Nutritional Strategies for the Preservation of Fat Free Mass at High Altitude
Abstract
:1. Introduction
2. Weight Loss and Body Composition Changes with Altitude Exposure
Body Composition Changes
| Reference | N | Conditions | Altitude (m) | Days at Altitude | Total Weight Loss (kg) | Body Composition Method | Body Fat Loss (kg) | FFM * Loss (kg) | % Weight Loss as FFM |
|---|---|---|---|---|---|---|---|---|---|
| Boyer and Blume [38] | 14 ** | Field | <5400 >5400 | 23 26 | 1.9 4.0 | Skinfolds | 1.34 1.2 | 0.56 2.8 | 29.5 70.0 |
| Rose et al. [1] | 8 males | Hypobaric chamber | Up to 8846 | 38 | 7.4 | Densitometry | 2.51 | 5.05 | 66.8 |
| Fulco et al. [35] | 16 males | Field | 3700–4300 | 16 | 5.9 | Densitometry Skinfolds Bioelectrical impedance | 3.46 2.53 1.34 | 2.44 3.37 4.56 | 41.4 57.1 77.3 |
| Westerterp et al. [2] | 3 male 2 female | Field | 5300–8872 | 30 † | 2.2 | Skinfolds | 1.4 | 0.8 | 36.4 |
| Westerterp et al. [3] | 4 male 2 female | Field | 6542 | 21 | 4.9 | Skinfolds | 3.5 | 1.3 | 27.0 |
| Pulfrey and Jones [9] | 5 male 1 female | Field | 5900–8046 | 40 † | 3.7 | Skinfolds | 0.9 | 1.9 | 51.4 |
| Armellini et al. [8] | 10 male 2 female | Field | ≥4500 | 16 | 3.3 | Bioelectrical impedance | 2.2 | 1.1 | 33.3 |
| Tanner et al. [7] | 5 male | Field | 2200–4300 | 21 | 4.2 | Densitometry Skinfolds Magnetic resonance | 3.2 1.1 1.7 | 1.0 3.2 2.5 | 23.0 75.0 62.0 |
| Wing-Gaia et al. [37] ‡ | 10 male 8 female | Field | 2835–5364 | 13 | Control: 1.9 Leucine: 1.8 | Ultrasound skinfolds | 0.6 1.1 | 1.2 0.8 | 66.0 42.0 |
3. Potential Mechanisms of Altitude-Induced Muscle Wasting

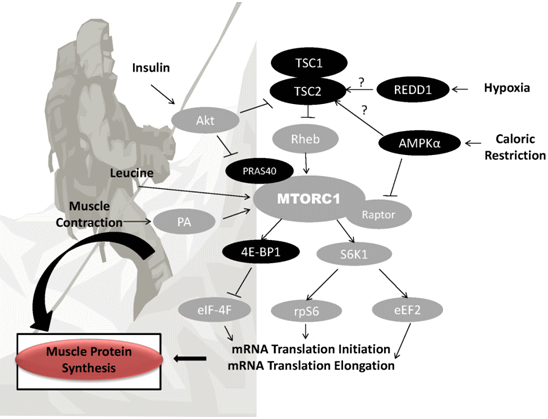
3.1. Hypoxia
3.2. Caloric Restriction and Suboptimal Protein Intake
4. Nutritional Strategies for Retention of FFM at Altitude
4.1. Protein and FFM Retention
4.2. Leucine and FFM Retention
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rose, M.S.; Houston, C.S.; Fulco, C.S.; Coates, G.; Sutton, J.R.; Cymerman, A. Operation Everest. II: Nutrition and body composition. J. Appl. Physiol. 1988, 65, 2545–2551. [Google Scholar]
- Westerterp, K.R.; Kayser, B.; Brouns, F.; Herry, J.P.; Saris, W.H. Energy expenditure climbing Mt. Everest. J. Appl. Physiol. 1992, 73, 1815–1819. [Google Scholar]
- Westerterp, K.R.; Kayser, B.; Wouters, L.; Le Trong, J.L.; Richalet, J.P. Energy balance at high altitude of 6542 m. J. Appl. Physiol. 1994, 77, 862–866. [Google Scholar]
- Westerterp, K.R.; Meijer, E.P.; Rubbens, M.; Robach, P.; Richalet, J.P. Operation Everest III: Energy and water balance. Pflug. Arch. 2000, 439, 483–488. [Google Scholar]
- Reynolds, R.D.; Lickteig, J.A.; Deuster, P.A.; Howard, M.P.; Conway, J.M.; Pietersma, A.; de Stoppelaar, J.; Deurenberg, P. Energy metabolism increases and regional body fat decreases while regional muscle mass is spared in humans climbing Mt. Everest. J. Nutr. 1999, 129, 1307–1314. [Google Scholar]
- Reynolds, R.D.; Lickteig, J.A.; Howard, M.P.; Deuster, P.A. Intakes of high fat and high carbohydrate foods by humans increased with exposure to increasing altitude during an expedition to Mt. Everest. J. Nutr. 1998, 128, 50–55. [Google Scholar]
- Tanner, D.A.; Stager, J.M. Partitioned weight loss and body composition changes during a mountaineering expedition: A field study. Wilderness Environ. Med. 1998, 9, 143–152. [Google Scholar]
- Armellini, F.; Zamboni, M.; Robbi, R.; Todesco, T.; Bissoli, L.; Mino, A.; Angelini, G.; Micciolo, R.; Bosello, O. The effects of high altitude trekking on body composition and resting metabolic rate. Horm. Metab. Res. 1997, 29, 458–461. [Google Scholar] [CrossRef]
- Pulfrey, S.M.; Jones, P.J. Energy expenditure and requirement while climbing above 6000 m. J. Appl. Physiol. 1996, 81, 1306–1311. [Google Scholar]
- Hoppeler, H.; Kleinert, E.; Schlegel, C.; Claassen, H.; Howald, H.; Kayar, S.R.; Cerretelli, P. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int. J. Sports Med. 1990, 11, S3–S9. [Google Scholar] [CrossRef]
- Sergi, G.; Imoscopi, A.; Sarti, S.; Perissinotto, E.; Coin, A.; Inelmen, E.M.; Zambon, S.; Busetto, L.; Seresin, C.; Manzato, E. Changes in total body and limb composition and muscle strength after a 6–8 weeks sojourn at extreme altitude (5000–8000 m). J. Sports Med. Phys. Fit. 2010, 50, 450–455. [Google Scholar]
- Fulco, C.S.; Rock, P.B.; Cymerman, A. Maximal and submaximal exercise performance at altitude. Aviat. Space Environ. Med. 1998, 69, 793–801. [Google Scholar]
- Murdoch, D.R. Symptoms of infection and altitude illness among hikers in the Mount Everest region of Nepal. Aviat. Space Environ. Med. 1995, 66, 148–151. [Google Scholar]
- Westerterp-Plantenga, M.S.; Westerterp, K.R.; Rubbens, M.; Verwegen, C.R.; Richelet, J.P.; Gardette, B. Appetite at “high altitude” [Operation Everest III (Comex-’97)]: A simulated ascent of Mount Everest. J. Appl. Physiol. 1999, 87, 391–399. [Google Scholar]
- Hamad, N.; Travis, S.P. Weight loss at high altitude: Pathophysiology and practical implications. Eur. J. Gastroenterol. Hepatol. 2006, 18, 5–10. [Google Scholar]
- Kayser, B. Nutrition and energetics of exercise at altitude. Theory and possible practical implications. Sports Med. 1994, 17, 309–323. [Google Scholar] [CrossRef]
- Butterfield, G.E.; Gates, J.; Fleming, S.; Brooks, G.A.; Sutton, J.R.; Reeves, J.T. Increased energy intake minimizes weight loss in men at high altitude. J. Appl. Physiol. 1992, 72, 1741–1748. [Google Scholar]
- Kayser, B.; Narici, M.; Milesi, S.; Grassi, B.; Cerretelli, P. Body composition and maximum alactic anaerobic performance during a one month stay at high altitude. Int. J. Sports Med. 1993, 14, 244–247. [Google Scholar]
- Consolazio, C.F.; Johnson, H.L.; Krzywicki, H.J.; Daws, T.A. Metabolic aspects of acute altitude exposure (4300 m) in adequately nourished humans. Am. J. Clin. Nutr. 1972, 25, 23–29. [Google Scholar]
- Richard, N.A.; Koehle, M.S. Differences in cardio-ventilatory responses to hypobaric and normobaric hypoxia: A review. Aviat. Space Environ. Med. 2012, 83, 677–684. [Google Scholar] [CrossRef]
- Degache, F.; Larghi, G.; Faiss, R.; Deriaz, O.; Millet, G. Hypobaric versus normobaric hypoxia: Same effects on postural stability? High Alt. Med. Biol. 2012, 13, 40–45. [Google Scholar] [CrossRef]
- Faiss, R.; Pialoux, V.; Sartori, C.; Faes, C.; Deriaz, O.; Millet, G.P. Ventilation, oxidative stress, and nitric oxide in hypobaric versus normobaric hypoxia. Med. Sci. Sports Exerc. 2013, 45, 253–260. [Google Scholar] [CrossRef]
- Fulco, C.S.; Beidleman, B.A.; Muza, S.R. Effectiveness of preacclimatization strategies for high-altitude exposure. Exerc. Sport Sci. Rev. 2013, 41, 55–63. [Google Scholar] [CrossRef]
- Savourey, G.; Launay, J.C.; Besnard, Y.; Guinet, A.; Travers, S. Normo- and hypobaric hypoxia: Are there any physiological differences? Eur. J. Appl. Physiol. 2003, 89, 122–126. [Google Scholar] [CrossRef]
- Aeberli, I.; Erb, A.; Spliethoff, K.; Meier, D.; Gotze, O.; Fruhauf, H.; Fox, M.; Finlayson, G.S.; Gassmann, M.; Berneis, K.; et al. Disturbed eating at high altitude: Influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 2013, 52, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Snyder, E.M.; Carr, R.D.; Deacon, C.F.; Johnson, B.D. Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans. Appl. Physiol. Nutr. Metab. 2008, 33, 929–935. [Google Scholar]
- Barnholt, K.E.; Hoffman, A.R.; Rock, P.B.; Muza, S.R.; Fulco, C.S.; Braun, B.; Holloway, L.; Mazzeo, R.S.; Cymerman, A.; Friedlander, A.L. Endocrine responses to acute and chronic high-altitude exposure (4300 m): Modulating effects of caloric restriction. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1078–E1088. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Saris, W.H.; van Es, M.; Ten Hoor, F. Use of the doubly labeled water technique in humans during heavy sustained exercise. J. Appl. Physiol. 1986, 61, 2162–2167. [Google Scholar]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression 1. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar]
- Mettler, S.; Mitchell, N.; Tipton, K.D. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef]
- Pikosky, M.A.; Smith, T.J.; Grediagin, A.; Castaneda-Sceppa, C.; Byerley, L.; Glickman, E.L.; Young, A.J. Increased protein maintains nitrogen balance during exercise-induced energy deficit. Med. Sci. Sports Exerc. 2008, 40, 505–512. [Google Scholar] [CrossRef]
- Fulco, C.S.; Friedlander, A.L.; Muza, S.R.; Rock, P.B.; Robinson, S.; Lammi, E.; Baker-Fulco, C.J.; Lewis, S.F.; Cymerman, A. Energy intake deficit and physical performance at altitude. Aviat. Space Environ. Med. 2002, 73, 758–765. [Google Scholar]
- Fulco, C.S.; Hoyt, R.W.; Baker-Fulco, C.J.; Gonzalez, J.; Cymerman, A. Use of bioelectrical impedance to assess body composition changes at high altitude. J. Appl. Physiol. 1992, 72, 2181–2187. [Google Scholar]
- Muller, W.; Horn, M.; Furhapter-Rieger, A.; Kainz, P.; Kropfl, J.M.; Maughan, R.J.; Ahammer, H. Body composition in sport: A comparison of a novel ultrasound imaging technique to measure subcutaneous fat tissue compared with skinfold measurement. Br. J. Sports Med. 2013, 47, 1028–1035. [Google Scholar] [CrossRef]
- Wing-Gaia, S.L.; Gershenoff, D.C.; Drummond, M.J.; Askew, E.W. Effect of leucine supplementation on fat free mass with prolonged hypoxic exposure during a 13-day trek to Everest Base Camp: A double-blind randomized study. Appl. Physiol. Nutr. Metab. 2014, 39. [Google Scholar] [CrossRef]
- Boyer, S.J.; Blume, F.D. Weight loss and changes in body composition at high altitude. J. Appl. Physiol. 1984, 57, 1580–1585. [Google Scholar]
- Matthews, D.E. Proteins and Amino Acids. In Modern Nutrition and Health and Disease, 9th ed.; Shils, M.O.J., Shike, M., Ross, A., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1999; pp. 11–48. [Google Scholar]
- Welle, S.; Thornton, C.; Statt, M.; McHenry, B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am. J. Physiol. 1994, 267, E599–E604. [Google Scholar]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Skeletal muscle responses to negative energy balance: Effects of dietary protein. Adv. Nutr. 2012, 3, 119–126. [Google Scholar]
- Brugarolas, J.; Lei, K.; Hurley, R.L.; Manning, B.D.; Reiling, J.H.; Hafen, E.; Witters, L.A.; Ellisen, L.W.; Kaelin, W.G., Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004, 18, 2893–2904. [Google Scholar] [CrossRef] [Green Version]
- Favier, F.B.; Costes, F.; Defour, A.; Bonnefoy, R.; Lefai, E.; Bauge, S.; Peinnequin, A.; Benoit, H.; Freyssenet, D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1659–R1666. [Google Scholar] [CrossRef]
- Preedy, V.R.; Smith, D.M.; Sugden, P.H. The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem. J. 1985, 228, 179–185. [Google Scholar]
- Schnakenberg, D.D.; Krabill, L.F.; Weiser, P.C. The anorexic effect of high altitude on weight gain, nitrogen retention and body composition of rats. J. Nutr. 1971, 101, 787–796. [Google Scholar]
- Bigard, A.X.; Douce, P.; Merino, D.; Lienhard, F.; Guezennec, C.Y. Changes in dietary protein intake fail to prevent decrease in muscle growth induced by severe hypoxia in rats. J. Appl. Physiol. 1996, 80, 208–215. [Google Scholar]
- Vigano, A.; Ripamonti, M.; de Palma, S.; Capitanio, D.; Vasso, M.; Wait, R.; Lundby, C.; Cerretelli, P.; Gelfi, C. Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics 2008, 8, 4668–4679. [Google Scholar]
- Etheridge, T.; Atherton, P.J.; Wilkinson, D.; Selby, A.; Rankin, D.; Webborn, N.; Smith, K.; Watt, P.W. Effects of hypoxia on muscle protein synthesis and anabolic signaling at rest and in response to acute resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E697–E702. [Google Scholar] [CrossRef]
- Imoberdorf, R.; Garlick, P.J.; McNurlan, M.A.; Casella, G.A.; Marini, J.C.; Turgay, M.; Bartsch, P.; Ballmer, P.E. Skeletal muscle protein synthesis after active or passive ascent to high altitude. Med. Sci. Sports Exerc. 2006, 38, 1082–1087. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Yeckel, C.W.; Volpi, E.; Wolf, S.E.; Morio, B.; Chinkes, D.L.; Paddon-Jones, D.; Wolfe, R.R. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E513–E522. [Google Scholar]
- Chaudhary, P.; Suryakumar, G.; Prasad, R.; Singh, S.N.; Ali, S.; Ilavazhagan, G. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: Role of ubiquitin-proteasome pathway and calpains. Mol. Cell. Biochem. 2012, 364, 101–113. [Google Scholar] [CrossRef]
- Holm, L.; Haslund, M.L.; Robach, P.; van Hall, G.; Calbet, J.A.; Saltin, B.; Lundby, C. Skeletal muscle myofibrillar and sarcoplasmic protein synthesis rates are affected differently by altitude-induced hypoxia in native lowlanders. PLoS One 2010, 5, e15606. [Google Scholar]
- McIver, C.M.; Wycherley, T.P.; Clifton, P.M. MTOR signaling and ubiquitin-proteosome gene expression in the preservation of fat free mass following high protein, calorie restricted weight loss. Nutr. Metab. 2012, 9, 83. [Google Scholar]
- Pasiakos, S.M.; Vislocky, L.M.; Carbone, J.W.; Altieri, N.; Konopelski, K.; Freake, H.C.; Anderson, J.M.; Ferrando, A.A.; Wolfe, R.R.; Rodriguez, N.R. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J. Nutr. 2010, 140, 745–751. [Google Scholar]
- Carbone, J.W.; Margolis, L.M.; McClung, J.P.; Cao, J.J.; Murphy, N.E.; Sauter, E.R.; Combs, G.F., Jr.; Young, A.J.; Pasiakos, S.M. Effects of energy deficit, dietary protein, and feeding on intracellular regulators of skeletal muscle proteolysis. FASEB J. 2013, 27, 5104–5111. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef]
- Bigard, A.X.; Satabin, P.; Lavier, P.; Canon, F.; Taillandier, D.; Guezennec, C.Y. Effects of protein supplementation during prolonged exercise at moderate altitude on performance and plasma amino acid pattern. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 5–10. [Google Scholar]
- Macdonald, J.H.; Oliver, S.J.; Hillyer, K.; Sanders, S.; Smith, Z.; Williams, C.; Yates, D.; Ginnever, H.; Scanlon, E.; Roberts, E.; et al. Body composition at high altitude: A randomized placebo-controlled trial of dietary carbohydrate supplementation. Am. J. Clin. Nutr. 2009, 90, 1193–1202. [Google Scholar] [CrossRef]
- Tappy, L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod. Nutr. Dev. 1996, 36, 391–397. [Google Scholar] [CrossRef]
- Kayser, B.; Acheson, K.; Decombaz, J.; Fern, E.; Cerretelli, P. Protein absorption and energy digestibility at high altitude. J. Appl. Physiol. 1992, 73, 2425–2431. [Google Scholar]
- Potier, M.; Darcel, N.; Tome, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 54–58. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Kayser, B. Body mass regulation at altitude. Eur. J. Gastroenterol. Hepatol. 2006, 18, 1–3. [Google Scholar]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar]
- Lynch, C.J.; Patson, B.J.; Anthony, J.; Vaval, A.; Jefferson, L.S.; Vary, T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E503–E513. [Google Scholar]
- Nakashima, K.; Ishida, A.; Yamazaki, M.; Abe, H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem. Biophys. Res. Commun. 2005, 336, 660–666. [Google Scholar] [CrossRef]
- Norton, L.E.; Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Garlick, P.J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. 2012, 9, 67. [Google Scholar]
- Burke, L.M.; Winter, J.A.; Cameron-Smith, D.; Enslen, M.; Farnfield, M.; Decombaz, J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 452–462. [Google Scholar]
- Jitomir, J.; Willoughby, D.S. Leucine for retention of lean mass on a hypocaloric diet. J. Med. Food 2008, 11, 606–609. [Google Scholar] [CrossRef]
- Balage, M.; Dardevet, D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 265–270. [Google Scholar] [CrossRef]
- Peters, S.J.; van Helvoort, A.; Kegler, D.; Argiles, J.M.; Luiking, Y.C.; Laviano, A.; van Bergenhenegouwen, J.; Deutz, N.E.; Haagsman, H.P.; Gorselink, M.; et al. Dose-dependent effects of leucine supplementation on preservation of muscle mass in cancer cachectic mice. Oncol. Rep. 2011, 26, 247–254. [Google Scholar]
- Gleason, J.E.; Corrigan, D.J.; Cox, J.E.; Reddi, A.R.; McGinnis, L.A.; Culotta, V.C. Analysis of hypoxia and hypoxia-like states through metabolite profiling. PLoS One 2011, 6, e24741. [Google Scholar]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010, 140, 1970–1976. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Ferrando, A.A.; Aarsland, A.A.; Wolfe, R.R. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl. Physiol. Nutr. Metab. 2009, 34, 151–161. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McClung, H.L.; McClung, J.P.; Margolis, L.M.; Andersen, N.E.; Cloutier, G.J.; Pikosky, M.A.; Rood, J.C.; Fielding, R.A.; Young, A.J. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am. J. Clin. Nutr. 2011, 94, 809–818. [Google Scholar] [CrossRef]
- Coffey, V.G.; Moore, D.R.; Burd, N.A.; Rerecich, T.; Stellingwerff, T.; Garnham, A.P.; Phillips, S.M.; Hawley, J.A. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur. J. Appl. Physiol. 2011, 111, 1473–1483. [Google Scholar] [CrossRef]
- Bigard, A.X.; Lavier, P.; Ullmann, L.; Legrand, H.; Douce, P.; Guezennec, C.Y. Branched-chain amino acid supplementation during repeated prolonged skiing exercises at altitude. Int. J. Sport Nutr. 1996, 6, 295–306. [Google Scholar]
- Schena, F.; Guerrini, F.; Tregnaghi, P.; Kayser, B. Branched-chain amino acid supplementation during trekking at high altitude. The effects on loss of body mass, body composition, and muscle power. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 394–398. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wing-Gaia, S.L. Nutritional Strategies for the Preservation of Fat Free Mass at High Altitude. Nutrients 2014, 6, 665-681. https://doi.org/10.3390/nu6020665
Wing-Gaia SL. Nutritional Strategies for the Preservation of Fat Free Mass at High Altitude. Nutrients. 2014; 6(2):665-681. https://doi.org/10.3390/nu6020665
Chicago/Turabian StyleWing-Gaia, Stacie L. 2014. "Nutritional Strategies for the Preservation of Fat Free Mass at High Altitude" Nutrients 6, no. 2: 665-681. https://doi.org/10.3390/nu6020665