Pinus densiflora Sieb. et Zucc. Alleviates Lipogenesis and Oxidative Stress during Oleic Acid-Induced Steatosis in HepG2 Cells
Abstract
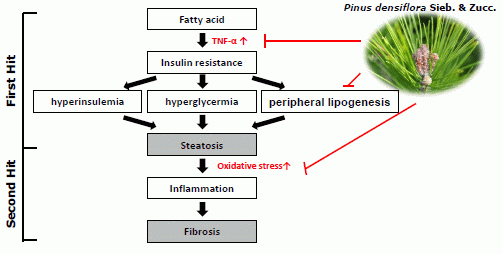
:1. Introduction
2. Experimental Section
2.1. Samples, Antibodies, and Reagents
2.2. OA/BSA Complex Solution Preparation
2.3. Cell Culture
2.4. Cytotoxicity
2.5. Staining Using Oil-red-O, BODIPY, and Nile Red
2.6. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analyses
| Gene | Forward | Reverse |
|---|---|---|
| SREBP-1 | 5′-GCGGAGCCATGGATTGCAC-3′ | 5′-TCTTCCTTGATACCAGGCCC-3′ |
| FAS | 5′-AGCTGCCAGAGTCGGAGAAC-3′ | 5′-TGTAGCCCACGAGTGTCTCG-3′ |
| SCD1 | 5′-CCAACACAATGGCATTCCAG-3′ | 5′-GGTGGTCACGAGCCCATTC-3′ |
| PPARγ | 5′-GAACAGATCCAGTGGTTGCAG-3′ | 5′-GGCATTATGAGACATCCCCAC-3′ |
| DGAT1 | 5′-GGCATCCTGAACTGGTGTGTG-3′ | 5′-GAGCTTGAGGAAGAGGATGGTG-3′ |
| ACC1 | 5′-GAGGGCTAGGTCTTTCTGGAAG-3′ | 5′-CCACAGTGAAATCTCGTTGAGA-3′ |
| PPARα | 5′-TCCGACTCCGTCTTCTTGAT-3′ | 5′-GCCTAAGGAAACCGTTCTGTG-3′ |
| CPT1 | 5′-TGAGCGACTGGTGGGAGGAG-3′ | 5′-GAGCCAGACCTTGAAGTAGCG-3′ |
| ACOX | 5′-TCCTGCCCACCTTGCTTCAC-3′ | 5′-TTGGGGCCGATGTCACCAAC-3′ |
| ACC2 | 5′-GCCAGAAGCCCCCAAGAAAC-3′ | 5′-CGACATGCTCGGCCTCATAG-3′ |
| TNFα | 5′-CAGCCTCTTCTCCTTCCTGAT-3′ | 5′-GCCAGAGGGCTGATTAGAGA-3′ |
2.7. Western Blot Analyses
2.8. Cytokine Determinations
2.9. Flow Cytometric Analyses of the Scavenging Activity of Intracellular ROS
2.10. Statistical Analyses
3. Results
3.1. Cytotoxic Effects of PSZ on HepG2 Cells

3.2. PSZ Reduces OA-Induced Steatosis in HepG2 Cells

3.3. PSZ Attenuates Hepatic Steatosis via Inhibition of Expression of Lipogenic Genes

3.4. PSZ Attenuates the Transcription Factors for Lipogenic Genes


3.5. PSZ Decreases Hepatic Inflammation by Attenuating TNFα Expression

3.6. PSZ Inhibits ROS Production

4. Discussion

5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Pais, R.; Ratziu, V. Epidemiology and natural history of nonalcoholic fatty liver disease. Rev. Prat. 2012, 62, 1416–1421. [Google Scholar]
- Tolman, K.G.; Dalpiaz, A.S. Treatment of non-alcoholic fatty liver disease. Ther. Clin. Risk Manag. 2007, 3, 1153–1163. [Google Scholar]
- Contos, M.J.; Choudhury, J.; Mills, A.S.; Sanyal, A.J. The histologic spectrum of nonalcoholic fatty liver disease. Clin. Liver. Dis. 2004, 8, 481–500. [Google Scholar] [CrossRef]
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar]
- Carter-Kent, C.; Zein, N.N.; Feldstein, A.E. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Am. J. Gastroenterol. 2008, 103, 1036–1042. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Lieber, C.S. CYP2E1: From ASH to NASH. Hepatol. Res. 2004, 28, 1–11. [Google Scholar]
- Mannaerts, G.P.; van Veldhoven, P.P.; Casteels, M. Peroxisomal lipid degradation via betaand alpha-oxidation in mammals. Cell Biochem. Biophys. 2000, 32, 73–87. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Morales, A.; Kaplowitz, N.; Fernandez-Checa, J.C. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: Studies with isolated mitochondria and rat hepatocytes. Mol. Pharmacol. 1995, 48, 825–834. [Google Scholar]
- Hensley, K.; Kotake, Y.; Sang, H.; Pye, Q.N.; Wallis, G.L.; Kolker, L.M.; Tabatabaie, T.; Stewart, C.A.; Konishi, Y.; Nakae, D.; et al. Dietary choline restriction causes complex I dysfunction and increased H(2)O(2) generation in liver mitochondria. Carcinogenesis 2000, 21, 983–989. [Google Scholar] [CrossRef]
- Park, H.J.; DiNatale, D.A.; Chung, M.Y.; Park, Y.K.; Lee, J.Y.; Koo, S.I.; O’Connor, M.; Manautou, J.E.; Bruno, R.S. Green tea extracts attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidants defences in ob/ob mice. J. Nutr. Biochem. 2011, 22, 393–400. [Google Scholar] [CrossRef]
- Shih, C.C.; Shlau, M.T.; Lin, C.H.; Wu, J.B. Momordica charantia ameliorates insulin resistance and dyslipidemia with altered hepatic glucose production and fatty acid synthesis and AMPK phosphorylation in high-fat-fed mice. Phytother. Res. 2014, 28, 363–371. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Sandeep Varma, R.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. In Vitro 2011, 27, 393–400. [Google Scholar]
- Kwon, J.H.; Kim, J.H.; Choi, S.E.; Park, K.H.; Lee, M.W. Inhibitory effects of phenolic compounds from needles of Pinus densiflora on nitric oxide and PGE2 production. Arch. Pharm. Res. 2010, 33, 2011–2016. [Google Scholar] [CrossRef]
- Jung, M.J.; Choi, J.H.; Chung, H.Y.; Jung, J.H.; Choi, J.S. A new C-methylated flavonoid glycoside from Pinus densiflora. Fitoterapia 2001, 72, 943–945. [Google Scholar] [CrossRef]
- Choi, E.M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 2007, 21, 471–475. [Google Scholar] [CrossRef]
- Ince, I.; Yesil-Celiktas, O.; Karabay-Yavasoglu, N.U.; Elgin, G. Effects of Pinus brutia bark extract and Pycnogenol® in a rat model of carrageenan induced inflammation. Phytomedicine 2009, 16, 1101–1104. [Google Scholar]
- Kwak, C.S.; Moon, S.C.; Lee, M.S. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora). Nutr. Cancer 2006, 56, 162–171. [Google Scholar] [CrossRef]
- Kim, C.S. A comparison study of antibacterial effects of pine needle extractson human skin pathogens. Kor. J. Aesthet Cosmetol. 2012, 10, 25–30. [Google Scholar]
- Kim, J.H.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kang, S.W.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells. Biosci. Biotechnol. Biochem. 2013, 77, 1595–1598. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhou, N.; Wang, X.; Liu, Q.; Bai, Y.; Bai, Y.; Liu, Z.; Yang, H.; Zou, J.; et al. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed. Rep. 2013, 1, 71–76. [Google Scholar]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in micefed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef]
- Cousin, S.P.; Hügl, S.R.; Wrede, C.E.; Kajio, H.; Myers, M.G.; Rhodes, C.J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-Induced deoxyribonucleic acid synthesis in the pancreatic beta-Cell Line INS-1. Endocrinology 2001, 142, 229–240. [Google Scholar]
- Hwang, Y.J.; Lee, E.J.; Kim, H.R.; Hwang, K.A. NF-κB-targeted anti-inflammatory activity of Prunella vulgaris var. lilacina in macrophages RAW 264.7. Int. J. Mol. Sci. 2013, 14, 21489–21503. [Google Scholar] [CrossRef]
- Hostanska, K.; Suter, A.; Melzer, J.; Saller, R.; Aller, R. Evaluation of cell death caused by an ethanolic extract of Serenoae repentis fructus (Prostasan®) on human carcinoma cell lines. Anticancer Res. 2007, 27, 873–882. [Google Scholar]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef]
- Gocze, P.M.; Freeman, D. A factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry 1994, 17, 151–158. [Google Scholar] [CrossRef]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes. Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-α expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef]
- Endo, M.; Masaki, T.; Seike, M.; Yoshimatsu, H. TNF-α induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp. Biol. Med. (Maywood) 2007, 232, 614–621. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Igata, M.; Motoshima, H.; Tsuruzoe, K.; Kojima, K.; Matsumura, T.; Kondo, T.; Taguchi, T.; Nakamaru, K.; Yano, M.; Kukidome, D.; et al. Adenosine monophosphateactivated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ. Res. 2005, 97, 837–844. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Huang, X.; Man, Y.; Zhou, Y.; Wang, S.; Wang, J.; Li, J. NOX3-derived reactive oxygen species promote TNF-α-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010, 584, 995–1000. [Google Scholar]
- Koek, G.H.; Liedorp, P.R.; Bast, A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 2011, 412, 1297–1305. [Google Scholar] [CrossRef]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar]
- Mavrogiannaki, A.N.; Migdalis, I.N. Nonalcoholic fatty liver disease, diabetes mellitus and cardiovascular disease: Newer Data. Int. J. Endocrinol. 2013, 2013, 450–639. [Google Scholar]
- Ibrahim, M.A.; Kelleni, M.; Geddawy, A. Nonalcoholic fatty liver disease: Current and potential therapies. Life Sci. 2013, 92, 114–118. [Google Scholar] [CrossRef]
- Del Prete, A.; Scalera, A.; Iadevaia, M.D.; Miranda, A.; Zulli, C.; Gaeta, L.; Tuccillo, C.; Federico, A.; Loguercio, C. Herbal products: Benefits, limits, and applications in chronic liver disease. Evid. Based Complement. Alternat. Med. 2012, 2012, 837939. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Atanasov, A.G.; Malainer, C.; Noha, S.M.; Kunert, O.; Schuster, D.; Heiss, E.H.; Oberlies, N.H.; Wagner, H.; Bauer, R.; et al. Identification of isosilybin a from milk thistle seeds as an agonist of peroxisome proliferator-activated receptor gamma. J. Nat. Prod. 2014, 77, 842–847. [Google Scholar] [CrossRef]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar]
- Laurent, T.; Okuda, Y.; Chijimatsu, T.; Umeki, M.; Kobayashi, S.; Kataoka, Y.; Tatsuguchi, I.; Mochizuki, S.; Oda, H. Freshwater clam extract ameliorates triglyceride and cholesterol metabolism through the expression of genes involved in hepatic lipogenesis and cholesterol degradation in rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 830684. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 50, 35832–35839. [Google Scholar]
- Menendez, J.A.; Lupu, R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: From anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch. Immunol. Ther. Exp. (Warsz) 2004, 52, 414–426. [Google Scholar]
- Schimmack, G.; Defronzo, R.A.; Musi, N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes. Metab. 2006, 8, 591–602. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecularanalysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty acid metabolism: Target for metabolic syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Wong, V.W.; Hui, A.Y.; Tsang, S.W.; Chan, J.L.; Tse, A.M.; Chan, K.F.; So, W.Y.; Cheng, A.Y.; Ng, W.F.; Wong, G.L.; et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1154–1161. [Google Scholar] [CrossRef]
- Bahcecioglu, I.H.; Yalniz, M.; Ataseven, H.; Ilhan, N.; Ozercan, I.H.; Seckin, D.; Sahin, K. Levels of serum hyaluronic acid, TNF-α and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology 2005, 52, 1549–1553. [Google Scholar]
- Cui, W.; Chen, S.L.; Hu, K.Q. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am. J. Transl. Res. 2010, 2, 95–104. [Google Scholar]
- Crespo, J.; Cayon, A.; Fernandez-Gil, P.; Hernández-Guerra, M.; Mayorga, M.; Domínguez-Díez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef]
- Francés, D.E.; Ingaramo, P.I.; Ronco, M.T.; Carnovale, C.E. Diabetes, an inflammatory process: Oxidative Stress and TNF-α involved in hepatic complication. J. Biomed. Sci. Eng. 2013, 6, 645–653. [Google Scholar] [CrossRef]
- Jung, M.J.; Chung, H.Y.; Choi, J.H.; Choi, J.S. Antioxidant principles from the needles of red pine, Pinus densiflora. Phytother. Res. 2003, 17, 1064–1068. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hwang, Y.-J.; Wi, H.-R.; Kim, H.-R.; Park, K.W.; Hwang, K.-A. Pinus densiflora Sieb. et Zucc. Alleviates Lipogenesis and Oxidative Stress during Oleic Acid-Induced Steatosis in HepG2 Cells. Nutrients 2014, 6, 2956-2972. https://doi.org/10.3390/nu6072956
Hwang Y-J, Wi H-R, Kim H-R, Park KW, Hwang K-A. Pinus densiflora Sieb. et Zucc. Alleviates Lipogenesis and Oxidative Stress during Oleic Acid-Induced Steatosis in HepG2 Cells. Nutrients. 2014; 6(7):2956-2972. https://doi.org/10.3390/nu6072956
Chicago/Turabian StyleHwang, Yu-Jin, Hae-Ri Wi, Haeng-Ran Kim, Kye Won Park, and Kyung-A Hwang. 2014. "Pinus densiflora Sieb. et Zucc. Alleviates Lipogenesis and Oxidative Stress during Oleic Acid-Induced Steatosis in HepG2 Cells" Nutrients 6, no. 7: 2956-2972. https://doi.org/10.3390/nu6072956