Walnut Oligopeptide Delayed Improved Aging-Related Learning and Memory Impairment in SAMP8 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal
2.3. Behavioral Tests
2.4. Biochemical Assays and Enzyme-Linked Immunosorbent Assay
2.5. Western Blot Analysis
2.6. Quantitative Real-Time PCR and Analyses of mtDNA Content
2.7. Statistical Methods
3. Results
3.1. Effects of WOPs on the Body Weight, Food and Water Consumption of Mice
3.2. Effects of WOPs on Learning and Memory Performance
3.2.1. The Space Exploration Capability of the Open-Field Test
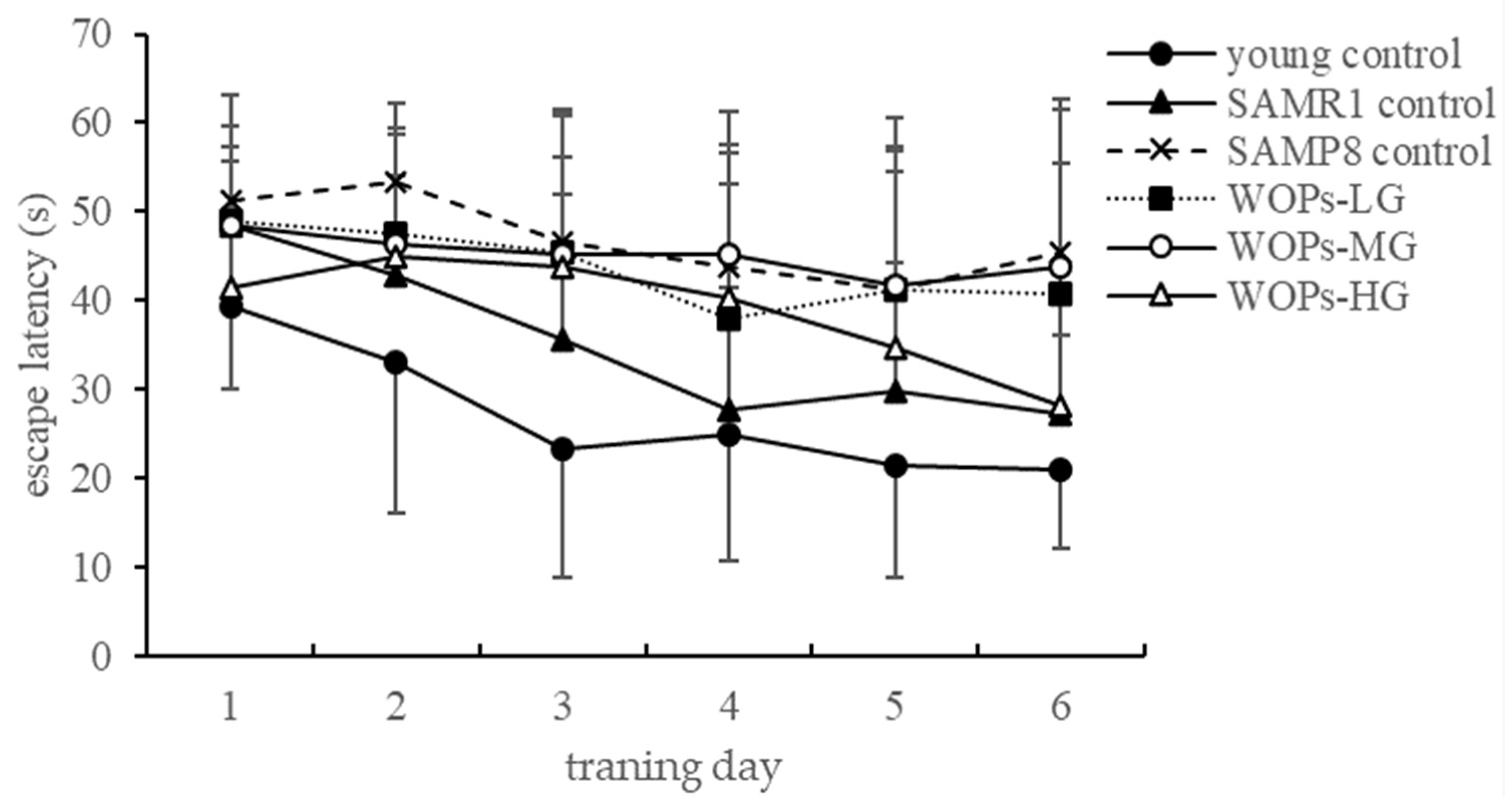
3.2.2. The Learning and Memory Capability in the Morris Water Maze Test
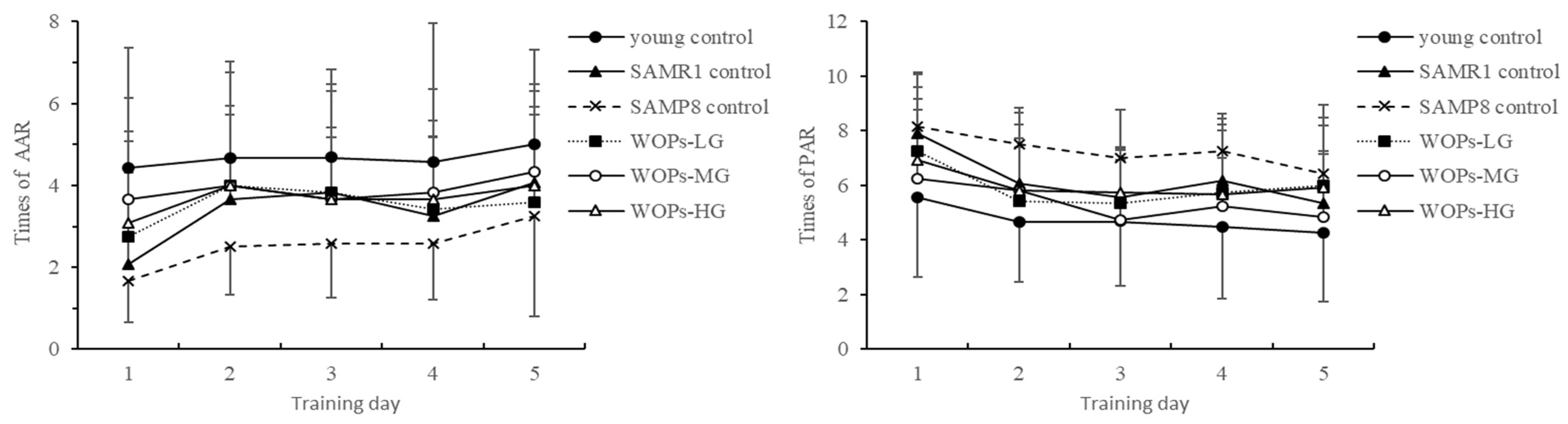
3.2.3. The Trend of AAR and PAR in the Shuttle Box Test
3.2.4. The Number of Errors and Latency in the Step-Down Test
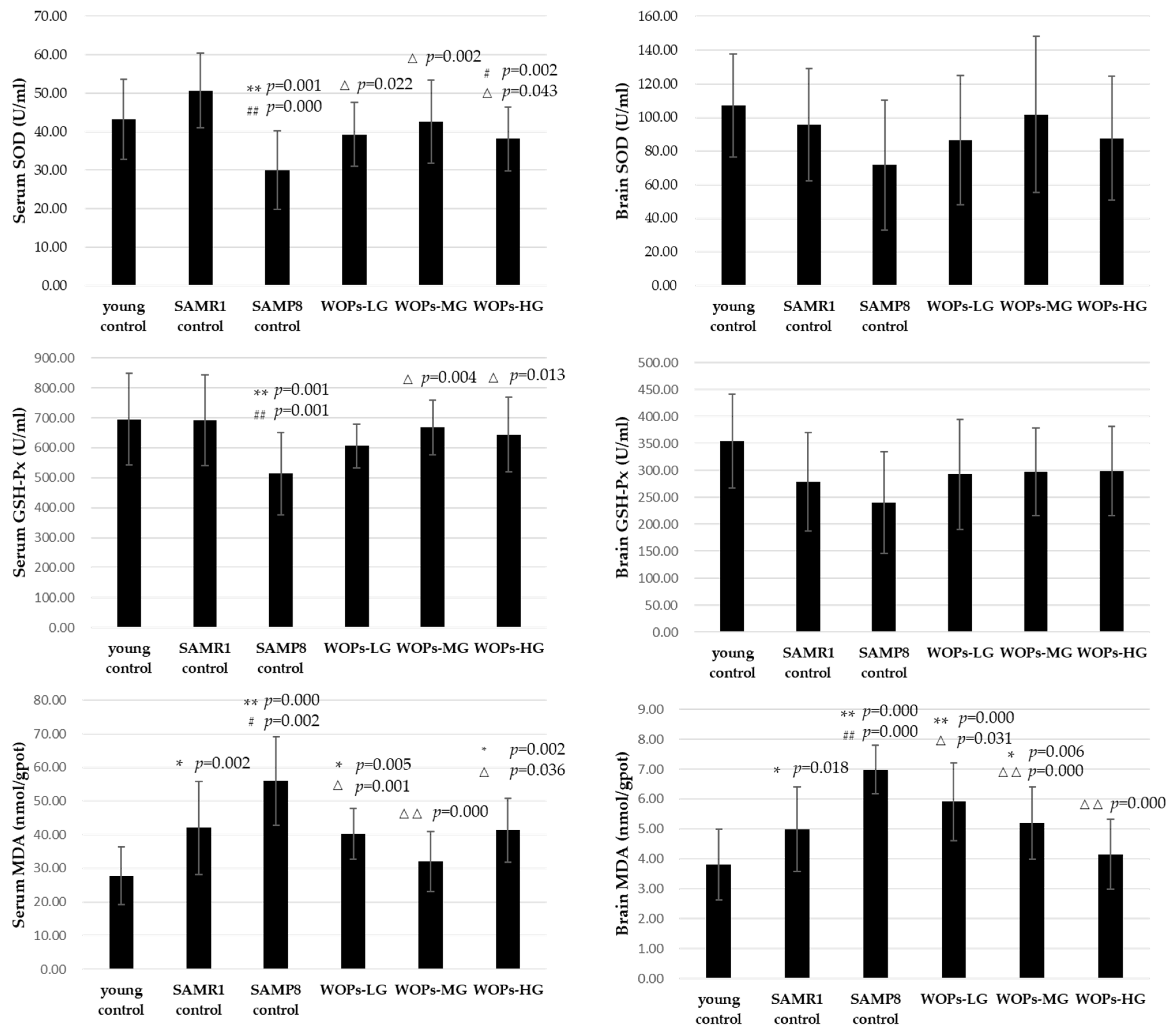
3.3. Effects of WOPs on Oxidative Stress in Serum and Hippocampus Tissue
3.4. Effects of WOPs on the Cholinergic Nervous System and Proinflammatory Factors in the Hippocampus Tissue
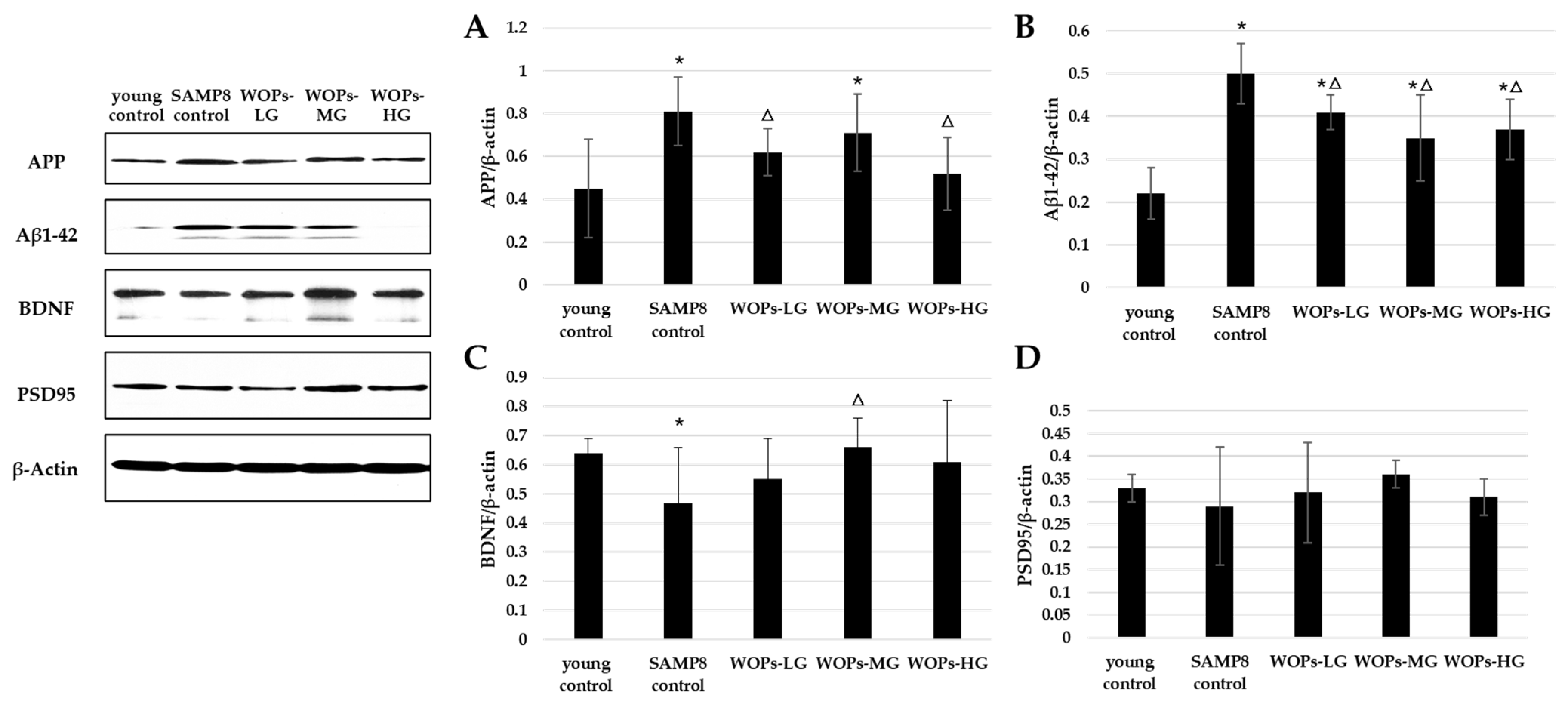
3.5. Effects of WOPs on Phosphorylate Synaptic Plasticity, Neurotrophic Factors, and Aβ Generation in the Hippocampus Tissue
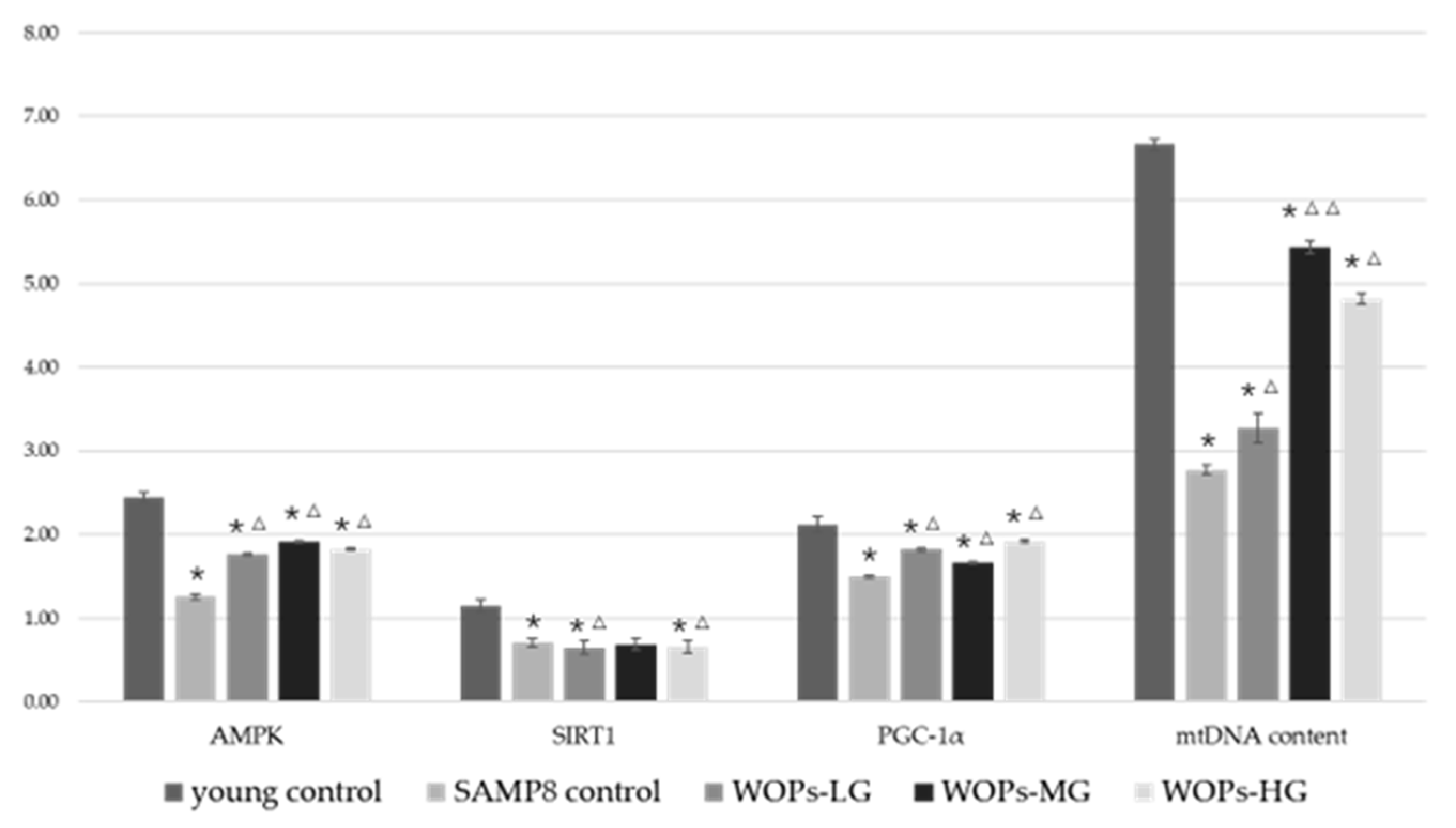
3.6. Effects of WOPs on Mitochondrial Function Factors and mtDNA Copy Number in the Hippocampus Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurd, M.D.; Martorell, P.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 369, 489–490. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [Green Version]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Chow, H.M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Strehler, E.E.; Thayer, S.A. Evidence for a role of plasma membrane calcium pumps in neurodegenerative disease: Recent developments. Neurosci. Lett. 2018, 663, 39–47. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630s–636s. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin Against Cerebral Ischemia-Reperfusion Via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef] [Green Version]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Liu, R.; He, L.; Mao, R.; Liu, X.; Zhang, T.; Hao, Y.; Fan, R.; Xu, M.; Li, Y. Radioprotective Effect of Walnut Oligopeptides against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice. Molecules 2019, 24, 1582. [Google Scholar]
- Li, D.; Ren, J.; Wang, T.; Wu, L.; Liu, P.; Li, Y. Anti-hypoxia effects of walnut oligopeptides (Juglans regia L.) in mice. Am. J. Transl. Res. 2021, 13, 4581–4590. [Google Scholar]
- Ting, D.; Yong, L. Beneficial effect and mechanism of walnut oligopeptide on Lactobacillus plantarum Z7. Food Sci. Nutr. 2021, 9, 672–681. [Google Scholar]
- Liu, R.; Hao, Y.; Zhu, N.; Liu, X.; Kang, J.; Mao, R.; Hou, C.; Li, Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2020, 12, 1138. [Google Scholar]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.; Chen, Q.; Di, L.; Mao, R.; Liu, X.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Palomera-Avalos, V.; Grinan-Ferre, C.; Puigoriol-Ilamola, D.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallas, M. Resveratrol Protects SAMP8 Brain Under Metabolic Stress: Focus on Mitochondrial Function and Wnt Pathway. Mol. Neurobiol. 2017, 54, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lu, X.; Zhu, W.; Liu, X.; Li, B.; Song, L.; Liu, H.; Cai, W.; Deng, Y.; Xu, T.; et al. Carnosine ameliorates age-related dementia via improving mitochondrial dysfunction in SAMP8 mice. Food Funct. 2020, 11, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Liu, X.; Hu, J.; Liu, R.; Zhu, N.; Li, Y. The Antioxidant Effects of Whey Protein Peptide on Learning and Memory Improvement in Aging Mice Models. Nutrients 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar]
- Downs, M.L.; Semic-Jusufagic, A.; Simpson, A.; Bartra, J.; Fernandez-Rivas, M.; Rigby, N.M.; Taylor, S.L.; Baumert, J.L.; Mills, E.N. Characterization of low molecular weight allergens from English walnut (Juglans regia). J. Agric. Food Chem. 2014, 62, 11767–11775. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, K.S. Walnut extract exhibits anti-fatigue action via improvement of exercise tolerance in mice. Lab. Anim. Res. 2013, 29, 190–195. [Google Scholar]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10 (Suppl. 4), S422–S436. [Google Scholar] [CrossRef] [Green Version]
- Esselun, C.; Dilberger, B.; Silaidos, C.V.; Koch, E.; Schebb, N.H.; Eckert, G.P. A Walnut Diet in Combination with Enriched Environment Improves Cognitive Function and Affects Lipid Metabolites in Brain and Liver of Aged NMRI Mice. Neuromol. Med. 2021, 23, 140–160. [Google Scholar] [CrossRef] [PubMed]
- Fidaleo, M.; Cavallucci, V.; Pani, G. Nutrients, neurogenesis and brain ageing: From disease mechanisms to therapeutic opportunities. Biochem. Pharmacol. 2017, 141, 63–76. [Google Scholar] [CrossRef]
- Garaschuk, O.; Semchyshyn, H.M.; Lushchak, V.I. Healthy brain aging: Interplay between reactive species, inflammation and energy supply. Ageing Res. Rev. 2018, 43, 26–45. [Google Scholar] [CrossRef]
- Louneva, N.; Cohen, J.W.; Han, L.Y.; Tallbot, K.; Wilson, R.S.; Bennett, D.A.; Trojanowski, J.Q.; Arnold, S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 2008, 173, 1488–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, K.; Grimm, A.; Kazmierczak, A.; Strosznajder, J.B.; Götz, J.; Eckert, A. Insights into mitochondrial dysfunction: Aging, amyloid-β, and tau-A deleterious trio. Antioxid. Redox Signal. 2012, 16, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Takata, K.; Takada, T.; Ito, A.; Asai, M.; Tawa, M.; Saito, Y.; Ashihara, E.; Tomimoto, H.; Kitamura, Y.; Shimohama, S. Microglial Amyloid-β1-40 Phagocytosis Dysfunction Is Caused by High-Mobility Group Box Protein-1: Implications for the Pathological Progression of Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 685739. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019, 30, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Gabelle, A.; Schraen, S.; Gutierrez, L.A.; Pays, C.; Rouaud, O.; Buée, L.; Touchon, J.; Helmer, C.; Lambert, J.C.; Berr, C. Plasma β-amyloid 40 levels are positively associated with mortality risks in the elderly. Alzheimer’s Dement. 2015, 11, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Ostojic, S.M. Exercise-induced mitochondrial dysfunction: A myth or reality? Clin. Sci. 2016, 130, 1407–1416. [Google Scholar] [CrossRef] [Green Version]
- Morozov, Y.M.; Datta, D.; Paspalas, C.D.; Arnsten, A.F.T. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol. Aging 2017, 51, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.K.; Craig, E.L.; Chakrabarti, L. Mitochondrial Complex 1 Activity Measured by Spectrophotometry Is Reduced across All Brain Regions in Ageing and More Specifically in Neurodegeneration. PLoS ONE 2016, 11, e0157405. [Google Scholar] [CrossRef] [Green Version]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 502–516. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Herskovits, A.Z.; Guarente, L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013, 23, 746–758. [Google Scholar]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Shi, X.; Ren, J.; Yin, H.; Li, D.; Song, L.; Zhang, Y. Mitochondrial homeostasis is involved in inhibiting hippocampus neuronal apoptosis during ZSWF ameliorate the cognitive dysfunction of SAMP8 mice. J. Funct. Foods 2022, 9, 105010. [Google Scholar] [CrossRef]
- Dong, W.; Quo, W.; Wang, F.; Li, C.; Xie, Y.; Zheng, X.; Shi, H. Electroacupuncture Upregulates SIRT1-Dependent PGC-1 alpha Expression in SAMP8 Mice. Med. Sci. Monit. 2015, 21, 3356–3362. [Google Scholar] [CrossRef]
- Izquierdo, V.; Palomera-Ávalos, V.; Pallàs, M.; Griñán-Ferré, C. Resveratrol Supplementation Attenuates Cognitive and Molecular Alterations under Maternal High-Fat Diet Intake: Epigenetic Inheritance over Generations. Int. J. Mol. Sci. 2021, 22, 1453. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef]
- Toricelli, M.; Pereira, A.; Souza Abrao, G.; Malerba, H.N.; Maia, J.; Buck, H.S.; Viel, T.A. Mechanisms of neuroplasticity and brain degeneration: Strategies for protection during the aging process. Neural Regen. Res. 2021, 16, 58–67. [Google Scholar] [PubMed]

| Groups | No. of Rearing | No. of Squares Crossed | No. Fecal Boli | No. of Inner Squares Entered | Time Spent in Inner Squares (s) |
|---|---|---|---|---|---|
| young control | 5.1 ± 8.4 | 61.9 ± 38.8 | 1.8 ± 1.7 | 3.1 ± 3.1 | 12.82 ± 15.00 |
| SAMR1 control | 4.1 ± 2.0 | 92.9 ± 25.9 | 1.8 ± 1.4 | 1.1 ± 1.0 | 2.35 ± 3.50 |
| SAMP8 control | 4.3 ± 5.6 | 71.8 ± 49.8 | 1.0 ± 1.3 | 1.2 ± 1.5 | 6.59 ± 8.50 |
| WOPs-LG | 1.8 ± 2.1 | 46.3 ± 36.9 # | 1.4 ± 1.2 | 1.1 ± 1.0 | 8.16 ± 9.09 |
| WOPs-MG | 2.3 ± 4.1 | 36.9 ± 33.2 ## | 2.1 ± 1.8 | 0.8 ± 1.0 | 5.77 ± 9.99 |
| WOPs-HG | 1.2 ± 1.5 * | 26.8 ± 18.3 *## | 2.3 ± 2.1 | 1.1 ± 1.2 | 5.50 ± 8.65 |
| Groups | Time Spent in the Target Quadrant (s) | Times of Platform Crossed |
|---|---|---|
| young control | 18.30 ± 4.14 | 3.4 ± 1.5 |
| SAMR1 control | 16.23 ± 6.30 | 1.8 ± 1.5 * |
| SAMP8 control | 6.61 ± 6.54 *# | 0.9 ± 1.6 ** |
| WOPs-LG | 13.69 ± 8.20 Δ | 0.3 ± 0.7 **# |
| WOPs-MG | 20.12 ± 9.28 ΔΔ | 0.8 ± 1.4 ** |
| WOPs-HG | 15.30 ± 7.93 Δ | 2.3 ± 1.9 Δ |
| Groups | the Number of Errors (No.) | Latency (s) |
|---|---|---|
| young control | 1.2 ± 0.4 | 206.08 ± 63.98 |
| SAMR1 control | 1.4 ± 0.5 | 171.63 ± 54.35 |
| SAMP8 control | 3.1 ± 1.2 *# | 71.49 ± 55.60 *## |
| WOPs-LG | 1.8 ± 1.0 | 147.46 ± 55.50 *Δ |
| WOPs-MG | 1.5 ± 0.7 Δ | 165.85 ± 45.54 ΔΔ |
| WOPs-HG | 1.6 ± 0.7 Δ | 163.34 ± 45.69 ΔΔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Xu, M.; Wu, L.; Fan, R.; Hao, Y.; Liu, X.; Mao, R.; Liu, R.; Li, Y. Walnut Oligopeptide Delayed Improved Aging-Related Learning and Memory Impairment in SAMP8 Mice. Nutrients 2022, 14, 5059. https://doi.org/10.3390/nu14235059
Du Q, Xu M, Wu L, Fan R, Hao Y, Liu X, Mao R, Liu R, Li Y. Walnut Oligopeptide Delayed Improved Aging-Related Learning and Memory Impairment in SAMP8 Mice. Nutrients. 2022; 14(23):5059. https://doi.org/10.3390/nu14235059
Chicago/Turabian StyleDu, Qian, Meihong Xu, Lan Wu, Rui Fan, Yuntao Hao, Xinran Liu, Ruixue Mao, Rui Liu, and Yong Li. 2022. "Walnut Oligopeptide Delayed Improved Aging-Related Learning and Memory Impairment in SAMP8 Mice" Nutrients 14, no. 23: 5059. https://doi.org/10.3390/nu14235059
APA StyleDu, Q., Xu, M., Wu, L., Fan, R., Hao, Y., Liu, X., Mao, R., Liu, R., & Li, Y. (2022). Walnut Oligopeptide Delayed Improved Aging-Related Learning and Memory Impairment in SAMP8 Mice. Nutrients, 14(23), 5059. https://doi.org/10.3390/nu14235059







