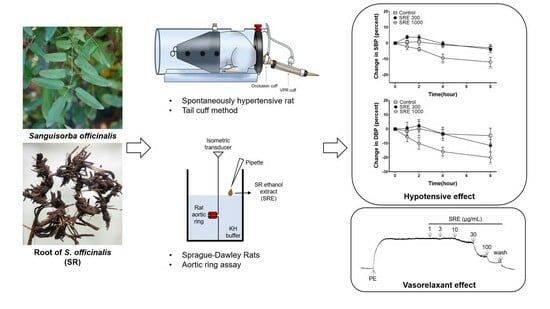
Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Animals
2.4. Measurement of Isotonic Changes in Blood Vessels
2.4.1. Preparation of Rat Aortic Rings
2.4.2. Vasodilatory Effect of SRE on the Rat Aortic Rings Constricted by PE
2.4.3. Vasodilatory Effect of SRE on Endothelium-Intact and -Removed Aortic Rings
2.4.4. Vasodilatory Effect of SRE When Pretreated with L-NAME and Indomethacin
2.4.5. Vasodilatory Effect of SRE When Pretreated with ODQ and MB
2.4.6. Vasodilatory Effect of SRE When Pretreated with K+ Channel Blockers
2.4.7. Inhibitory Effect of SRE on Extracellular Ca2+-Induced Contraction
2.4.8. Inhibitory Effect of SRE on Ang II-Induced Contraction
2.5. Blood Pressure Measurement of SHR
2.6. Statistical Analysis
3. Results
3.1. Vasodilatory Effect of SRE on the Aortic Rings Constricted by PE
3.2. Vasodilatory Effect of SRE on Endothelium-Intact and -Removed Aortic Rings
3.3. Effects of L-NAME and Indomethacin Pretreatment on Vasorelaxation
3.4. Effects of ODQ and MB Pretreatment on Vasorelaxation
3.5. Effect of K+ Channel Blocker Pretreatment on Vasorelaxation
3.6. Inhibitory Effect of SRE on Extracellular Ca2+-Induced Contraction
3.7. Inhibitory Effect of SRE on Angiotensin II-Induced Contraction
3.8. Blood Pressure-Lowering Effect of SRE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Olsen, H.; Klemetsrud, T.; Stokke, H.P.; Tretli, S.; Westheim, A. Adverse drug reactions in current antihypertensive therapy: A general practice survey of 2586 patients in Norway. Blood Press. 1999, 8, 94–101. [Google Scholar]
- Jiao, X.-F.; Song, K.; Jiao, X.; Li, H.; Zeng, L.; Zou, K.; Zhang, W.; Wang, H.; Zhang, L. Hyperuricaemia, gout and related adverse events associated with antihypertensive drugs: A real-world analysis using the FDA adverse event reporting system. Front. Pharmacol. 2023, 13, 1045561. [Google Scholar] [CrossRef]
- Lee, S.; Jo, C.; Choi, H.-Y.; Lee, K. Effect of Co-Administration of Curcumin with Amlodipine in Hypertension. Nutrients 2021, 13, 2797. [Google Scholar] [CrossRef]
- Markovics, A.; Biro, A.; Kun-Nemes, A.; Fazekas, M.É.; Rácz, A.A.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Effect of Anthocyanin-Rich Extract of Sour Cherry for Hyperglycemia-Induced Inflammatory Response and Impaired Endothelium-Dependent Vasodilation. Nutrients 2020, 12, 3373. [Google Scholar] [CrossRef]
- Alsayed, A.M.A.; Zhang, B.L.; Bredeloux, P.; Boudesocque-Delaye, L.; Yu, A.; Peineau, N.; Enguehard-Gueiffier, C.; Ahmed, E.M.; Pasqualin, C.; Maupoil, V. Aqueous Fraction from Hibiscus sabdariffa Relaxes Mesenteric Arteries of Normotensive and Hypertensive Rats through Calcium Current Reduction and Possibly Potassium Channels Modulation. Nutrients 2020, 12, 1782. [Google Scholar] [CrossRef]
- Nakamura, K.; Suzuki, Y.; Goto, K.; Yamaguchi, S.; Hiramitsu, M. Antihypertensive and Vasorelaxant Effects of Citric Acid and Lemon Juice in Spontaneously Hypertensive Rats: In Vivo and Ex Vivo Studies. Nutrients 2023, 15, 3849. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirose, M.; Takahashi, K.; Koyama, K.; Shimomura, K. Sanguisorba officinalis L. (Great Burnet): In Vitro Culture and Production of Sanguiin, Tannins, and Other Secondary Metabolites. In Medicinal and Aromatic Plants VIII, 1st ed.; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1995; Volume 33, pp. 427–441. [Google Scholar]
- Bączek, K. Accumulation of biomass and phenolic compounds in Polish and Mongolian great burnet (Sanguisorba officinalis L.) populations. Herba Pol. 2014, 60, 44–55. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Chen, Q.; Wang, L.; Yang, J.; Wu, A.; Jiang, N.; Liu, Y.; Chen, J.; Zou, W.; et al. A Comprehensive Review of Genus Sanguisorba: Traditional Uses, Chemical Constituents and Medical Applications. Front. Pharmacol. 2021, 12, 750165. [Google Scholar] [CrossRef]
- Yu, T.; Lee, Y.J.; Yang, H.M.; Han, S.; Kim, J.H.; Lee, Y.; Kim, C.; Han, M.H.; Kim, M.-Y.; Lee, J.; et al. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE2 production is mediated by suppression of NF-κB and AP-1 activation signaling cascade. J. Ethnopharmacol. 2011, 134, 11–17. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, M.; Erdenebileg, S.; Cha, K.H.; Ryu, D.H.; Kim, H.Y.; Lee, S.H.; Jung, J.H.; Nho, C.W. Sanguisorba officinalis L. Ameliorates Hepatic Steatosis and Fibrosis by Modulating Oxidative Stress, Fatty Acid Oxidation, and Gut Microbiota in CDAHFD-Induced Mice. Nutrients 2023, 15, 3779. [Google Scholar] [CrossRef]
- Wang, Z.; Loo, W.T.; Wang, N.; Chow, L.W.; Wang, D.; Han, F.; Zheng, X.; Chen, J.-P. Effect of Sanguisorba officinalis L on breast cancer growth and angiogenesis. Expert. Opin. Ther. Targets 2012, 16 (Suppl. S1), S79–S89. [Google Scholar] [CrossRef]
- Son, D.J.; Hwang, S.Y.; Kim, M.-H.; Park, U.K.; Kim, B.S. Anti-Diabetic and Hepato-Renal Protective Effects of Ziyuglycoside II Methyl Ester in Type 2 Diabetic Mice. Nutrients 2015, 7, 5469–5483. [Google Scholar] [CrossRef]
- Tu, J.; Li, Q.; Zhou, B. The Tannins from Sanguisorba officinalis L. (Rosaceae): A Systematic Study on the Metabolites of Rats Based on HPLC-LTQ-Orbitrap MS2 Analysis. Molecules 2021, 26, 4053. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.-J.; Wang, L.-Q.; Wu, J.; Dai, C.; Yuan, Y.-M.; Li, G.Q.; Yao, M.-C. A tannin compound from Sanguisorba officinalis blocks Wnt/β-catenin signaling pathway and induces apoptosis of colorectal cancer cells. Chin. Med. 2019, 14, 22. [Google Scholar] [CrossRef]
- Russell, J.A.; Rohrbach, M.S. Tannin induces endothelium-dependent contraction and relaxation of rabbit pulmonary artery. Am. Rev. Respir. Dis. 1989, 139, 498–503. [Google Scholar] [CrossRef]
- Liu, J.-C.; Hsu, F.-L.; Tsai, J.-C.; Chan, P.; Liu, J.Y.-H.; Thomas, G.N.; Tomlinson, B.; Lo, M.-Y.; Lin, J.-Y. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef]
- Ando, J.; Kamiya, A. Blood flow and vascular endothelial cell function. Front. Med. Biol. Eng. 1993, 5, 245–264. [Google Scholar]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina 2003, 39, 333–341. [Google Scholar]
- Sharma, V.; Berkelhamer, S.; Lakshminrusimha, S. Persistent pulmonary hypertension of the newborn. Matern. Health Neonatol. Perinatol. 2015, 1, 14. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Reeve, H.L.; Huang, J.M.; Tolarova, S.; Nelson, D.P.; Weir, E.K.; Archer, S.L. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 889–897. [Google Scholar] [CrossRef]
- Nieves-Cintrón, M.; Syed, A.U.; Nystoriak, M.A.; Navedo, M.F. Regulation of voltage-gated potassium channels in vascular smooth muscle during hypertension and metabolic disorders. Microcirculation 2018, 25, e12423. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Cat, A.N.D.; Touyz, R.M. Cell signaling of angiotensin II on vascular tone: Novel mechanisms. Curr. Hypertens. Rep. 2011, 13, 122–128. [Google Scholar]
- Biancardi, V.C.; Bomfim, G.F.; Reis, W.L.; Al-Gassimi, S.; Nunes, K.P. The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol. Res. 2017, 120, 88–96. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Zhonghua Bencao Edit Committee. Zhonghua Bencao; Shanghai Science and Technology Publications: Shanghai, China, 1999. [Google Scholar]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2012, 35, 1039–1047. [Google Scholar] [CrossRef]
- Hongo, K.; Nakagomi, T.; Kassell, N.F.; Sasaki, T.; Lehman, M.; Vollmer, D.G.; Tsukahara, T.; Ogawa, H.; Torner, J. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke 1988, 19, 892–897. [Google Scholar] [CrossRef]
- Moţoc, A.-C.T.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of Polyphenolic Composition and Antimicrobial Properties of Sanguisorba officinalis L. and Sanguisorba minor Scop. Plants 2022, 11, 3561. [Google Scholar]
- Gawron-Gzella, A.; Witkowska-Banaszczak, E.; Bylka, W.; Dudek-Makuch, M.; Odwrot, A.; Skrodzka, N. Chemical Composition, Antioxidant and Antimicrobial Activities of Sanguisorba officinalis L. Extracts. Pharm. Chem. J. 2016, 50, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yan, H.-L.; Wang, L.-X.; Xu, J.-F.; Peng, C.; Ao, H.; Tan, Y.-Z. Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, K.-W.; Lee, S.; Jo, C.; Lee, K.; Ham, I.; Choi, H.-Y. Endothelium-Dependent Vasorelaxant Effect of Prunus persica Branch on Isolated Rat Thoracic Aorta. Nutrients 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Kudo, R.; Yuui, K.; Kasuda, S. Endothelium-Independent Relaxation of Vascular Smooth Muscle Induced by Persimmon-Derived Polyphenol Phytocomplex in Rats. Nutrients 2021, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Feng, T.; Shan, L.; Cai, B.; Chu, W.; Niu, H.; Lu, Y.; Yang, B. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res. 2008, 22, 1428–1433. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. https://doi.org/10.3390/nu15214510
Jung J, Shin S, Park J, Lee K, Choi H-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients. 2023; 15(21):4510. https://doi.org/10.3390/nu15214510
Chicago/Turabian StyleJung, Jaesung, Sujin Shin, Junkyu Park, Kyungjin Lee, and Ho-Young Choi. 2023. "Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats" Nutrients 15, no. 21: 4510. https://doi.org/10.3390/nu15214510