IL-10 and IL-1β Serum Levels, Genetic Variants, and Metabolic Syndrome: Insights into Older Adults’ Clinical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Sample Collection
2.3. Metabolic Syndrome Defining Condition
2.4. Laboratory Analysis and Cytokine Dosage
2.5. DNA Extraction
2.6. Genotyping
2.7. Statistical Analysis
3. Results
3.1. IL-10 and IL-1β Serum Concentrations’ Associations with Hematological, Biochemical, and Anthropometric Measurements
3.2. Participants’ Serum IL-10 and IL-1β Levels and Other Clinical Signs and Symptoms
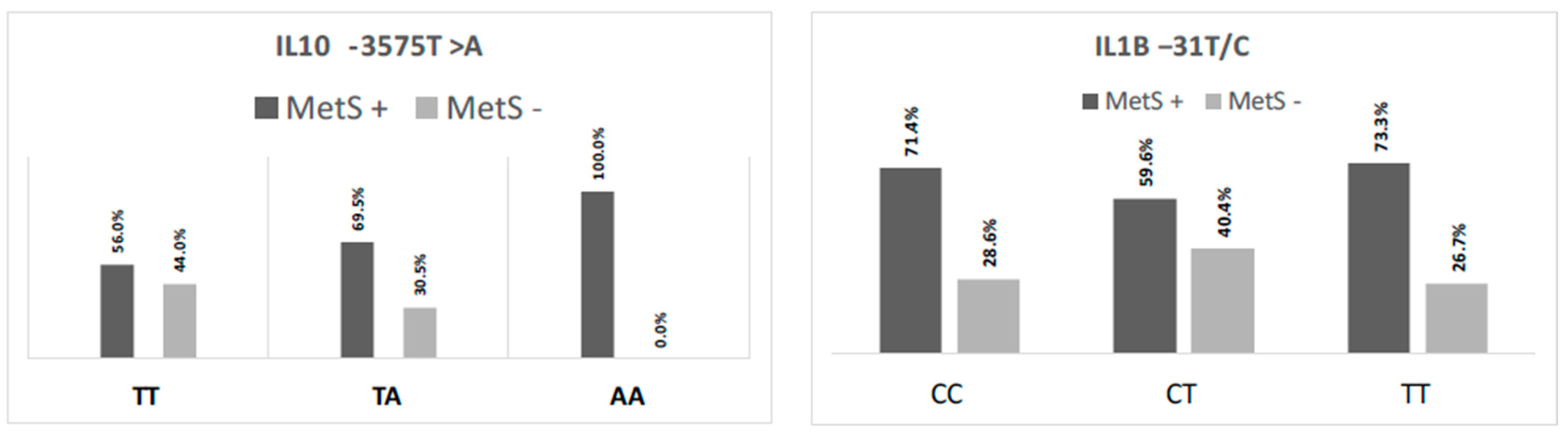
3.3. IL10 and IL1B Gene Polymorphisms’ Genotype Frequency Distribution and Their Relationship with Their Serum Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Instituto Brasileiro de Geografia e Estatística–IBGE.|Censo 2022: Número de Pessoas Com 65 Anos Ou Mais de Idade Cresceu 57,4% Em 12 Anos. 2023. Available online: https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-noticias/noticias/38186-censo-2022-numero-de-pessoas-com-65-anos-ou-mais-de-idade-cresceu-57-4-em-12-anos (accessed on 1 April 2023).
- Oliveira, A.S. Transição Demográfica, Transição Epidemiológica e Envelhecimento Populacional no Brasil. Hygeia Rev. Bras. Geogr. Médica Saúde 2019, 15, 69–79. [Google Scholar] [CrossRef]
- McKeown, R.E. The Epidemiologic Transition: Changing Patterns of Mortality and Population Dynamics. Am. J. Lifestyle Med. 2009, 3, 19S–26S. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Santos, A.J.; Kislaya, I.; Nunes, B.; Freire, A.C. Síndrome Metabólica Em Portugal: Prevalência e Fatores Associados. Acta Med. Port. 2022, 35, 633–643. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Barbalho, Y.G.; Stival, M.M.; de Lima, L.R.; da Silva, I.C.R.; de Oliveira Silva, A.; da Costa, M.V.G.; Rehem, T.C.M.S.B.; Funghetto, S.S. Impact of Metabolic Syndrome Components in High-Risk Cardiovascular Disease Development in Older Adults. Clin. Interv. Aging 2020, 15, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.F.; Precoma, D.B.; Andrade, J.P.; Correa Filho, H.; Saraiva, J.F.K.; Oliveira, G.M.M.; Murro, A.L.B.; Campos, A.; Alessi, A.; Avezum Junior, A.; et al. I Diretriz Brasileira de Prevenção Cardiovascular. Arq. Bras. Cardiol. 2013, 101, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; Zito, M.C.; et al. From Metabolic Syndrome to Neurological Diseases: Role of Autophagy. Front. Cell Dev. Biol. 2021, 9, 651021. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, Endothelial Stress, Inflammation, and the Metabolic Syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Maintinguer Norde, M.; Oki, E.; Ferreira Carioca, A.A.; Teixeira Damasceno, N.R.; Fisberg, R.M.; Lobo Marchioni, D.M.; Rogero, M.M. Influence of IL1B, IL6 and IL10 Gene Variants and Plasma Fatty Acid Interaction on Metabolic Syndrome Risk in a Cross-Sectional Population-Based Study. Clin. Nutr. 2018, 37, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, H.R.; Hosseini, R.; Bijani, A.; Akhavan-Niaki, H.; Parsian, H.; Pouramir, M.; Saravi, M.; Bagherzadeh, M.; Mosapour, A.; Saleh-Moghaddam, M.; et al. Interleukin 10, Lipid Profile, Vitamin D, Selenium, Metabolic Syndrome, and Serum Antioxidant Capacity in Elderly People with and without Cardiovascular Disease: Amirkola Health and Ageing Project Cohort-Based Study. ARYA Atheroscler. 2019, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, C.; Ma, Y.; Bu, L.; Yan, L.; Liu, D. Hydrodynamic Delivery of MIL10 Gene Protects Mice From High-Fat Diet-Induced Obesity and Glucose Intolerance. Mol. Ther. 2013, 21, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, D.; Yin, C.; Wang, S.; Wang, M.; Xiao, Y. IL-10/STAT3 Is Reduced in Childhood Obesity with Hypertriglyceridemia and Is Related to Triglyceride Level in Diet-Induced Obese Rats. BMC Endocr. Disord. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.-L.; Zhao, S.-J.; Lin, X.-X.; Liao, A.-H. IL-10: A Bridge between Immune Cells and Metabolism during Pregnancy. J. Reprod. Immunol. 2022, 154, 103750. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Skadow, M.H.; Qu, R.; Oh, J.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; Kluger, Y.; Crawford, J.M.; et al. IL-10 Constrains Sphingolipid Metabolism via Fatty Acid Desaturation to Limit Inflammation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Barcelos, A.L.V.; de Oliveira, E.A.; Haute, G.V.; Costa, B.P.; Pedrazza, L.; Donadio, M.V.F.; de Oliveira, J.R.; Bodanese, L.C. Association of IL-10 to Coronary Disease Severity in Patients with Metabolic Syndrome. Clin. Chim. Acta 2019, 495, 394–398. [Google Scholar] [CrossRef]
- LaMarche, N.M.; Kane, H.; Kohlgruber, A.C.; Dong, H.; Lynch, L.; Brenner, M.B. Distinct INKT Cell Populations Use IFNγ or ER Stress-Induced IL-10 to Control Adipose Tissue Homeostasis. Cell Metab. 2020, 32, 243–258.e6. [Google Scholar] [CrossRef]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and Inflammatory Cytokines and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Dharmadhikari, G.; Schumann, D.M.; Størling, J. Interleukin-Targeted Therapy for Metabolic Syndrome and Type 2 Diabetes. In Diabetes—Perspectives in Drug Therapy; Springer: Berlin/Heidelberg, Germany, 2011; pp. 257–278. [Google Scholar]
- Netea, M.G.; Dinarello, C.A. More than Inflammation: Interleukin-1β Polymorphisms and the Lipid Metabolism. J. Clin. Endocrinol. Metab. 2011, 96, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, S.J.; Trimigliozzi, K.; Dror, E.; Meier, D.T.; Molina-Tijeras, J.A.; Rachid, L.; Le Foll, C.; Magnan, C.; Schulze, F.; Stawiski, M.; et al. The Cephalic Phase of Insulin Release Is Modulated by IL-1β. Cell Metab. 2022, 34, 991–1003.e6. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 Family Members in the Pathogenesis and Treatment of Metabolic Disease: Focus on Adipose Tissue Inflammation and Insulin Resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, Z.; Cao, S.; Gao, W.; Liu, L.; Yin, Y.; Liu, P.; Shu, Y. Systematic Review and Meta-Analysis on the Association between IL-1B Polymorphisms and Cancer Risk. PLoS ONE 2013, 8, e63654. [Google Scholar] [CrossRef]
- Dai, Z.-M.; Liu, J.; Cao, X.-M.; Zhang, Y.; Wang, M.; Liu, X.-H.; Li, C.-J.; Dai, Z.-J.; Zhang, W.-G. Association Between Interleukin-10-3575T>A (Rs1800890) Polymorphism and Cancer Risk. Genet. Test. Mol. Biomark. 2015, 19, 324–330. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Lucena Pereira da Fonseca, H. A Reforma Da Saúde de Brasília, Brasil. Cien. Saude Colet. 2019, 24, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Lins, M.E.M.; Marques, A.P.d.O.; Leal, M.C.C.; Barros, R.L.d.M. Risco de Fragilidade Em Idosos Comunitários Assistidos Na Atenção Básica de Saúde e Fatores Associados. Saúde Debate 2019, 43, 520–529. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S.; Muc-Wierzgoń, M. Inflammation and Oxidative Stress in Frailty and Metabolic Syndromes—Two Sides of the Same Coin. Metabolites 2023, 13, 475. [Google Scholar] [CrossRef]
- Subramanian, N.; Tavira, B.; Hofwimmer, K.; Gutsmann, B.; Massier, L.; Abildgaard, J.; Juul, A.; Rydén, M.; Arner, P.; Laurencikiene, J. Sex-Specific Regulation of IL-10 Production in Human Adipose Tissue in Obesity. Front. Endocrinol. 2022, 13, 996954. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.K. The Relationship Between IL-10 and Dislipidemia in Type 2 Diabetics. Al-Mustansiriyah J. Sci. 2011, 22, 202–213. [Google Scholar]
- Moraitis, A.G.; Freeman, L.A.; Shamburek, R.D.; Wesley, R.; Wilson, W.; Grant, C.M.; Price, S.; Demosky, S.; Thacker, S.G.; Zarzour, A.; et al. Elevated Interleukin-10: A New Cause of Dyslipidemia Leading to Severe HDL Deficiency. J. Clin. Lipidol. 2015, 9, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.S.; Pereira, Í.S.; Santos, D.P.; Lopes, D.N.; Prado, A.O.; Calado, S.P.M.; Gonçalves, C.V.; Galantini, M.P.L.; Muniz, I.P.R.; Santos, G.S.; et al. Association between Body Composition and Inflammation: A Central Role of IL-17 and IL-10 in Diabetic and Hypertensive Elderly Women. Exp. Gerontol. 2019, 127, 110734. [Google Scholar] [CrossRef]
- Acosta, J.R.; Tavira, B.; Douagi, I.; Kulyté, A.; Arner, P.; Rydén, M.; Laurencikiene, J. Human-Specific Function of IL-10 in Adipose Tissue Linked to Insulin Resistance. J. Clin. Endocrinol. Metab. 2019, 104, 4552–4562. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and Human Longevity in the Omics Era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Fasshauer, M.; Tönjes, A.; Kratzsch, J.; Schön, M.; Paschke, R. Association of Interleukin-6, C-Reactive Protein, Interleukin-10 and Adiponectin Plasma Concentrations with Measures of Obesity, Insulin Sensitivity and Glucose Metabolism. Exp. Clin. Endocrinol. Diabetes 2005, 113, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.S.; Daneshpour, M.S.; Akbarzadeh, M.; Hedayati, M.; Azizi, F.; Zarkesh, M. Association of Baseline and Changes in Adiponectin, Homocysteine, High-Sensitivity C-Reactive Protein, Interleukin-6, and Interleukin-10 Levels and Metabolic Syndrome Incidence: Tehran Lipid and Glucose Study. Heliyon 2023, 9, e19911. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.W.; Arnesen, H.; Seljeflot, I. Components of the Interleukin-6 Transsignalling System Are Associated with the Metabolic Syndrome, Endothelial Dysfunction and Arterial Stiffness. Metabolism 2013, 62, 1008–1013. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic Syndrome and Cardiovascular Diseases: Going beyond Traditional Risk Factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Francischetti, I.; Moreno, J.B.; Scholz, M.; Yoshida, W.B. Os Leucócitos e a Resposta Inflamatória Na Lesão de Isquemia-Reperfusão. Rev. Bras. Cir. Cardiovasc. 2010, 25, 575–584. [Google Scholar] [CrossRef]
- Cruvinel, W.d.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; da Silva, N.P.; Andrade, L.E.C. Sistema Imunitário: Parte I. Fundamentos Da Imunidade Inata Com Ênfase Nos Mecanismos Moleculares e Celulares Da Resposta Inflamatória. Rev. Bras. Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated Plasma Cytokines in Autism Spectrum Disorders Provide Evidence of Immune Dysfunction and Are Associated with Impaired Behavioral Outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Cho, J.S.; Guo, Y.; Ramos, R.I.; Hebroni, F.; Plaisier, S.B.; Xuan, C.; Granick, J.L.; Matsushima, H.; Takashima, A.; Iwakura, Y.; et al. Neutrophil-Derived IL-1β Is Sufficient for Abscess Formation in Immunity against Staphylococcus Aureus in Mice. PLoS Pathog. 2012, 8, e1003047. [Google Scholar] [CrossRef]
- Garcia, L.P.; Sanchez, Z.M. Consumo de Álcool Durante a Pandemia Da COVID-19: Uma Reflexão Necessária Para o Enfrentamento Da Situação. Cad. Saude Publica 2020, 36, e00124520. [Google Scholar] [CrossRef]
- Afshar, M.; Richards, S.; Mann, D.; Cross, A.; Smith, G.B.; Netzer, G.; Kovacs, E.; Hasday, J. Acute Immunomodulatory Effects of Binge Alcohol Ingestion. Alcohol 2015, 49, 57–64. [Google Scholar] [CrossRef]
- Achur, R.N.; Freeman, W.M.; Vrana, K.E. Circulating Cytokines as Biomarkers of Alcohol Abuse and Alcoholism. J. Neuroimmune Pharmacol. 2010, 5, 83–91. [Google Scholar] [CrossRef]
| Parameters | [IL-10] pg/mL | [IL-1 β] pg/mL | |
|---|---|---|---|
| [IL-10] pg/mL | ρ | 1.000 | 0.016 |
| p-value | - | 0.825 | |
| [IL-1 β] pg/mL | ρ | 0.016 | 1.000 |
| p-value | 0.825 | - | |
| Total leukocytes | ρ | −0.06 | 0.213 ** |
| p-value | 0.406 | 0.003 | |
| Rod neutrophils | ρ | 0.011 | 0.093 |
| p-value | 0.878 | 0.201 | |
| Segmented neutrophils | ρ | −0.02 | 0.163 * |
| p-value | 0.781 | 0.024 | |
| Eosinophils | ρ | −0.096 | 0.046 |
| p-value | 0.187 | 0.530 | |
| Basophils | ρ | −0.104 | 0.028 |
| p-value | 0.154 | 0.703 | |
| Lymphocytes | ρ | 0.053 | −0.131 |
| p-value | 0.467 | 0.070 | |
| Monocytes | ρ | −0.068 | −0.104 |
| p-value | 0.350 | 0.153 | |
| Total cholesterol | ρ | 0.008 | 0.050 |
| p-value | 0.910 | 0.492 | |
| Triglycerides | ρ | −0.268 ** | 0.079 |
| p-value | <0.001 | 0.272 | |
| HDL | ρ | 0.365 ** | 0.038 |
| p-value | <0.001 | 0.598 | |
| LDL | ρ | 0.003 | 0.034 |
| p-value | 0.972 | 0.648 | |
| Total lipids | ρ | −0.023 | 0.106 |
| p-value | 0.830 | 0.311 | |
| Glucose | ρ | −0.370 ** | 0.017 |
| p-value | <0.001 | 0.817 | |
| HbA1c | ρ | −0.379 ** | 0.052 |
| p-value | <0.001 | 0.476 | |
| Estimated average blood glucose | ρ | −0.377 ** | 0.056 |
| p-value | <0.001 | 0.442 | |
| Glutamic oxaloacetic transaminase | ρ | 0.115 | −0.010 |
| p-value | 0.111 | 0.894 | |
| Pyruvic glutamic transaminase | ρ | 0.016 | −0.113 |
| p-value | 0.821 | 0.116 | |
| Time with T2DM | ρ | −0.264 ** | −0.031 |
| p-value | <0.001 | 0.677 | |
| Time with SAH | ρ | −0.071 | −0.053 |
| p-value | 0.332 | 0.471 | |
| BMI | ρ | 0.109 | 0.038 |
| p-value | 0.146 | 0.608 | |
| Waist circumference | ρ | −0.150 | 0.073 |
| p-value | 0.062 | 0.366 | |
| Fat mass | ρ | 0.293 ** | 0.011 |
| p-value | <0.001 | 0.896 | |
| Lean Mass | ρ | −0.263 ** | −0.03 |
| p-value | <0.001 | 0.707 | |
| Bone mineral content | ρ | −0.328 ** | −0.012 |
| p-value | <0.001 | 0.879 | |
| [IL-10] pg/mL | [IL-1β] pg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P25 | Median | P75 | p Value | N | P25 | Median | P75 | p Value | ||
| MetS | Yes | 127 | 4.28 | 4.59 | 4.84 | <0.001 * | 127 | 3.53 | 6.23 | 8.66 | 0.482 |
| No | 66 | 5.24 | 5.35 | 5.57 | 66 | 4.74 | 7.47 | 8.75 | |||
| Sarcopenia (DEXA) | Yes | 23 | 4.25 | 4.84 | 5.28 | 0.722 | 23 | 4.83 | 7.44 | 8.75 | 0.537 |
| No | 170 | 4.51 | 4.85 | 5.23 | 170 | 3.53 | 7.30 | 8.75 | |||
| SAH | Yes | 147 | 4.35 | 4.84 | 5.22 | 0.266 | 147 | 3.53 | 6.23 | 8.75 | 0.231 |
| No | 46 | 4.37 | 5.09 | 5.34 | 46 | 4.77 | 7.48 | 8.78 | |||
| T2DM | Yes | 108 | 4.34 | 4.83 | 5.12 | 0.007 * | 108 | 3.53 | 7.30 | 8.68 | 0.821 |
| No | 85 | 4.51 | 5.02 | 5.38 | 85 | 3.54 | 7.40 | 8.76 | |||
| Age | 60 to 65 | 81 | 4.51 | 4.84a | 5.29 | 0.042 * | 81 | 3.54 | 6.25 | 7.53 | 0.691 |
| 66 to 69 | 43 | 4.51 | 4.83a | 5.06 | 43 | 3.53 | 7.47 | 8.84 | |||
| 70 to 75 | 40 | 4.35 | 5.045a | 5.31 | 40 | 3.52 | 6.02 | 8.76 | |||
| 76 to 79 | 16 | 4.67 | 5.035a | 5.27 | 16 | 4.86 | 7.51 | 8.81 | |||
| ≥80 | 13 | 4.11 | 4.48b | 4.84 | 13 | 3.65 | 7.53 | 8.66 | |||
| Smoking | Yes | 14 | 4.31 | 4.94 | 5.24 | 0.808 | 14 | 3.50 | 6.85 | 8.75 | 0.644 |
| No | 179 | 4.37 | 4.84 | 5.24 | 179 | 3.54 | 7.40 | 8.75 | |||
| Use of alcoholic beverages | Yes | 9 | 4.48 | 5.01 | 5.24 | 0.709 | 9 | 3.51 | 3.53 | 5.94 | 0.037 * |
| No | 184 | 4.37 | 4.84 | 5.24 | 184 | 3.55 | 7.42 | 8.75 | |||
| Perform physical exercises | Yes | 135 | 4.37 | 4.86 | 5.27 | 0.573 | 135 | 3.54 | 7.30 | 8.75 | 0.853 |
| No | 58 | 4.48 | 4.84 | 5.22 | 58 | 3.53 | 7.40 | 8.76 | |||
| Altered BP | Yes | 80 | 4.35 | 4.84 | 5.22 | 0.358 | 80 | 3.52 | 7.41 | 8.77 | 0.596 |
| No | 95 | 4.51 | 4.86 | 5.24 | 95 | 4.74 | 7.48 | 8.76 | |||
| Altered SBP | Yes | 56 | 4.33 | 4.79 | 5.16 | 0.241 | 56 | 3.52 | 6.24 | 8.73 | 0.288 |
| No | 116 | 4.51 | 4.86 | 5.24 | 116 | 4.71 | 7.48 | 8.77 | |||
| Altered DBP | No | 116 | 4.37 | 4.86 | 5.23 | 0.954 | 116 | 4.71 | 7.48 | 8.75 | 0.301 |
| Yes | 53 | 4.48 | 4.83 | 5.22 | 53 | 3.52 | 6.25 | 8.79 | |||
| HDL | Altered | 40 | 4.24 | 4.51 | 4.93 | <0.001 * | 40 | 3.53 | 7.46 | 8.81 | 0.709 |
| Normal | 152 | 4.59 | 5.01 | 5.28 | 152 | 3.54 | 7.30 | 8.75 | |||
| Glucose | Altered | 98 | 4.29 | 4.76 | 5.01 | <0.001 * | 98 | 3.53 | 6.27 | 7.65 | 0.567 |
| Normal | 95 | 4.79 | 5.22 | 5.40 | 95 | 4.68 | 7.41 | 8.76 | |||
| HbA1c | Altered | 110 | 4.29 | 4.77 | 5.02 | <0.001 * | 110 | 3.54 | 7.46 | 8.75 | 0.341 |
| Normal | 82 | 4.79 | 5.19 | 5.38 | 82 | 3.52 | 6.19 | 8.69 | |||
| Total cholesterol | Altered | 102 | 4.48 | 4.86 | 5.24 | 0.806 | 102 | 3.54 | 7.41 | 8.76 | 0.911 |
| Normal | 91 | 4.35 | 4.84 | 5.22 | 91 | 3.53 | 6.29 | 8.75 | |||
| LDL | Altered | 100 | 4.37 | 4.84 | 5.24 | 0.945 | 100 | 3.54 | 7.46 | 8.79 | 0.891 |
| Normal | 87 | 4.35 | 4.84 | 5.22 | 87 | 3.53 | 6.29 | 8.75 | |||
| Waist circumference | Altered | 123 | 4.51 | 4.84 | 5.07 | 0.851 | 123 | 3.53 | 7.30 | 8.75 | 0.343 |
| Normal | 32 | 4.28 | 4.82 | 5.30 | 32 | 3.52 | 6.23 | 7.50 | |||
| Biological sex | Female | 154 | 4.74 | 5.01 | 5.28 | <0.001 * | 154 | 3.54 | 7.41 | 8.75 | 0.588 |
| Male | 39 | 4.25 | 4.31 | 5.18 | 39 | 3.53 | 6.09 | 8.79 | |||
| IL10 -3575T>A | IL1Β −31T>C | |||||
|---|---|---|---|---|---|---|
| TT | TA | AA | CC | CT | TT | |
| [IL-10] pg/mL | [IL-10] pg/mL | [IL-10] pg/mL | [IL-1Β] pg/mL | [IL-1Β] pg/mL | [IL-1Β] pg/mL | |
| N | 109 | 59 | 25 | 84 | 94 | 15 |
| % | 56.5 | 30.6 | 12.9 | 43.5 | 48.7 | 7.8 |
| P25 | 4.76 | 4.28 | 4.02 | 3.49 | 7.48 | 17.46 |
| Median | 5.02a | 4.84b | 4.23c | 3.52a | 7.53b | 17.52c |
| P75 | 5.34 | 5.23 | 4.29 | 4.76 | 8.82 | 18.66 |
| p-value | <0.001 * | <0.001 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, R.d.S.; de Souza Silva, C.M.; Ferreira Fratelli, C.; Ramos de Lima, L.; Morato Stival, M.; Schwerz Funghetto, S.; Rodrigues da Silva, I.C.; Vieira de Andrade, R. IL-10 and IL-1β Serum Levels, Genetic Variants, and Metabolic Syndrome: Insights into Older Adults’ Clinical Characteristics. Nutrients 2024, 16, 1241. https://doi.org/10.3390/nu16081241
Freitas RdS, de Souza Silva CM, Ferreira Fratelli C, Ramos de Lima L, Morato Stival M, Schwerz Funghetto S, Rodrigues da Silva IC, Vieira de Andrade R. IL-10 and IL-1β Serum Levels, Genetic Variants, and Metabolic Syndrome: Insights into Older Adults’ Clinical Characteristics. Nutrients. 2024; 16(8):1241. https://doi.org/10.3390/nu16081241
Chicago/Turabian StyleFreitas, Renata de Souza, Calliandra Maria de Souza Silva, Caroline Ferreira Fratelli, Luciano Ramos de Lima, Marina Morato Stival, Silvana Schwerz Funghetto, Izabel Cristina Rodrigues da Silva, and Rosângela Vieira de Andrade. 2024. "IL-10 and IL-1β Serum Levels, Genetic Variants, and Metabolic Syndrome: Insights into Older Adults’ Clinical Characteristics" Nutrients 16, no. 8: 1241. https://doi.org/10.3390/nu16081241








