides to suckling piglets promotes the growth of fiber-degrading gut bacterial populations during the lactation and nursery periods. Sci. Rep. 2022, 12, 11594. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, version 2.6-4; GitHub, Inc.: San Francisco, CA, USA, 2020. [Google Scholar]
- Paulson, J.N. metagenomeSeq: Statistical Analysis for Sparse High-Throughput Sequencing. Bioconductor Package. Available online: http://www.cbcb.umd.edu/software/metagenomeSeq (accessed on 2 November 2022).
- Paulson, J.N.; Colin Stine, O.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Montoro, J.C.; Manzanilla, E.G.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Clear, O.; Díaz, J.A.C. Predicting Productive Performance in Grow-Finisher Pigs Using Birth and Weaning Body Weight. Animals 2020, 10, 1017. [Google Scholar] [CrossRef]
- Gondret, F.; Lefaucheur, L.; Louveau, I.; Lebret, B.; Pichodo, X.; le Cozler, Y. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod. Sci. 2005, 93, 137–146. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Michiels, J.; de Vos, M.; Missotten, J.; Ovyn, A.; de Smet, S.; van Ginneken, C. Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br. J. Nutr. 2013, 109, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Huting, A.M.S.; Sakkas, P.; Wellock, I.; Almond, K.; Kyriazakis, I. Once small always small? To what extent morphometric characteristics and postweaning starter regime affect pig lifetime growth performance. Porcine Health Manag. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.L.; Nannoni, E.; Govoni, N.; Scorrano, F.; Zannoni, A.; Forni, M.; Martelli, G.; Sardi, L. Hair cortisol determination in sows in two consecutive reproductive cycles. Reprod. Biol. 2014, 14, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Stubsjøen, S.M.; Bohlin, J.; Dahl, E.; Knappe-Poindecker, M.; Fjeldaas, T.; Lepschy, M.; Palme, R.; Langbein, J.; Ropstad, E. Assessment of chronic stress in sheep (part I): The use of cortisol and cortisone in hair as non-invasive biological markers. Small Rumin. Res. 2015, 132, 25–31. [Google Scholar] [CrossRef]
- Vanaelst, B.; Michels, N.; de Vriendt, T.; Huybrechts, I.; Vyncke, K.; Sioen, I.; Bammann, K.; Rivet, N.; Raul, J.S.; Molnar, D.; et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur. J. Pediatr. 2013, 172, 843–846. [Google Scholar] [CrossRef] [PubMed]
- La Marca-Ghaemmaghami, P.; la Marca, R.; Dainese, S.M.; Haller, M.; Zimmermann, R.; Ehlert, U. The association between perceived emotional support, maternal mood, salivary cortisol, salivary cortisone, and the ratio between the two compounds in response to acute stress in second trimester pregnant women. J. Psychosom. Res. 2013, 75, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Xu, Y.; Yang, J.; Chen, Z.; Deng, H. Characteristics of novel hair-based biomarker for the activity assessment of 11β-hydroxysteroid dehydrogenase. Clin. Chim. Acta 2013, 426, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mousikou, M.; Kyriakou, A.; Skordis, N. Stress and Growth in Children and Adolescents. Horm. Res. Paediatr. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Shimanoe, C.; Matsumoto, A.; Hara, M.; Akao, C.; Nishida, Y.; Horita, M.; Nanri, H.; Higaki, Y.; Tanaka, K. Perceived stress, depressive symptoms, and cortisol-to-cortisone ratio in spot urine in 6878 older adults. Psychoneuroendocrinology 2021, 125, 105125. [Google Scholar] [CrossRef]
- Atlaoui, D.; Duclos, M.; Gouarne, C.; Lacoste, L.; Barale, F.; Chatard, J.C. The 24-h urinary cortisol/cortisone ratio for monitoring training in elite swimmers. Med. Sci. Sports Exerc. 2004, 36, 218–224. [Google Scholar] [CrossRef]
- Hennebert, O.; Chalbot, S.; Alran, S.; Morfin, R. Dehydroepiandrosterone 7α-hydroxylation in human tissues: Possible interference with type 1 11β-hydroxysteroid dehydrogenase-mediated processes. J. Steroid Biochem. Mol. Biol. 2007, 104, 326–333. [Google Scholar] [CrossRef]
- Krozowski, Z.; Li, K.X.Z.; Koyama, K.; Smith, R.E.; Obeyesekere, V.R.; Stein-Oakley, A.; Sasano, H.; Coulter, C.; Cole, T.; Sheppard, K.E. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J. Steroid Biochem. Mol. Biol. 1999, 69, 391–401. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Melo-Durán, D.; Gonzalez-Solè, F.; D’Angelo, M.; Pérez-Cano, F.J.; Solà-Oriol, D. Body weight of newborn and suckling piglets affects their intestinal gene expression. J. Anim. Sci. 2022, 100, skac161. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Kang, D.K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.; et al. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef] [Green Version]
- Holman, D.B.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-Analysis to Define a Core Microbiota in the Swine Gut. mSystems 2017, 2, e00004-17. [Google Scholar] [CrossRef] [Green Version]
- Saladrigas-García, M.; D’Angelo, M.; Ko, H.L.; Nolis, P.; Ramayo-Caldas, Y.; Folch, J.M.; Llonch, P.; Solà-Oriol, D.; Pérez, J.F.; Martín-Orúe, S.M. Understanding host-microbiota interactions in the commercial piglet around weaning. Sci. Rep. 2021, 11, 23488. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Chae, J.P.; Pajarillo, E.A.B.; Kim, S.H.; Kwak, M.J.; Eun, J.S.; Chee, S.W.; Whang, K.Y.; Kim, S.H.; Kang, D.K. Association between the body weight of growing pigs and the functional capacity of their gut microbiota. Anim. Sci. J. 2020, 91, e13418. [Google Scholar] [CrossRef]
- Han, G.G.; Lee, J.Y.; Jin, G.D.; Park, J.; Choi, Y.H.; Chae, B.J.; Kim, E.B.; Choi, Y.J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Stephens, D.A.; Moodie, E.E.M.; Prendergast, A.J.; Stoltzfus, R.J.; Humphrey, J.H.; Manges, A.R. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panasevich, M.R.; Wankhade, U.D.; Chintapalli, S.V.; Shankar, K.; Rector, R.S. Cecal versus fecal microbiota in Ossabaw swine and implications for obesity. Physiol. Genom. 2018, 50, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Ramayo-Caldas, Y.; Mach, N.; Lepage, P.; Levenez, F.; Denis, C.; Lemonnier, G.; Leplat, J.J.; Billon, Y.; Berri, M.; Doré, J.; et al. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 2016, 10, 2973–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homann, C.M.; Rossel, C.A.J.; Dizzell, S.; Bervoets, L.; Simioni, J.; Li, J.; Gunn, E.; Surette, M.G.; de Souza, R.J.; Mommers, M.; et al. Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients 2021, 13, 2639. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schokker, D.; Zhang, J.; Zhang, L.L.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS ONE 2014, 9, e1000040. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Kallus, S.J.; Brandt, L.J. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigors, S.; O’Doherty, J.V.; Kelly, A.K.; O’Shea, C.J.; Sweeney, T. The Effect of Divergence in Feed Efficiency on the Intestinal Microbiota and the Intestinal Immune Response in Both Unchallenged and Lipopolysaccharide Challenged Ileal and Colonic Explants. PLoS ONE 2016, 11, e0148145. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.L.; Sun, H.; Wu, J.; Niu, H.H.; Feng, J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. 2014, 98, 680–685. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Li, M.; Xu, Q.; Zhang, X.; Tang, Z.; Chen, J.; Zhen, J.; Sun, Z. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J. Appl. Microbiol. 2020, 128, 1613–1623. [Google Scholar] [CrossRef]
- Lu, J.J.; Zou, X.T.; Wang, Y.M. Effects of sodium butyrate on the growth performance, intestinal microflora and morphology of weanling pigs. J. Anim. Feed Sci. 2008, 17, 568–578. [Google Scholar] [CrossRef]

| Group 1 | Group Size (n) | Lactation ADG, g/d | Nursery ADG, g/d | Birth BW, kg | Weaning BW, kg | Nursery BW, kg |
|---|---|---|---|---|---|---|
| Fast_Fast | 19 | 246 ± 5.2 a | 273 ± 7.1 a | 1.45 ± 0.026 a | 6.6 ± 0.09 a | 17.9 ± 0.33 a |
| Fast_Slow | 19 | 244 ± 4.6 a | 128 ± 3.7 c | 1.31 ± 0.069 ab | 6.4 ± 0.09 a | 11.7 ± 0.20 c |
| Slow_Fast | 19 | 148 ± 3.3 b | 238 ± 5.7 b | 1.35 ± 0.074 ab | 4.4 ± 0.11 b | 14.2 ± 0.31 b |
| Slow_Slow | 18 | 138 ± 5.9 b | 112 ± 3.5 d | 1.21 ± 0.056 b | 4.1 ± 0.11 b | 8.7 ± 0.17 d |
| p-value | <0.001 | <0.001 | 0.041 | <0.001 | <0.001 |
| Ingredient, % | Creep Feed | Pre-Starter | Starter |
|---|---|---|---|
| Wheat | 17.8 | 23.0 | 17.5 |
| Barley | 7.0 | 10.0 | 30.7 |
| Maize | 1.2 | 1.4 | 21.7 |
| Rapeseed | - | - | 3.0 |
| Sweet milk whey | 15.0 | 10.0 | - |
| Broken rice | 15.0 | 15.0 | - |
| Spray dried yogurt | 13.2 | - | - |
| Soy protein concentrate | 8.4 | 3.7 | - |
| Soybean meal 47% crude protein (CP) | - | 13.0 | - |
| Soybean meal 44% CP | - | - | 12.3 |
| Extruded soybeans | - | 6.0 | - |
| Porcine digestible peptides 62% CP | 5.8 | 2.1 | - |
| Porcine digestible peptides 50% CP | - | - | 5.7 |
| Animal plasma 80% CP | 1.7 | 1.7 | - |
| Skimmed milk | 3.3 | - | |
| Extruded cereals 1 | 3.0 | - | - |
| Lard | 1.0 | 1.5 | 3.3 |
| Lactose | - | 2.8 | - |
| Sucrose | 2.2 | 1.7 | - |
| Sugar beet pulp | 2 | 1.8 | 0.5 |
| Wheat bran | 1.6 | 3.8 | - |
| Lignocellulose 65% crude fiber (CF) | - | - | 1 |
| Vit-Min premix 2 | 0.5 | 0.5 | 0.5 |
| Liquid lysine 50% | 0.61 | 0.62 | 0.99 |
| DL-Methionine | 0.26 | 0.24 | 0.22 |
| L-Threonine | 0.19 | 0.18 | 0.27 |
| L-Valine | 0.16 | 0.08 | 0.09 |
| L-Tryptophane | 0.10 | 0.07 | 0.07 |
| Histidine | - | - | 0.11 |
| Isoleucine | - | - | 0.03 |
| Mono calcium phosphate | - | 0.42 | 1.10 |
| Calcium carbonate | - | 0.19 | 0.71 |
| Salt | - | 0.26 | 0.25 |
| Calculated composition | |||
| NE, kcal/kg | 2558 | 2430 | 2500 |
| Ash, % | 5.1 | 5.0 | 5.2 |
| Crude protein, % | 19.7 | 18.9 | 17.5 |
| Ether extract, % | 4.7 | 4.1 | 6.6 |
| Crude fiber, % | 1.9 | 3.0 | 5.4 |
| Starch, % | 29.8 | 30.0 | 39.1 |
| Calcium, % | 0.550 | 0.650 | 0.700 |
| Total p, % | 0.484 | 0.515 | 0.626 |
| Digestible p, % | 0.333 | 0.300 | 0.423 |
| Digestible amino acids | |||
| Lys, % | 1.372 | 1.294 | 1.187 |
| Met, % | 0.578 | 0.497 | 0.453 |
| Met + Cys, % | 0.822 | 0.774 | 0.712 |
| Thr, % | 0.891 | 0.839 | 0.794 |
| Trp, % | 0.301 | 0.284 | 0.251 |
| Item | Cortisol (pg/mg) | Cortisone (pg/mg) | Cortisol/Cortisone | |
|---|---|---|---|---|
| Lactation Growth | Nursery Growth | |||
| Fast | Fast | 19.2 | 188 | 0.27 |
| Slow | 17.4 | 86 | 0.54 | |
| Slow | Fast | 20.3 | 105 | 0.56 |
| Slow | 19.1 | 115 | 0.67 | |
| SEM 1 | 1.06 | 33.1 | 0.163 | |
| Lactation growth | ||||
| Fast | 18.3 | 140 | 0.40 | |
| Slow | 19.7 | 110 | 0.62 | |
| SEM | 0.74 | 22.3 | 0.115 | |
| Nursery growth | ||||
| Fast | 19.7 | 153 | 0.39 | |
| Slow | 18.2 | 99 | 0.60 | |
| SEM | 0.74 | 23.4 | 0.110 | |
| p-value 2 | ||||
| Lactation growth | 0.130 | 0.329 | 0.227 | |
| Nursery growth | 0.463 | 0.041 | 0.069 | |
| Lactation × Nursery growth | 0.852 | 0.174 | 0.157 | |
| Item | Observed OTUs | Evenness | Shannon |
|---|---|---|---|
| Lactation growth | |||
| Fast | 354 | 0.786 | 6.63 |
| Slow | 343 | 0.781 | 6.55 |
| SEM 1 | 14.9 | 0.0078 | 0.109 |
| Nursery growth | |||
| Fast | 353 | 0.797 | 6.72 |
| Slow | 345 | 0.770 | 6.46 |
| SEM | 15.1 | 0.0074 | 0.105 |
| p-value 2 | |||
| Lactation growth | 0.330 | 0.568 | 0.384 |
| Nursery growth | 0.969 | 0.016 | 0.127 |
| Lactation × Nursery growth | 0.526 | 0.985 | 0.904 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Solé, F.; Solà-Oriol, D.; Villagómez-Estrada, S.; Melo-Durán, D.; López, L.V.; Villarroel Román, N.; López-Arjona, M.; Pérez, J.F. Fecal Microbiota and Hair Glucocorticoid Concentration Show Associations with Growth during Early Life in a Pig Model. Nutrients 2022, 14, 4639. https://doi.org/10.3390/nu14214639
González-Solé F, Solà-Oriol D, Villagómez-Estrada S, Melo-Durán D, López LV, Villarroel Román N, López-Arjona M, Pérez JF. Fecal Microbiota and Hair Glucocorticoid Concentration Show Associations with Growth during Early Life in a Pig Model. Nutrients. 2022; 14(21):4639. https://doi.org/10.3390/nu14214639
Chicago/Turabian StyleGonzález-Solé, Francesc, David Solà-Oriol, Sandra Villagómez-Estrada, Diego Melo-Durán, Laura Victoria López, Nathaly Villarroel Román, Marina López-Arjona, and José Francisco Pérez. 2022. "Fecal Microbiota and Hair Glucocorticoid Concentration Show Associations with Growth during Early Life in a Pig Model" Nutrients 14, no. 21: 4639. https://doi.org/10.3390/nu14214639





