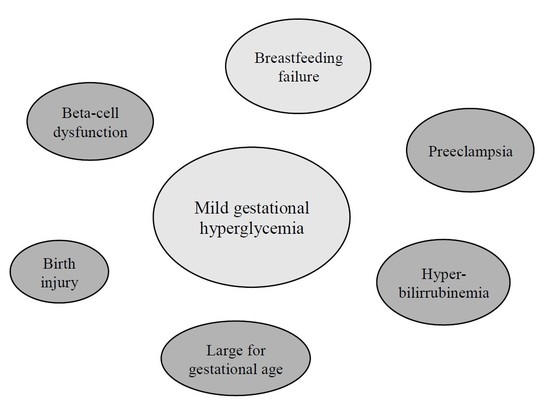
The Effects of Mild Gestational Hyperglycemia on Exclusive Breastfeeding Cessation
Abstract
:1. Introduction
2. Experimental Section
2.1. Enrollment
2.2. Selection Criteria and Data Collection
2.3. Glucose Challenge Test
- (i)
- Normal glucose tolerance (NGT), defined by normal 1-hOGTT results (1-h plasma glucose < 7.8 mmol/L);
- (ii)
- Mild impairment of glucose tolerance (MIGT), defined by a single abnormal value greater than or equal to 7.8 mmol/L, but less than 10.6 mmol/L;
- (iii)
- GDM, requires at least two of the following on the 3-hOGTT: fasting glucose 5.8 mmol/L, 1-h glucose 10.6 mmol/L, 2-h glucose 9.2 mmol/L, or 3-h glucose 8.1 mmol/L.
2.4. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. EBF Outcome: Univariate Analysis
3.3. EBF Outcome: Multivariate Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1-hOGTT | 1-h post-glucose challenge |
| 3-hOGTT | 3-h glucose tolerance test |
| ACOG | American College of Obstetricians and Gynecologists |
| DLII | delayed onset of lactogenesis stage II |
| EBF | exclusive breastfeeding |
| GDM | gestational diabetes |
| IADPSG | International Association of Diabetes and Pregnancy Study Groups |
| LII | lactogenesis stage II |
| MIGT | mild impairment of glucose tolerance |
| NGT | normal glucose tolerance |
References
- Herring, S.J.; Rich-Edwards, J.W.; Oken, E.; Rifas-Shiman, S.L.; Kleinman, K.P.; Gillman, M.W. Association of postpartum depression with weight retention 1 year after childbirth. Obesity 2008, 16, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.L.; McQueen, K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr. 2007, 96, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P., Jr.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Nordskar, J.J.; Leithe, H.; Hjorth-Hansen, A.K.; Martinussen, M.; Carlsen, S.M. Breast size increment during pregnancy and breastfeeding in mothers with polycystic ovary syndrome: A follow-up study of a randomised controlled trial on metformin versus placebo. BJOG 2012, 119, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Horton, B.J.; Chetwynd, E.; Watkins, S.; Grewen, K.; Meltzer-Brody, S. Prevalence and Risk Factors for Early, Undesired Weaning Attributed to Lactation Dysfunction. J. Women’s Health 2014, 23, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lemay, D.G.; Ballard, O.A.; Hughes, M.A.; Morrow, A.L.; Horseman, N.D.; Nommsen-Rivers, L.A. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE 2013, 8, e67531. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A.; Dolan, L.M.; Huang, B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed. Med. 2012, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Brownell, E.; Howard, C.R.; Lawrence, R.A.; Dozier, A.M. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J. Pediatr. 2012, 161, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Hiersch, L.; Hod, M.; Chen, R.; Wiznitzer, A.; Yogev, Y. Is abnormal 50-g glucose-challenge testing an independent predictor of adverse pregnancy outcome? J. Matern. Fetal Neonatal Med. 2012, 25, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Verd, S.; Nadal-Amat, J.; Gich, I.; Leshem, M. Salt preference of nursing mothers is associated with earlier cessation of exclusive breastfeeding. Appetite 2010, 54, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Verd, S.; Barriuso, L.; Gich, I.; Gutiérrez, A.; Nadal-Amat, J.; Carreras, E. Risk of early breastfeeding cessation among symmetrical, small for gestational age infants. Ann. Hum. Biol. 2013, 40, 146–151. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122, 406–416. [Google Scholar]
- Chapman, D.J.; Perez-Escamilla, R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J. Hum. Lact. 1999, 5, 107–111. [Google Scholar] [CrossRef]
- Hruschka, D.J.; Sellen, D.W.; Stein, A.D.; Martorell, R. Delayed onset of lactation and risk of ending full breast-feeding early in rural Guatemala. J. Nutr. 2003, 133, 2592–2599. [Google Scholar] [PubMed]
- Stuebe, A.M.; Landon, M.B.; Lai, Y.; Klebanoff, M.; Ramin, S.M.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Sciscione, A.; Catalano, P.; et al. Is There a Threshold Oral Glucose Tolerance Test Value for Predicting Adverse Pregnancy Outcome? Am. J. Perinatol. 2015, 32, 833–838. [Google Scholar] [PubMed]
- Retnakaran, R.; Qi, Y.; Sermer, M.; Connelly, P.W.; Zinman, B.; Hanley, A.J. Isolated hyperglycemia at 1 h on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care 2008, 31, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M. Hyperlycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [PubMed]
- De Bortoli, J.; Amir, L.H. Is onset of lactation delayed in women with diabetes in pregnancy? A systematic review. Diabet. Med. 2016, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, R.; Goulet, C.; Cgagnon, M. Infant and maternal factors influencing breastmilk sodium among primiparous mothers. Breastfeed. Med. 2012, 7, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Riddle, S.W.; Nommsen-Rivers, L.A. A case control study of diabetes during pregnancy and low milk supply. Breastfeed. Med. 2016, 11, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bromiker, R.; Rachamim, A.; Hammerman, C.; Schimmel, M.; Kaplan, M.; Medoff-Cooper, B. Immature suckling patterns in infants of mothers with diabetes. J. Pediatr. 2006, 49, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Haile, Z.T.; Oza-Frank, R.; Azulay Chertok, I.R.; Passen, N. Association between history of gestational diabetes and exclusive breastfeeding at hospital discharge. J. Hum. Lact. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.; Melamed, N.; Vandenbergh, H.; Berger, H. The impact of adoption of the international association of diabetes in pregnancy study group criteria for the screening and diagnosis of gestational diabetes. Am. J. Obstet. Gynecol. 2015, 212, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Vandorten, J.P.; Dodson, W.C.; Espeland, M.A.; Grobman, W.A.; Guise, J.M.; Mercer, B.M.; Minkoff, H.L.; Poindexter, B.; Prosser, L.A.; Sawaya, G.F.; et al. NIH consensus development conference: Diagnosing gestational diabetes mellitus. NIH Consens. State Sci. Statements 2013, 29, 1–31. [Google Scholar]
- Capula, C.; Chiefari, E.; Borelli, M.; Oliverio, R.; Vero, A.; Foti, D.; Puccio, L.; Vero, R.; Brunetti, A. A new predictive tool for the early risk assessment of gestational diabetes mellitus. Prim Care Diabetes 2016, 10, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A. Does insulin explain the relationship between maternal obesity and poor lactation outcomes? An overview of the literature. Adv. Nutr. 2016, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
| Variable | NGT: 1-h Plasma Glucose < 7.8 mmol/L (N = 616) | MIGT: 10.6 mmol/L > 1-hOGTT Results ≥ 7.8 mmol/L (N = 152) | p |
|---|---|---|---|
| Gender: | 0.72 | ||
| Male | 51% | 53% | |
| Female | 49% | 47% | |
| Parity: | 1 | ||
| 1 | 64% | 65% | |
| >1 | 36% | 35% | |
| Mother’s age (years) | 33 (20–45) | 33 (25–42) | 0.064 |
| Gestational weight gain | 12 (1–39) | 12 (4–27) | 0.84 |
| Weeks of gestation | 40 (37–42) | 40 (37–42) | 0.79 |
| Delivery type: | 0.67 | ||
| Eutocic | 82% | 18% | |
| Instrumental | 80% | 20% | |
| C-section | 79% | 21% | |
| Birth weight | 3272 (1995–4800) | 3395 (2050–4390) | 0.018 |
| Birth height | 49.5 (33–54) | 50 (45.5–53.5) | 0.13 |
| Birth head circumference | 34.5 (31–37) | 34.5 (31–37.5) | 0.74 |
| Percent of loss of birth weight to discharge | 6 (−0.32–0.21) | 7 (−7–13) | 0.41 |
| EBF Discontinuation | Before Day 100 (N = 384) | Equal or Later than Day 100 (N = 384) | p |
|---|---|---|---|
| MIGT | 58% | 42% | 0.03 * |
| NGT | 48% | 52% | |
| Gender: | 0.001 ** | ||
| Male | 214 (54%) | 169 (43%) | |
| Female | 179 (45%) | 225 (57%) | |
| Parity: | <0.001 *** | ||
| 1 | 271(72%) | 214 (57%) | |
| >1 | 107 (28%) | 159 (28%) | |
| Mother’s age (years) | 33 (18–42) | 33 (21–45) | 0.92 |
| Gestational weight gain | 12 (1–30) | 12 (4–39) | 0.05 |
| Weeks of gestation | 40 (37–42) | 40 (37–42) | 0.27 |
| Delivery type: | 0.38 | ||
| Eutocic | 184 (50%) | 207 (57%) | |
| Instrumental | 61 (17%) | 55 (15%) | |
| C-section | 119 (33%) | 110 (30%) | |
| Birth weight | 3252 (1995–4390) | 3330 (2310–4800) | 0.02 * |
| Birth height | 49.5 (33–54) | 50 (45.5–54) | 0.32 |
| Birth head circumference | 34.5 (31–37) | 34.5 (31–37.5) | 0.36 |
| Percent of loss of birth weight to discharge | 7 (−7–21) | 6 (−32–20) | <0.001 *** |
| AOR (95% CI) | p | |
|---|---|---|
| Mildly impaired glucose tolerance | 1.65 (1.11–2.45) | 0.01 |
| Early neonatal weight loss | 1.73 (1.26–2.36) | 0.001 |
| Admission to neonatal ward | 3.32 (1.04–10.60) | 0.04 |
| Parity | 0.57 (0.41–0.79) | 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verd, S.; De Sotto, D.; Fernández, C.; Gutiérrez, A. The Effects of Mild Gestational Hyperglycemia on Exclusive Breastfeeding Cessation. Nutrients 2016, 8, 742. https://doi.org/10.3390/nu8110742
Verd S, De Sotto D, Fernández C, Gutiérrez A. The Effects of Mild Gestational Hyperglycemia on Exclusive Breastfeeding Cessation. Nutrients. 2016; 8(11):742. https://doi.org/10.3390/nu8110742
Chicago/Turabian StyleVerd, Sergio, Diego De Sotto, Consuelo Fernández, and Antonio Gutiérrez. 2016. "The Effects of Mild Gestational Hyperglycemia on Exclusive Breastfeeding Cessation" Nutrients 8, no. 11: 742. https://doi.org/10.3390/nu8110742