Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review
Abstract
:1. Introduction
2. Post-Resection Changes and Complications in SBS
2.1. Intestinal Adaptation
2.2. Small Bowel Bacterial Overgrowth (SBBO)
2.3. Blood Stream Infection
2.4. Intestinal Failure Associated Liver Disease (IFALD)
2.5. Probiotics
2.5.1. Role in Gut Maturation and Adaptation
2.5.2. Enhancement of Gut Barrier Function
2.5.3. Suppression of Pathogens
2.5.4. Immune Modulating Effects
2.5.5. Effect on IFALD
| Animal model used | Probiotic used | Results | |
|---|---|---|---|
| Eizaguirre et al. [63] | Adult Wistar rats (80% bowel resection) | Bifidobacterium lactis | BT rate in SBS group 87% vs. 50% in SBS-Probiotic group (p < 0.05) (RRR was 0.43) |
| Garcia-Urkia et al. [64] | Adult Wistar rats (80% bowel resection) | Bifidobacterium lactis | BT rate in SBS probiotic group 44% vs. 93% in non-probiotic group |
| Mogilner et al. [65] | Sprague-Dawley rats (75% bowel resection) | Lactobacillus GG | BT to liver (60% vs. 40%); BT to peripheral blood (40% vs. 20%). SBS-Probiotic rats showed a significant increase in crypt depth in ileum and a mild decrease in apoptotic index in jejunum and ileum |
| Eizaguirre [66]. | Adult Wistar rats (80% bowel resection) | Bifidobacterium lactis | BT in probiotic group 35% vs. 67% in non-probiotic group. Intestinal epithelial proliferation index and proliferation to apoptosis rate higher in probiotic group |
| Muftoglu et al. [67] | Wistar-Albino rats (75% intestinal resection) | Lactobacillus acidophilus, Bifidobacteria, Streptococcus thermophilus | Intestinal diameter, mitotic index, villus length, crypt depth, goblet cell count and immunohistochemical staining for trophic effect significantly increased in jejunum of the SBS-Probiotic group and insignificant increase in ileum |
| Eizaguirre et al. [68] | Adult Wistar rats (80% bowel resection) | Bifidobacterium lactis | BT (E. coli) rate of 33% (bacterial culture and PCR) as against a rate of 73% by bacterial culture and 87% by PCR in non-probiotic group |
| Category | Criteria |
|---|---|
| Study design | RCT, quasi-RCT |
| Participants | Infants and children with SBS |
| Interventions | Oral probiotics of any strain, dose or duration, in any form |
| Comparisons | Probiotics in conjunction with conventional treatment vs. conventional treatment with or without placebo |
| Outcomes | Primary: time to full enteral feeds, duration of parenteral nutrition support, growth parameters (weight, height), survival |
| Secondary: episodes SBBO, episodes of enterocolitis, episodes of culture proven sepsis, adverse effects of probiotics |
| Search terminologies | Yield |
|---|---|
| Pubmed: “Short Bowel Syndrome” [Mesh] AND “Probiotics” [Mesh]. | 25 |
| Pubmed: “Lactobacillus” [Mesh] AND “Short Bowel Syndrome” [Mesh]. | 26 |
| Pubmed: “Short Bowel Syndrome” [Mesh] AND “Bifidobacterium” [Mesh]. | 10 |
| Embase: “Short bowel syndrome” AND “Bifidobacterium OR Lactobacillus OR probiotic agent OR probiotics” | 93 |
| Final yield after removing overlapping articles | 67 |
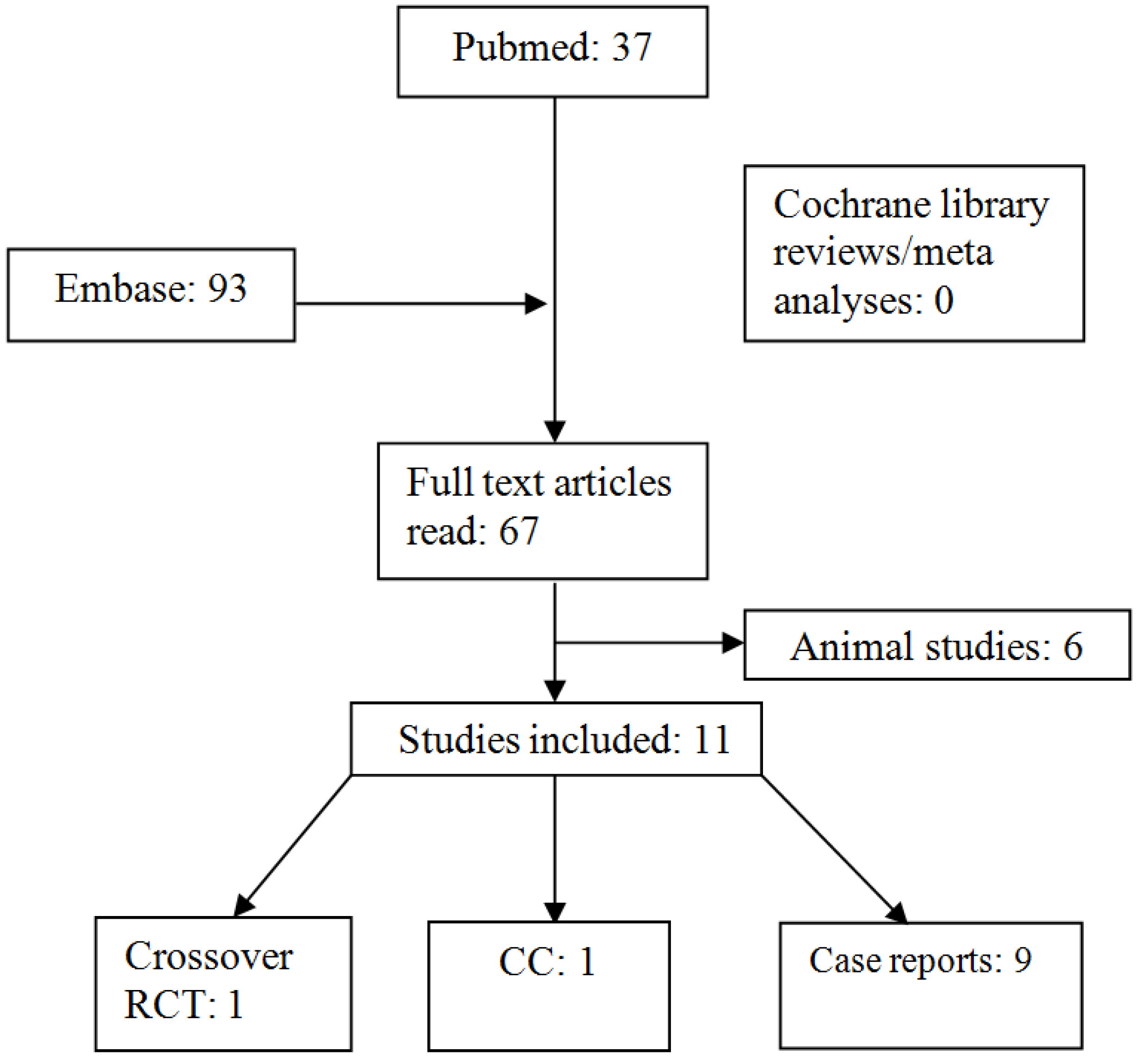
3. Results
| Type of study | Age at start of probiotic therapy | Age at bowel resection | Cause of SBS/Small intestine length | Problem before starting probiotics | Probiotics used | Clinical effects reported | |
|---|---|---|---|---|---|---|---|
| Uchida et al. (2007) [71] | Case control study Objective: study immunonutritional effects (prealbumin lymphocyte count); faecal flora, faecal SCFA, weight and height velocity after synbiotic therapy in SBS | (1) 2 year | <1 month | (1) Jejunal atresia, 40 cm | Growth retardation home parenteral nutrition abnormal faecal flora | Bifidobacterium breve Yakult Lactobacillus casei Shirota galactooligosaccharides |
|
| Vanderhoof et al. (1998) [72] | Case report | (1) 7 year | Infancy | (1) Midgut volvulus | SBBO diarrhoea abdominal distension | Lactobacillus plantarum 299V |
|
| (2) 14 year | 5 year | (2) Midgut volvulus | SBBO diarrhoea abdominal distension arthritis PN | Lactobacillus plantarum 299V Lactobacillus GG | |||
| Kanamori et al. (2001) [73] | Case report | (1) 2 year | 1 day | (1) Gastroschisis, 25 cm | enterocolitis, metabolic acidosis and fever episodes poor growth | Bifidobacterium breve Yakult Lactobacillus casei Shirota galactooligosaccharides |
|
| Kanamori et al. (2004) [74] | Case series | (1) 1 year 3 month | (1) Hirschsprung’s disease | Refractory enterocolitis in all central venous catheter sepsis abnormal intestinal flora | Bifidobacterium breve Yakult Lactobacillus casei Shirota galactooligosaccharides |
| |
| (2) 1 year 4 month | (2) Refractory enterocolitis, 85 cm | TPN | |||||
| (3) 2 year 2 moth | (3) Malrotation, 15 cm | PN | |||||
| (4) 3 year 4 month | (4) Gastroschisis, 25 cm | TPN | |||||
| (5) 4 year 8 month | (5) Hirschsprung’s disease, 100 cm | ||||||
| (6) 7 year | (6) Hirschsprung’s disease, 140 cm | ||||||
| (7) 20 year 8 month | (7) Malrotation, 60 cm | ||||||
| Shiau et al. (2007) [75] | Case report | (1) 1 month | (1) Midgut volvulus, 10 cm | Diarrhoea PN | Lactobacillus acidophilus Bifidobacterium infanti |
| |
| Candy et al. (2001) [76] | Case report | (1) 11 month | <1 month | (1) NEC, 60 cm | SBBO diarrhoea abnormal small bowel flora negative Na+ balance extremely low urine sodium 8 ± 5 mmol/L | Lactobacillus casei Shirota |
|
3.1. Case Reports on Clinical Benefits of Probiotics in SBS
3.2. Case Reports on Complications of Probiotics in SBS
4. Discussion
5. Conclusions
Conflict of Interest
References
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16–S28. [Google Scholar] [CrossRef]
- O’Keefe, S.J.; Buchman, A.L.; Fishbein, T.M.; Jeejeebhoy, K.N.; Jeppesen, P.B.; Shaffer, J. Short bowel syndrome and intestinal failure: Consensus definitions and overview. Clin. Gastroenterol. Hepatol. 2006, 4, 6–10. [Google Scholar] [CrossRef]
- Diamanti, A.; Basso, M.S.; Castro, M.; Di Ciommo, V.; Bracci, F.; Ferretti, F.; Pietrobattista, A.; Gambarara, M. Irreversible intestinal failure: Prevalence and prognostic factors. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 450–457. [Google Scholar] [CrossRef]
- Grant, D. Intestinal transplantation: 1997 report of the international registry. Transplantation 1999, 67, 1061–1064. [Google Scholar] [CrossRef]
- Spencer, A.U.; Kovacevich, D.; McKinney-Barnett, M.; Hair, D.; Canham, J.; Maksym, C.; Teitelbaum, D.H. Pediatric short-bowel syndrome: The cost of comprehensive care. Am. J. Clin. Nutr. 2008, 88, 1552–1559. [Google Scholar] [CrossRef]
- Wales, P.W.; de Silva, N.; Kim, J.H.; Lecce, L.; Sandhu, A.; Moore, A.M. Neonatal short bowel syndrome: A cohort study. J. Pediatr. Surg. 2005, 40, 755–762. [Google Scholar] [CrossRef]
- Spencer, A.U.; Neaga, A.; West, B.; Safran, J.; Brown, P.; Btaiche, I.; Kuzma-O’Reilly, B.; Teitelbaum, D.H. Pediatric short bowel syndrome: Redefining predictors of success. Ann. Surg. 2005, 242, 403–409; discussion 409–412. [Google Scholar]
- Wales, P.W.; Christison-Lagay, E.R. Short bowel syndrome: Epidemiology and etiology. Semin. Pediatr. Surg. 2010, 19, 3–9. [Google Scholar] [CrossRef]
- Salvia, G.; Guarino, A.; Terrin, G.; Cascioli, C.; Paludetto, R.; Indrio, F.; Lega, L.; Fanaro, S.; Stronati, M.; Corvaglia, L.; et al. Neonatal onset intestinal failure: An Italian multicenter study. J. Pediatr. 2008, 153, 674–676. [Google Scholar] [CrossRef]
- Cole, C.R.; Hansen, N.I.; Higgins, R.D.; Ziegler, T.R.; Stoll, B.J. Very low birth weight preterm infants with surgical short bowel syndrome: Incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008, 122, e573–e582. [Google Scholar] [CrossRef]
- Wales, P.W.; de Silva, N.; Kim, J.; Lecce, L.; To, T.; Moore, A. Neonatal short bowel syndrome: Population-based estimates of incidence and mortality rates. J. Pediatr. Surg. 2004, 39, 690–695. [Google Scholar] [CrossRef]
- Martinez, M.; Fabeiro, M.; Dalieri, M.; Barcellandi, P.; Prozzi, M.; Hernandez, J.; Alberti, M.; Fernandez, A. Outcome and survival of pediatric short bowel syndrome (SBS). Nutr. Hosp. 2011, 26, 239–242. [Google Scholar]
- Cole, C.R.; Frem, J.C.; Schmotzer, B.; Gewirtz, A.T.; Meddings, J.B.; Gold, B.D.; Ziegler, T.R. The rate of bloodstream infection is high in infants with short bowel syndrome: Relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J. Pediatr. 2010, 156, 941–947. [Google Scholar] [CrossRef]
- Olieman, J.F.; Poley, M.J.; Gischler, S.J.; Penning, C.; Escher, J.C.; van den Hoonaard, T.L.; van Goudoever, J.B.; Bax, N.M.; Tibboel, D.; IJsselstijn, H. Interdisciplinary management of infantile short bowel syndrome: Resource consumption, growth, and nutrition. J. Pediatr. Surg. 2010, 45, 490–498. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Siplovich, L.; Shiloni, E.; Mor-Vaknin, N.; Harmon, C.M.; Coran, A.G. Intestinal adaptation in short-bowel syndrome in infants and children: A collective review. Pediatr. Surg. Int. 2002, 18, 258–263. [Google Scholar] [CrossRef]
- Weale, A.R.; Edwards, A.G.; Bailey, M.; Lear, P.A. Intestinal adaptation after massive intestinal resection. Postgrad. Med. J. 2005, 81, 178–184. [Google Scholar] [CrossRef]
- Cole, C.R.; Ziegler, T.R. Small bowel bacterial overgrowth: A negative factor in gut adaptation in pediatric SBS. Curr. Gastroenterol. Rep. 2007, 9, 456–462. [Google Scholar] [CrossRef]
- Serrano, M.S.; Schmidt-Sommerfeld, E. Nutrition support of infants with short bowel syndrome. Nutrition 2002, 18, 966–970. [Google Scholar] [CrossRef]
- Kaufman, S.S.; Loseke, C.A.; Lupo, J.V.; Young, R.J.; Murray, N.D.; Pinch, L.W.; Vanderhoof, J.A. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J. Pediatr. 1997, 131, 356–361. [Google Scholar] [CrossRef]
- D’Antiga, L.; Dhawan, A.; Davenport, M.; Mieli-Vergani, G.; Bjarnason, I. Intestinal absorption and permeability in paediatric short-bowel syndrome: A pilot study. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 588–593. [Google Scholar]
- Kurkchubasche, A.G.; Smith, S.D.; Rowe, M.I. Catheter sepsis in short-bowel syndrome. Arch. Surg. 1992, 127, 21–24; discussion 24–25. [Google Scholar] [CrossRef]
- Terra, R.M.; Plopper, C.; Waitzberg, D.L.; Cukier, C.; Santoro, S.; Martins, J.R.; Song, R.J.; Gama-Rodrigues, J. Remaining small bowel length: Association with catheter sepsis in patients receiving home total parenteral nutrition: Evidence of bacterial translocation. World J. Surg. 2000, 24, 1537–1541. [Google Scholar] [CrossRef]
- Kelly, D.A. Intestinal failure-associated liver disease: What do we know today? Gastroenterology 2006, 130, S70–S77. [Google Scholar] [CrossRef]
- Sondheimer, J.M.; Asturias, E.; Cadnapaphornchai, M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 131–137. [Google Scholar]
- Carter, B.A.; Shulman, R.J. Mechanisms of disease: Update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 277–287. [Google Scholar] [CrossRef]
- Kelly, D.A. Preventing parenteral nutrition liver disease. Early Hum. Dev. 2010, 86, 683–687. [Google Scholar] [CrossRef]
- Shanahan, F. The host-microbe interface within the gut. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 915–931. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef]
- Barc, M.C.; Charrin-Sarnel, C.; Rochet, V.; Bourlioux, F.; Sandre, C.; Boureau, H.; Dore, J.; Collignon, A. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: Influence of Saccharomyces boulardii. Anaerobe 2008, 14, 229–233. [Google Scholar] [CrossRef]
- Koruda, M.J.; Rolandelli, R.H.; Settle, R.G.; Zimmaro, D.M.; Rombeau, J.L. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology 1988, 95, 715–720. [Google Scholar]
- Tappenden, K.A.; Thomson, A.B.; Wild, G.E.; McBurney, M.I. Short-chain fatty acid-supplemented total parenteral nutrition enhances functional adaptation to intestinal resection in rats. Gastroenterology 1997, 112, 792–802. [Google Scholar] [CrossRef]
- Tappenden, K.A.; McBurney, M.I. Systemic short-chain fatty acids rapidly alter gastrointestinal structure, function, and expression of early response genes. Dig. Dis. Sci. 1998, 43, 1526–1536. [Google Scholar] [CrossRef]
- Bartholome, A.L.; Albin, D.M.; Baker, D.H.; Holst, J.J.; Tappenden, K.A. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J. Parenter. Enteral. Nutr. 2004, 28, 210–222; discussion 222–223. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Fasano, A.; Nataro, J.P. Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins. Adv. Drug Deliv. Rev. 2004, 56, 795–807. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; de Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef]
- Parassol, N.; Freitas, M.; Thoreux, K.; Dalmasso, G.; Bourdet-Sicard, R.; Rampal, P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res. Microbiol. 2005, 156, 256–262. [Google Scholar] [CrossRef]
- Otte, J.M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006, 130, 731–746. [Google Scholar] [CrossRef]
- Mangell, P.; Nejdfors, P.; Wang, M.; Ahrne, S.; Westrom, B.; Thorlacius, H.; Jeppsson, B. Lactobacillus plantarum 299V inhibits escherichia coli-induced intestinal permeability. Dig. Dis. Sci. 2002, 47, 511–516. [Google Scholar] [CrossRef]
- Ewaschuk, J.; Endersby, R.; Thiel, D.; Diaz, H.; Backer, J.; Ma, M.; Churchill, T.; Madsen, K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology 2007, 46, 841–850. [Google Scholar] [CrossRef]
- Sherman, P.M.; Johnson-Henry, K.C.; Yeung, H.P.; Ngo, P.S.; Goulet, J.; Tompkins, T.A. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect. Immun. 2005, 73, 5183–5188. [Google Scholar] [CrossRef]
- Lee, Y.K.; Puong, K.Y.; Ouwehand, A.C.; Salminen, S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 2003, 52, 925–930. [Google Scholar] [CrossRef]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef]
- Caballero-Franco, C.; Keller, K.; de Simone, C.; Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G315–G322. [Google Scholar] [CrossRef]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate mapks and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar]
- Schlee, M.; Harder, J.; Koten, B.; Stange, E.F.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 2008, 151, 528–535. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.H.; Lievin-Le Moal, V.; Servin, A.L. Ph-, lactic acid-, and non-lactic acid-dependent activities of probiotic lactobacilli against Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013. [Google Scholar] [CrossRef]
- Lievin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.R.; Servin, A.L. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef]
- Ogawa, M.; Shimizu, K.; Nomoto, K.; Tanaka, R.; Hamabata, T.; Yamasaki, S.; Takeda, T.; Takeda, Y. Inhibition of in vitro growth of shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 2001, 68, 135–140. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczynski, M.; Radzikowski, A. Probiotics in the prevention of antibiotic-associated diarrhea in children: A meta-analysis of randomized controlled trials. J. Pediatr. 2006, 149, 367–372. [Google Scholar] [CrossRef]
- Yasui, H.; Nagaoka, N.; Mike, A.; Hayakawa, K.; Ohwaki, M. Detection of Bifidobacterium strains that induce large quantities of IGA. Microb. Ecol. Health Dis. 1992, 5, 155–162. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigon, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Shu, Q.; Gill, H.S. A dietary probiotic (Bifidobacterium lactis HN019) reduces the severity of Escherichia coli O157:H7 infection in mice. Med. Microbiol. Immunol. 2001, 189, 147–152. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; Nanno, M.; Okumura, K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; des Robert, C.; Fang, M.; Liboni, K.; McMahon, R.; Caicedo, R.A.; Neu, J. Lactobacillus Rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 545–552. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar]
- Segawa, S.; Wakita, Y.; Hirata, H.; Watari, J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int. J. Food Microbiol. 2008, 128, 371–377. [Google Scholar] [CrossRef]
- Eizaguirre, I.; Urkia, N.G.; Asensio, A.B.; Zubillaga, I.; Zubillaga, P.; Vidales, C.; Garcia-Arenzana, J.M.; Aldazabal, P. Probiotic supplementation reduces the risk of bacterial translocation in experimental short bowel syndrome. J. Pediatr. Surg. 2002, 37, 699–702. [Google Scholar] [CrossRef]
- Garcia-Urkia, N.; Asensio, A.B.; Zubillaga Azpiroz, I.; Zubillaga Huici, P.; Vidales, C.; Garcia-Arenzana, J.M.; Aldazabal, P.; Eizaguirre, I. Beneficial effects of Bifidobacterium lactis in the prevention of bacterial translocation in experimental short bowel syndrome. Cir. Pediatr. 2002, 15, 162–165. [Google Scholar]
- Mogilner, J.G.; Srugo, I.; Lurie, M.; Shaoul, R.; Coran, A.G.; Shiloni, E.; Sukhotnik, I. Effect of probiotics on intestinal regrowth and bacterial translocation after massive small bowel resection in a rat. J. Pediatr. Surg. 2007, 42, 1365–1371. [Google Scholar] [CrossRef]
- Eizaguirre, I.; Garcia Urkia, N.; Asensio, A.B.; Hijona, E.; Garcia Arenzana, J.M.; Bachiller, P.; Aldazabal, P. Adaptation in the small intestine: Effect of minimal enteral nutrition and probiotics on proliferation and apoptosis in the intestinal wall. Cir. Pediatr. 2010, 23, 118–121. [Google Scholar]
- Tolga Muftuoglu, M.A.; Civak, T.; Cetin, S.; Civak, L.; Gungor, O.; Saglam, A. Effects of probiotics on experimental short-bowel syndrome. Am. J. Surg. 2011, 202, 461–468. [Google Scholar] [CrossRef]
- Eizaguirre, I.; Aldazabal, P.; Urkia, N.G.; Asensio, A.; Arenzxana, J.M. Escherichia coli translocation in experimental short bowel syndrome: Probiotic supplementation and detection by polymerase chain reaction. Pediatr. Surg. Int. 2011, 27, 1301–1305. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011); The Cochrane Collaboration, 2011. Available online: http://www.cochrane-handbook.org (accessed on 5 February 2013).
- ClinicalTrials Web site. Available online: http://www.clinicaltrials.gov/ (accessed on 5 February 2013).
- Uchida, K.; Takahashi, T.; Inoue, M.; Morotomi, M.; Otake, K.; Nakazawa, M.; Tsukamoto, Y.; Miki, C.; Kusunoki, M. Immunonutritional effects during synbiotics therapy in pediatric patients with short bowel syndrome. Pediatr. Surg. Int. 2007, 23, 243–248. [Google Scholar] [CrossRef]
- Vanderhoof, J.A.; Young, R.J.; Murray, N.; Kaufman, S.S. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 155–160. [Google Scholar] [CrossRef]
- Kanamori, Y.; Hashizume, K.; Sugiyama, M.; Morotomi, M.; Yuki, N. Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: A novel synbiotics therapy for intestinal failure. Dig. Dis. Sci. 2001, 46, 2010–2016. [Google Scholar] [CrossRef]
- Kanamori, Y.; Sugiyama, M.; Hashizume, K.; Yuki, N.; Morotomi, M.; Tanaka, R. Experience of long-term synbiotic therapy in seven short bowel patients with refractory enterocolitis. J. Pediatr. Surg. 2004, 39, 1686–1692. [Google Scholar] [CrossRef]
- Shiau, S.L.; Su, B.H.; Lin, K.J.; Lin, H.C.; Lin, J.N. Possible effect of probiotics and breast milk in short bowel syndrome: Report of one case. Acta Paediatr. Taiwan 2007, 48, 89–92. [Google Scholar]
- Candy, D.C.; Densham, L.; Lamont, L.S.; Greig, M.; Lewis, J.; Bennett, H.; Griffiths, M. Effect of administration of Lactobacillus casei Shirota on sodium balance in an infant with short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 506–508. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Cohran, V.; Korff, S.; Sullivan, C.; Iyer, K.; Zheng, X. Intestinal permeability and effects of Lactobacillus Rhamnosus therapy in children with short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 41–47. [Google Scholar] [CrossRef]
- Kunz, A.N.; Noel, J.M.; Fairchok, M.P. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 457–458. [Google Scholar] [CrossRef]
- de Groote, M.A.; Frank, D.N.; Dowell, E.; Glode, M.P.; Pace, N.R. Lactobacillus Rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005, 24, 278–280. [Google Scholar] [CrossRef]
- Munakata, S.; Arakawa, C.; Kohira, R.; Fujita, Y.; Fuchigami, T.; Mugishima, H. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev. 2010, 32, 691–694. [Google Scholar] [CrossRef]
- Ku, W.H.; Lau, D.C.; Huen, K.F. Probiotics provoked D-lactic acidosis in short bowel syndrome: Case report and literature review. Hong Kong J. Paediatr. 2006, 11, 246–254. [Google Scholar]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar]
- Apostolou, E.; Kirjavainen, P.V.; Saxelin, M.; Rautelin, H.; Valtonen, V.; Salminen, S.J.; Ouwehand, A.C. Good adhesion properties of probiotics: A potential risk for bacteremia? FEMS Immunol. Med. Microbiol. 2001, 31, 35–39. [Google Scholar] [CrossRef]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M.; et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 2010, 156, 679–681. [Google Scholar] [CrossRef]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef]
- Uchida, H.; Yamamoto, H.; Kisaki, Y.; Fujino, J.; Ishimaru, Y.; Ikeda, H. D-lactic acidosis in short-bowel syndrome managed with antibiotics and probiotics. J. Pediatr. Surg. 2004, 39, 634–636. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264; quiz 1446–1447. [Google Scholar]
- Haschke-Becher, E.; Brunser, O.; Cruchet, S.; Gotteland, M.; Haschke, F.; Bachmann, C. Urinary D-lactate excretion in infants receiving Lactobacillus johnsonii with formula. Ann. Nutr. Metab. 2008, 53, 240–244. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reddy, V.S.; Patole, S.K.; Rao, S. Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review. Nutrients 2013, 5, 679-699. https://doi.org/10.3390/nu5030679
Reddy VS, Patole SK, Rao S. Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review. Nutrients. 2013; 5(3):679-699. https://doi.org/10.3390/nu5030679
Chicago/Turabian StyleReddy, Vudum S., Sanjay K. Patole, and Shripada Rao. 2013. "Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review" Nutrients 5, no. 3: 679-699. https://doi.org/10.3390/nu5030679
APA StyleReddy, V. S., Patole, S. K., & Rao, S. (2013). Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review. Nutrients, 5(3), 679-699. https://doi.org/10.3390/nu5030679




