Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome
Abstract
:1. Introduction
2. Experimental Section
2.1. Analysis of Green Cardamom and Black Cardamom
2.2. Animals and Diets
| Ingredient, g/kg | C | H |
|---|---|---|
| Corn starch | 570.0 | - |
| Powdered rat feed | 155.0 | 155.0 |
| HMW salt mixture | 25.0 | 25.0 |
| Fructose | - | 175.0 |
| Beef tallow | - | 200.0 |
| Condensed milk | - | 395.0 |
| Water | 250.0 | 50.0 |
| Energy, kJ/g | 11.23 | 17.93 |
2.3. Oral Glucose Tolerance Test
2.4. Systolic Blood Pressure
2.5. Echocardiography
2.6. Isolated Heart Preparation
2.7. Aortic Contractility
2.8. Body Composition Measurements
2.9. Organ Weights
2.10. Histology
2.11. Plasma Biochemistry
2.12. Statistical Analysis
3. Results
3.1. Cardamom Analysis
| Variable | Green Cardamom | Black Cardamom |
|---|---|---|
| Gas chromatography-mass spectrometry (GC-MS) area (%) | ||
| α-terpinyl acetate | 72.73 | -* |
| 1,8-cineole | 10.61 | 65.52 |
| α-terpineol | 0.86 | 3.29 |
| limonene | 0.38 | 3.59 |
| α-pinene | 1.50 | 2.84 |
| β-pinene | 0.23 | 3.43 |
| Composition | ||
| Energy (KJ/100 g) | 1557 | 1477 |
| Protein (% w/w) | 10.8 | 9.3 |
| Total fat (% w/w) | 10.3 | 1.7 |
| Moisture (% w/w) | 12.2 | 9.4 |
| Total carbohydrate (%) | 58.4 | 73.9 |
3.2. Metabolic Parameters
3.3. Cardiovascular Structure and Function
| Variable | C | CG | CB | H | HG | HB | p values | ||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||||
| Food intake (g/day) | 33.8 ± 0.7 a | 35.1 ± 0.7 a | 34.6 ± 0.8 a | 26.9 ± 0.7 ab | 24.2 ± 0.5 b | 25.0 ± 0.6 ab | <0.0001 | 0.55 | 0.0157 |
| Water intake (mL/day) | 23.9 ± 1.4 b | 36.8 ± 2.0 a | 27.2 ± 2.0 b | 26.6 ± 1.2 b | 28.0 ± 1.0 b | 35.0 ± 1.3 a | 0.65 | <0.0001 | <0.0001 |
| Cardamom intake (g/day) | 0.0 ± 0.0 c | 1.1 ± 0.0 a | 1.1 ± 0.0 a | 0.0 ± 0.0 c | 0.7 ± 0.0 b | 0.8 ± 0.0 b | <0.0001 | <0.0001 | <0.0001 |
| Cumulative energy intake from water (kJ) | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 6230 ± 700 b | 6,450 ± 350 b | 7,950 ± 490 a | <0.0001 | 0.0554 | 0.0554 |
| Cumulative energy intake from food (kJ) | 21,640 ± 730 | 22,440 ± 690 | 22,150 ± 420 | 24,760 ± 670 | 24,680 ± 1290 | 24,890 ± 670 | 0.0001 | 0.88 | 0.85 |
| Cumulative energy intake (kJ) | 21,640 ± 730 b | 22,440 ± 690 b | 22,150 ± 420 b | 30,990 ± 730 a | 31,130 ± 1420 a | 32,840 ± 930 a | <0.0001 | 0.40 | 0.51 |
| Feed conversion efficiency (%) | 1.6 ± 0.3 c | 3.1 ± 0.2c | 1.3 ± 0.5 c | 8.4 ± 1.2 a | 8.1 ± 0.5 a | 4.8 ± 1.2 bc | <0.0001 | 0.0029 | 0.0202 |
| Initial body weight (g) | 336 ± 3 | 338 ± 2 | 337 ± 2 | 336 ± 2 | 339 ± 2 | 336 ± 1 | >0.99 | 0.59 | 0.89 |
| Body weight at 8 weeks (g) | 392 ± 7 b | 401 ± 10 b | 390 ± 5 b | 477 ± 15 a | 488 ± 14 a | 462 ± 12 a | <0.0001 | 0.25 | 0.78 |
| Body weight at 16 weeks (g) | 417 ± 8 c | 435 ± 9 c | 409 ± 6 c | 561 ± 18 a | 574 ± 19 a | 506 ± 11 b | <0.0001 | 0.0023 | 0.15 |
| Body weight gained (8–16 weeks) (%) * | 6.4 ± 1.3 b | 8.3 ± 1.2 b | 4.8 ± 1.2 b | 17.2 ± 1.3 a | 17.6 ± 1.1 a | 9.5 ± 2.4 b | <0.0001 | 0.0003 | 0.0578 |
| Visceral adiposity index (%) | 4.5 ± 0.2 b | 4.2 ± 0.4 b | 4.4 ± 0.2 b | 7.2 ± 0.6 ab | 8.7 ± 0.5 a | 5.7 ± 0.4 b | <0.0001 | 0.0057 | 0.0015 |
| Abdominal circumference (cm) | 20.0 ± 0.2 b | 21.5 ± 0.3 ab | 18.3 ± 0.2c | 23.9 ± 0.4 a | 23.1 ± 0.3 a | 20.7 ± 0.3b | <0.0001 | <0.0001 | 0.0011 |
| Body mass index (kg/m2) | 5.6 ± 0.2 b | 5.9 ± 0.2 b | 5.6 ± 0.1b | 6.7 ± 0.2 a | 7.1 ± 0.1 a | 5.9 ± 0.1 b | <0.0001 | 0.06 | 0.78 |
| Bone mineral content (g) | 12.7 ± 0.4 c | 13.1 ± 0.6 c | 12.0 ± 0.3 c | 16.0 ± 0.7 ab | 17.9 ± 0.4 a | 13.8 ± 0.6c | <0.0001 | <0.0001 | 0.0223 |
| Total body lean mass (g) | 295 ± 7 b | 297 ± 7 b | 297 ± 7 b | 329 ± 9 a | 267 ± 10 c | 311 ± 6 b | 0.36 | 0.0019 | 0.0008 |
| Total body fat mass (g) | 100 ± 7 c | 112 ± 19 c | 77 ± 8 d | 203 ± 19 b | 270 ± 15 a | 140 ± 17 c | <0.0001 | <0.0001 | 0.0108 |
| Tissue wet weight (mg/mm) | |||||||||
| Retroperitoneal adipose tissue | 171 ± 10 c | 153 ± 16 c | 137 ± 9c | 375 ± 48 b | 488 ± 43 a | 234 ± 28 c | <0.0001 | 0.0002 | 0.0011 |
| Epididymal adipose tissue | 119 ± 8 b | 109 ± 12 b | 95 ± 9 b | 225 ± 23 a | 268 ± 28 a | 144 ± 15 b | <0.0001 | 0.0008 | 0.0113 |
| Omental adipose tissue | 85 ± 7 b | 96 ± 10 b | 100 ± 8 b | 190 ± 19 a | 227 ± 27 a | 137 ± 13 b | <0.0001 | 0.0282 | 0.0129 |
| Liver | 261.8 ± 10.1 b | 248.6 ± 11.3 b | 226.3 ± 4.7 c | 336.3 ± 12.3 a | 345.9 ± 10.4 a | 282.4 ± 11.0 b | <0.0001 | <0.0001 | 0.15 |
| Glucose metabolism and plasma biochemistry | |||||||||
| OGTT-AUC (mmol/L·min) | 659 ± 13 c | 722 ± 18 b | 715 ± 28 bc | 799 ± 9 a | 818 ± 19 a | 763 ± 7 a | <0.001 | 0.0574 | 0.0364 |
| Plasma insulin (μmol/L) | 2.0 ± 0.2 b | 0.9 ± 0.1 b | 1.3 ± 0.2 b | 5.7 ± 1.3 a | 2.2 ± 0.5 b | 2.8 ± 0.5 b | <0.001 | 0.0014 | 0.11 |
| Plasma leptin (ng/mL) | 3.3 ± 0.5 b | 5.3 ± 0.8 b | 1.9 ± 0.5 b | 7.9 ± 1.0 a | 9.1 ± 0.6 a | 3.4 ± 0.5 b | <0.001 | <0.0001 | 0.07 |
| Plasma ALP (U/L) | 131 ± 7 cd | 170 ± 13 cd | 113 ± 4 | 214 ± 18 b | 261 ± 23 a | 178 ± 15 c | <0.001 | 0.0001 | 0.66 |
| Plasma ALT (U/L) | 30.1 ± 2.0 c | 34.8 ± 2.3 bc | 25.5 ± 0.8 c | 39.0 ± 3.8 a | 38.3 ± 1.6 bc | 35.4 ± 2.4 bc | 0.0003 | 0.0376 | 0.35 |
| Plasma AST (U/L) | 61.5 ± 1.5 b | 67.2 ± 3.5 b | 59.2 ± 1.7 b | 90.2 ± 5.6 a | 62.2 ± 2.0 b | 59.3 ± 1.2 b | 0.0024 | <0.0001 | <0.0001 |
| Plasma total cholesterol (mmol/L) | 1.8 ± 0.1 bc | 1.5 ± 0.1 c | 1.7 ± 0.1 c | 2.2 ± 0.1 a | 2.0 ± 0.1 ab | 1.8 ± 0.1 bc | <0.001 | 0.0042 | 0.0487 |
| Plasma triglycerides (mmol/L) | 0.9 ± 0.1 b | 0.7 ± 0.1 b | 0.5 ± 0.1 b | 2.2 ± 0.4 a | 2.3 ± 0.3 a | 1.1 ± 0.2 b | <0.001 | 0.0032 | 0.1 |
| Plasma NEFA (mmol/L) | 3.8 ± 0.6 bc | 2.1 ± 0.2 c | 2.2 ± 0.2 c | 6.6 ± 0.8 a | 6.2 ± 0.4 a | 4.1 ± 0.5 bc | <0.001 | 0.0008 | 0.1 |
| Variable | C | CG | CB | H | HG | HB | p values | ||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||||
| Heart rate (bpm) | 268.8 ± 21.5 b | 236.0 ± 10.2 b | 299.4 ± 22.1 b | 352.6 ± 22.9 a | 255.1 ± 19.3 b | 317.5 ± 19.8 ab | 0.0164 | 0.0024 | 0.18 |
| LVIDd (mm) | 6.61 ± 0.27 b | 7.81 ± 0.16 a | 7.07 ± 0.18 b | 7.86 ± 0.36 a | 8.11 ± 0.16 a | 7.24 ± 0.26 b | 0.006 | 0.003 | 0.06 |
| LVIDs (mm) | 3.46 ± 0.12 b | 4.44 ± 0.18 a | 3.35 ± 0.16 b | 4.20 ± 0.16 a | 4.45 ± 0.28 a | 3.48 ± 0.24 b | 0.08 | <0.0001 | 0.15 |
| Fractional shortening (%) | 53.0 ± 1.5 a | 43.3 ± 1.5 b | 55.3 ± 1.9 a | 51.4 ± 3.6 ab | 45.0 ± 3.3 b | 50.4 ± 2.3 a | 0.44 | 0.0016 | 0.42 |
| (+)dP/dt (mmHg/S) | 1298 ± 56 a | 1200 ± 63 a | 1265 ± 54 a | 842 ± 42 b | 883 ± 59 b | 1186 ± 61 a | <0.0001 | 0.0045 | 0.0063 |
| (−)dP/dt (mmHg/S) | −858. ± 38 a | −687 ± 42 c | −734 ± 27 cb | −437 ± 38 d | −532 ± 39 d | −712 ± 38 cb | <0.0001 | 0.0125 | <0.0001 |
| Diastolic stiffness (k) | 22.7 ± 0.7 b | 23.6 ± 0.9 b | 22.2 ± 0.8 b | 28.5 ± 0.5 a | 27.3 ± 0.5 a | 24.1 ± 0.8 b | <0.0001 | 0.0014 | 0.0286 |
| Diastolic volume (μL) | 313 ± 36 b | 504 ± 29 a | 377 ± 29 b | 530 ± 68 a | 562 ± 33 a | 408 ± 42 b | 0.0043 | 0.0037 | 0.07 |
| Systolic volume (μL) | 44 ± 5 b | 95 ± 11 a | 42 ± 6 b | 80 ± 9 a | 100 ± 16 a | 49 ± 10 b | 0.06 | <0.0001 | 0.24 |
| Stroke volume (μL) | 269 ± 38 b | 409 ± 22 ab | 335 ± 28 b | 445 ± 63 a | 463 ± 31 a | 359 ± 37 b | 0.0092 | 0.06 | 0.11 |
| Cardiac output (mL/min) | 71.9 ± 13.0 b | 96.0 ± 5.5 b | 101.2 ± 10.6 b | 157.2 ± 23.7 a | 119.6 ± 16.1 ab | 115.4 ± 14.4 ab | 0.0017 | 0.88 | 0.0451 |
| Estimated LV mass, Litwin (g) | 0.80 ± 0.03 b | 1.04 ± 0.04 a | 0.90 ± 0.04 ab | 1.15 ± 0.07 a | 1.15 ± 0.06a | 1.03 ± 0.07 a | <0.0001 | 0.0364 | 0.06 |
| LV + septum wet weight * | 17.9 ± 1.7 b | 19.6 ± 0.7 ab | 16.6 ± 0.5 b | 21.9 ± 0.7 a | 22.8 ± 1.0 a | 18.0 ± 0.8 b | 0.0009 | 0.001 | 0.41 |
| Relative wall thickness | 0.56 ± 0.04 | 0.48 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.03 | 0.48 ± 0.01 | 0.56 ± 0.02 | 0.86 | 0.0122 | 0.32 |
| Systolic blood pressure (mmHg) | 132 ± 3 c | 135 ± 2 c | 132 ± 2c | 161 ± 3 a | 151 ± 2 b | 136 ± 1 c | <0.0001 | <0.0001 | <0.0001 |
| Systolic wall stress (mmHg) | 80.4 ± 5.1 b | 111.5 ± 5.7 a | 72.9 ± 5.2 b | 105.5 ± 8.1 a | 112.8 ± 9.2 a | 81.2 ± 7.2 b | 0.047 | <0.0001 | 0.22 |
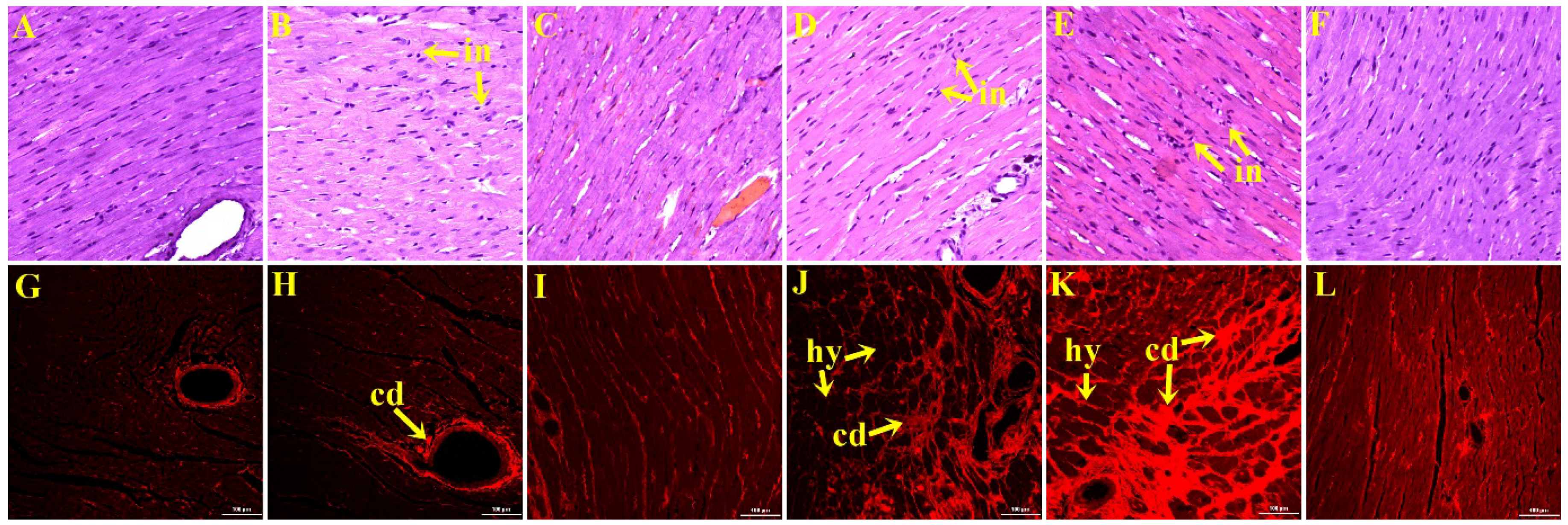
3.4. Liver Structure and Function


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iyer, A.; Panchal, S.; Poudyal, H.; Brown, L. Potential health benefits of Indian spices in the symptoms of the metabolic syndrome: A Review. Indian J. Biochem. Biophys. 2009, 46, 467–481. [Google Scholar] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185 (Suppl. 4), S4–S24. [Google Scholar] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2011, 51, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Non-alcoholic fatty liver disease from pathogenesis to management: An update. Obes. Rev. 2010, 11, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012, 8, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Al Rashdan, I.; Al Nesef, Y. Prevalence of overweight, obesity, and metabolic syndrome among adult Kuwaitis: Results from community-based national survey. Angiology 2010, 61, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 13, 1–7. [Google Scholar]
- Janus, E.D.; Laatikainen, T.; Dunbar, J.A.; Kilkkinen, A.; Bunker, S.J.; Philpot, B.; Tideman, P.A.; Tirimacco, R.; Heistaro, S. Overweight, obesity and metabolic syndrome in rural southeastern Australia. Med. J. Aust. 2007, 187, 147–152. [Google Scholar] [PubMed]
- Joshi, R.; Sharma, P.; Sharma, V.; Prasad, R.; Sud, R.K.; Gulati, A. Analysis of the essential oil of large cardamom (Amomum subulatum Roxb.) growing in different agro-climatic zones of Himachal Pradesh, India. J. Sci. Food Agric. 2013, 93, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Padmakumari Amma, K.P.; Rani, M.P.; Sasidharan, I.; Nisha, V.N.P. Chemical composition, flavonoid-phenolic contents and radical scavenging activity of four major varieties of cardamom. Int. J. Biol. Med. Res. 2010, 1, 20–24. [Google Scholar]
- Chempakam, B.; Sunitha, S. Small cardamom. In Chemistry of Spices; Parthasarathy, V., Chempakam, B., Eds.; CABI: Cambridge, UK, 2008. [Google Scholar]
- Al-Zuhair, H.; el-Sayeh, B.; Ameen, H.A.; al-Shoora, H. Pharmacological studies of cardamom oil in animals. Pharmacol. Res. 1996, 34, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bisht, V.K.; Negi, J.S.; Bhandari, A.K.; Sundriyal, R.C. Amomum subulatum Roxb: Traditional, phytochemical and biological activities—An overview. Afr. J. Agric. Res. 2011, 6, 5386–5390. [Google Scholar] [CrossRef]
- Gilani, A.H.; Jabeen, Q.; Khan, A.U.; Shah, A.J. Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. J. Ethnopharmacol. 2008, 115, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Arif-ullah, K.; Qaiser Jabeen, K.; Anwar-Hassan, G. Pharmacological basis for the medicinal use of cardamom in asthma. Bangladesh. J. Pharmacol. 2011, 6, 34–37. [Google Scholar]
- Jamal, A.; Farah; Siddiqui, A.; Aslam, M.; Javed, K.; Jafri, M.A. Antiulcerogenic activity of Elettaria cardamomum Maton. and Amomum subulatum Roxb. seeds. Indian J. Traditional Knowl. 2005, 4, 298–302. [Google Scholar]
- Alam, K.; Pathak, D.; Ansari, S.H. Evaluation of anti-inflammatory activity of Ammomum subulatum fruit extract. Int. J. Pharm. Sci. Drug Res. 2011, 3, 35–37. [Google Scholar]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khitan, Z.; Kim, D.H. Fructose: A key factor in the development of metabolic syndrome and hypertension. J. Nutr. Metab. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Whaley-Connell, A.T.; Sowers, J.R. Fructose and uric acid: Is there a role in endothelial function? Curr. Hypertens Rep. 2014, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jain, V.; Katewa, S.S. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J. Biochem. Biophys. 2009, 46, 503–506. [Google Scholar] [PubMed]
- Parmar, M.Y.; Shah, P.; Thakkar, V.; Gandhi, T.R. Hepatoprotective activity of Amomum subulatum Roxb against ethanol-induced liver damage. Int. J. Green Pharm. 2009, 3, 250–254. [Google Scholar] [CrossRef]
- Bairwa, G.; Jasuja, N.; Joshi, S. Lipid lowering and antioxidant effects of Amomum subulatum seeds (Family Zingiberaceae) in cholesterol fed rabbits. Arch. Phytopathol. Plant Prot. 2011, 44, 1425–1431. [Google Scholar] [CrossRef]
- Joshi, S.; Bairwa, G.; Sharma, N. Effect of Amomum subulatum on oxidative stress and serum lipids in cholesterol fed rabbits. Int. J. Nat. Prod. 2012, 1, 1–6. [Google Scholar]
- Vavaiya, R.; Patel, A.; Manek, R. Anti-diabetic activity of Amomum subulatum Roxb. fruit constituents. Int. J. Parm. Innov. 2010, 2, 50–65. [Google Scholar]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.; Leal-Cardoso, J.H. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can. J. Physiol. Pharmacol. 2002, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.K.; Kang, P.; Lee, H.S.; Min, S.S.; Seol, G.H. Effects of 1,8-cineole on hypertension induced by chronic exposure to nicotine in rats. J. Pharm. Pharmacol. 2014, 66, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.V.; Assreuy, A.M.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhaes, P.J.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Suzuki, J.; Nakagawa, K.; Hayashi, S.; Enomoto, T.; Fujita, T.; Yamaji, R.; Inui, H.; Nakano, Y. Eucalyptus leaf extract inhibits intestinal fructose absorption, and suppresses adiposity due to dietary sucrose in rats. Br. J. Nutr. 2005, 93, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Peterson, M.; Su, G.L.; Wang, S.C. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am. J. Clin. Nutr. 2015, 101, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.Y.; Shah, P.A.; Gao, J.; Gandhi, T.R. Hepatoprotection through regulation of voltage dependent anion channel expression by Amomum subulatum Roxb seeds extract. Indian J. Pharmacol. 2011, 43, 671–675. [Google Scholar] [PubMed]
- Porter, S.A.; Pedley, A.; Massaro, J.M.; Vasan, R.S.; Hoffmann, U.; Fox, C.S. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Maha, M.B.; Randa, M.S. Arabic coffee with two doses of cardamom: Effects on health biomarkers in healthy women. Int. J. Nut. Food Sci. 2013, 2, 280–286. [Google Scholar]
- Sainsbury, C.A.; Sattar, N.; Connell, J.M.; Hillier, C.; Petrie, J.R. Non-esterified fatty acids impair endothelium-dependent vasodilation in rat mesenteric resistance vessels. Clin. Sci. (Lond.) 2004, 107, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jain, V.; Singh, D.P. Effect of greater cardamom (Amomum subulatum Roxb.) on blood lipids, fibrinolysis and total antioxidant status in patients with ischemic heart disease. Asian Pac. J. Trop. Dis. 2012, 2, S739–S743. [Google Scholar] [CrossRef]
- El Malti, J.; Mountassif, D.; Amarouch, H. Antimicrobial activity of Elettaria cardamomum: Toxicity, biochemical and histological studies. Food Chem. 2007, 104, 1560–1568. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Papathanasopoulos, A.; Camilleri, M. Dietary fiber supplements: Effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010, 138, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B. Dietary fiber and energy regulation. J. Nutr. 2000, 130, 272S–275S. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaswant, M.; Poudyal, H.; Mathai, M.L.; Ward, L.C.; Mouatt, P.; Brown, L. Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome. Nutrients 2015, 7, 7691-7707. https://doi.org/10.3390/nu7095360
Bhaswant M, Poudyal H, Mathai ML, Ward LC, Mouatt P, Brown L. Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome. Nutrients. 2015; 7(9):7691-7707. https://doi.org/10.3390/nu7095360
Chicago/Turabian StyleBhaswant, Maharshi, Hemant Poudyal, Michael L. Mathai, Leigh C. Ward, Peter Mouatt, and Lindsay Brown. 2015. "Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome" Nutrients 7, no. 9: 7691-7707. https://doi.org/10.3390/nu7095360
APA StyleBhaswant, M., Poudyal, H., Mathai, M. L., Ward, L. C., Mouatt, P., & Brown, L. (2015). Green and Black Cardamom in a Diet-Induced Rat Model of Metabolic Syndrome. Nutrients, 7(9), 7691-7707. https://doi.org/10.3390/nu7095360






