Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons
Abstract
:1. Introduction
2. Materials and Methods
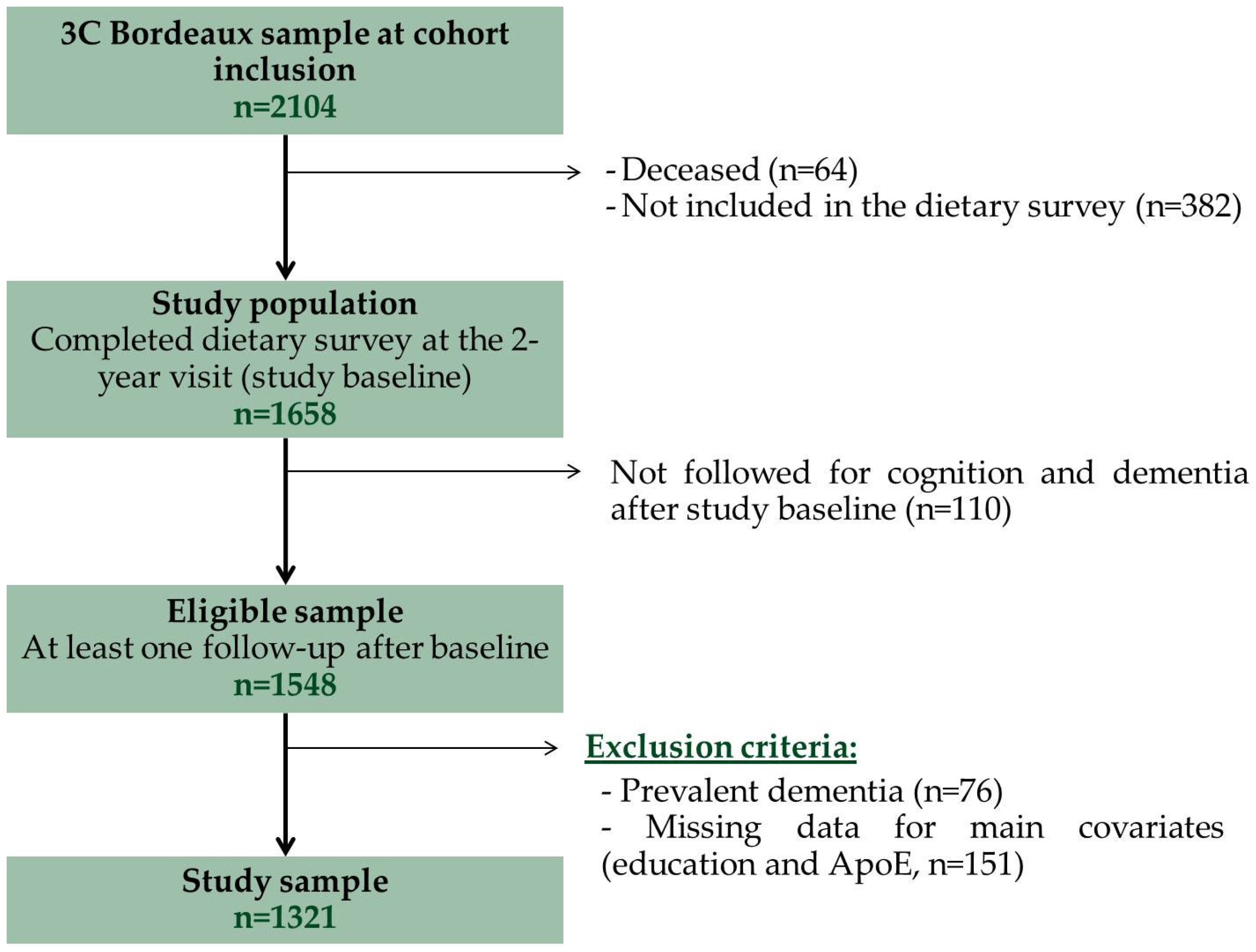
2.1. Study Population
2.2. Diagnosis of Dementia
2.3. Assessment of Diet and Intake of B-Vitamins
2.4. Other Variables
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Sample
3.2. Multivariate Associations between Intake of B Vitamins and Risk of Dementia
3.3. Secondary Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S. Homocysteine and Alzheimer’s disease. Lancet Neurol. 2003, 2, 425–428. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Troen, A.; Rosenberg, I.H. B vitamins and the aging brain. Nutr. Rev. 2010, 68 (Suppl. 2), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Martel, F.; Borges, N.; Araújo, J.M.; Keating, E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S. The Role of B Vitamins in Preventing and Treating Cognitive Impairment and Decline. Adv. Nutr. Int. Rev. J. 2012, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Martelli, M.; Servadei, L.; Brunetti, N.; Porcellini, E.; Licastro, F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005, 82, 636–643. [Google Scholar] [PubMed]
- Ramos, M.I.; Allen, L.H.; Mungas, D.M.; Jagust, W.J.; Haan, M.N.; Green, R.; Miller, J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005, 82, 1346–1352. [Google Scholar] [PubMed]
- De Lau, L.M.L.; Refsum, H.; Smith, A.D.; Johnston, C.; Breteler, M.M.B. Plasma folate concentration and cognitive performance: Rotterdam Scan Study. Am. J. Clin. Nutr. 2007, 86, 728–734. [Google Scholar] [PubMed]
- Agnew-Blais, J.C.; Wassertheil-Smoller, S.; Kang, J.H.; Hogan, P.E.; Coker, L.H.; Snetselaar, L.G.; Smoller, J.W. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women’s Health Initiative Memory Study. J. Acad. Nutr. Diet. 2015, 115, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Corrada, M.M.; Kawas, C.H.; Hallfrisch, J.; Muller, D.; Brookmeyer, R. Reduced risk of Alzheimer’s disease with high folate intake: The Baltimore Longitudinal Study of Aging. Alzheimers Dement. J. Alzheimers Assoc. 2005, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.-X.; Miller, J.; Green, R.; Mayeux, R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch. Neurol. 2007, 64, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.; Wengreen, H.J.; Munger, R.G.; Corcoran, C.D. Dietary folate, vitamin B-12, vitamin B-6 and incident Alzheimer’s disease: The cache county memory, health and aging study. J. Nutr. Health Aging 2009, 13, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Schneider, J.A.; Tangney, C.C.; Bienia, J.L.; Aggarwal, N.T. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 435–443. [Google Scholar] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Hebert, L.E.; Scherr, P.A.; Schneider, J.A. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch. Neurol. 2005, 62, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, M.; Fischer, P. Folate and Alzheimer: When time matters. J. Neural Transm. 2013, 120, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.P.M.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. B-Vitamin Treatment Trialists’ Collaboration Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.E.; Nichols, T.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.F.; Rosenberg, I.H. The Effect of Folic Acid Fortification on Plasma Folate and Total Homocysteine Concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- 3C Study Group. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Jutand, M.A.; Larrieu, S.; Letenneur, L.; Delcourt, C.; Combe, N.; Barberger-Gateau, P. Energy, macronutrient and fatty acid intake of French elderly community dwellers and association with socio-demographic characteristics: Data from the Bordeaux sample of the Three-City Study. Br. J. Nutr. 2007, 98, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Deheeger, M.; Preziosi, P. Portions Alimentaires: Manuel Photos Pour L’estimation des Quantités; Economica: Paris, France, 2000. [Google Scholar]
- Favier, J.-C.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Répertoire Général des Aliments: Table de Composition, 2nd ed.; Technique & Documentation; Institut National de la Recherche Agronomique: Paris, France, 1995. [Google Scholar]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables; Medpharm Scientific Publishers: Stuttgart, Germany, 2000. [Google Scholar]
- Renaud, S.; Godsey, F.; Ortchanian, E.; Baudier, F. Table de Composition des Aliments; ASTRA-CALVE: Courbevoie, France, 1979. [Google Scholar]
- Agence Nationale de Sécurité Sanitaire, de l’alimentation, de l’environnement et du travail (ANSES). AVIS Relatif à L’évaluation des Apports en Vitamines et Minéraux Issus de L’alimentation non Enrichie, de L’alimentation Enrichie et des Compléments Alimentaires dans la Population Française: Estimation des Apports Usuels, des Prévalences D’inadéquation et des Risques de Dépassement des Limites de Sécurité; Avis de l’ANSES. Saisine n°2012-SA-0142; ANSES: Maisons-Alfort, France, 2015. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Fuhrer, R.; Rouillon, F. The French version of the CES-D (Center for Epidemiologic Studies-Depression Scale). Psychiatr. Psychobiol. 1989, 4, 163–166. [Google Scholar]
- Lamarca, R.; Alonso, J.; Gómez, G.; Muñoz, Á. Left-truncated Data with Age as Time Scale: An Alternative for Survival Analysis in the Elderly Population. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M337–M343. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Chandler, C.J. Metabolic Interactions of Alcohol and Folate. J. Nutr. 2002, 132, 2367S–2372S. [Google Scholar] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [PubMed]
- Moore, E.M.; Ames, D.; Mander, A.G.; Carne, R.P.; Brodaty, H.; Woodward, M.C.; Boundy, K.; Ellis, K.A.; Bush, A.I.; Faux, N.G.; et al. Among vitamin B12 deficient older people, high folate levels are associated with worse cognitive function: Combined data from three cohorts. J. Alzheimers Dis. 2014, 39, 661–668. [Google Scholar] [PubMed]
- Jernerén, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith, A.D. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.; Ferrario, E.; Massaia, M.; Cappa, G.; Rivolta, G.; Grossi, E.; Buckley, A.E. Low folate levels in the cognitive decline of elderly patients and the efficacy of folate as a treatment for improving memory deficits. Arch. Gerontol. Geriatr. 1997, 26, 1–13. [Google Scholar] [CrossRef]
- Andreeva, V.A.; Kesse-Guyot, E.; Barberger-Gateau, P.; Fezeu, L.; Hercberg, S.; Galan, P. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: Ancillary findings from the SU.FOL.OM3 randomized trial. Am. J. Clin. Nutr. 2011, 94, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.M.; Bradley, K.M.; de Jager, C.; Budge, M.M.; Smith, A.D. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kåreholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Mühlmann, M.; Ertl-Wagner, B.; et al. Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: A longitudinal population-based study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and Folate Status in Relation to Decline in Scores on the Mini-Mental State Examination in the Framingham Heart Study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Birks, J.; Nexo, E.; Ueland, P.M.; Schneede, J.; Scott, J.; Molloy, A.; Evans, J.G. Low vitamin B-12 status and risk of cognitive decline in older adults. Am. J. Clin. Nutr. 2007, 86, 1384–1391. [Google Scholar] [PubMed]
- Moorthy, D.; Peter, I.; Scott, T.M.; Parnell, L.D.; Lai, C.-Q.; Crott, J.W.; Ordovás, J.M.; Selhub, J.; Griffith, J.; Rosenberg, I.H.; et al. Status of Vitamins B-12 and B-6 but Not of Folate, Homocysteine, and the Methylenetetrahydrofolate Reductase C677T Polymorphism Are Associated with Impaired Cognition and Depression in Adults123. J. Nutr. 2012, 142, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Scott, J.M. Intake and status of folate and related B-vitamins: Considerations and challenges in achieving optimal status. Br. J. Nutr. 2008, 99, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- ANSES. Food Consumption Data from the Individual and National Study on Food Consumption 2 (INCA2). Available online: Data.gouv.fr/fr/datasets/donnees-de-consommations-et-habitudes-alimentaires-de-letude-inca-2-3/ (accessed on 2 June 2016).
- Bates, C.J.; Prentice, A.; van der Pols, J.C.; Walmsley, C.; Pentieva, K.D.; Finch, S.; Smithers, G.; Clarke, P.C. Estimation of the use of dietary supplements in the National Diet and Nutrition Survey: People aged 65 years and Over. An observed paradox and a recommendation. Eur. J. Clin. Nutr. 1998, 52, 917–923. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Overall Sample (n = 1321) | Incident Dementia (n = 197) | No Dementia (n = 1124) | p for Risk of Dementia a |
|---|---|---|---|---|
| Age (years) | 75.8 ± 4.8 | 78.3 ± 4.6 | 75.4 ± 4.7 | <0.001 |
| Gender, female | 822 (62.2) | 140 (71.1) | 682 (60.7) | 0.30 |
| Education ≥high school | 540 (40.9) | 72 (36.5) | 468 (41.6) | 0.24 |
| ApoEε4, carrier | 240 (18.2) | 49 (24.9) | 191 (17.0) | <0.001 |
| Alcohol intake (g/day) | 13.9 ± 15.5 | 12.9 ± 14.0 | 14.1 ± 15.8 | 0.86 |
| Tobacco consumption (pack-year) | ||||
| 0 | 861 (65.2) | 142 (72.1) | 719 (64.0) | 0.75 |
| <10 | 156 (11.8) | 20 (10.2) | 136 (12.1) | |
| (10–20) | 84 (6.4) | 10 (5.1) | 74 (6.6) | |
| (20–30) | 71 (5.4) | 7 (3.6) | 64 (5.7) | |
| ≥30 | 149 (11.3) | 18 (9.1) | 131 (11.7) | |
| Regular exercise b | 412 (35.3) | 46 (28.2) | 366 (36.5) | 0.14 |
| BMI (kg/m2) | 26.6 ± 4.1 | 26.1 ± 4.3 | 26.7 ± 4.1 | 0.23 |
| Hypercholesterolemia | 762 (57.7) | 125 (63.5) | 637 (56.7) | 0.16 |
| Diabetes | 125 (9.5) | 33 (16.8) | 92 (8.2) | <0.001 |
| History of cardiovascular diseases | 428 (32.4) | 63 (32.0) | 365 (32.5) | 0.94 |
| Hypertension | 998 (75.5) | 151 (76.6) | 847 (75.4) | 0.63 |
| High depressive symptomatology | 98 (7.4) | 22 (11.2) | 76 (6.8) | 0.10 |
| Number of drugs consumed | 4.8 ± 2.9 | 5.8 ± 3.2 | 4.6 ± 2.8 | <0.001 |
| B vitamin/multivitamin supplement use | 19 (1.4) | 2 (1.0) | 17 (1.5) | 0.59 |
| Energy intake (Kcal/day) | 1623.0 ± 514.0 | 1565.0 ± 500.2 | 1633.0 ± 515.9 | 0.44 |
| Vitamin B6 intake (mg/day) | 1.5 ± 0.6 | 1.4 ± 0.6 | 1.5 ± 0.6 | 0.52 |
| Folate intake (µg/day) | 278.3 ± 134.8 | 251.9 ± 126.1 | 283.0 ± 135.8 | 0.01 |
| Vitamin B12 intake (µg/day) | 5.7 ± 11.4 | 4.9 ± 9.9 | 5.8 ± 11.7 | 0.30 |
| Number of Dementia Cases | Risk of Dementia (HR [95% CI]) a | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Vitamin B6 (mg/day) | |||
| Q1 <1.0 | 50 | 1.0 (reference) | 1.0 (reference) |
| Q2 (1.0–1.2) | 36 | 0.81 (0.51–1.28) | 0.86 (0.54–1.36) |
| Q3 (1.2–1.5) | 36 | 1.02 (0.63–1.65) | 1.08 (0.66–1.77) |
| Q4 (1.5–1.9) | 42 | 1.26 (0.78–2.04) | 1.40 (0.85–2.31) |
| Q5 ≥1.9 | 33 | 1.02 (0.58–1.78) | 1.08 (0.60–1.94) |
| p for trend | 0.52 | 0.38 | |
| Folate (µg/day) | |||
| Q1 <168.3 | 56 | 1.0 (reference) | 1.0 (reference) |
| Q2 (168.3–225.4) | 37 | 0.66 (0.43–1.02) | 0.66 (0.42–1.02) |
| Q3 (225.4–281.4) | 40 | 0.67 (0.43–1.03) | 0.73 (0.47–1.15) |
| Q4 (281.4–375.6) | 35 | 0.69 (0.43–1.10) | 0.76 (0.47–1.24) |
| Q5 ≥375.6 | 29 | 0.47 (0.28–0.79) | 0.47 (0.28–0.81) |
| p for trend | 0.01 | 0.02 | |
| Vitamin B12 (µg/day) | |||
| Q1 <1.8 | 38 | 1.0 (reference) | 1.0 (reference) |
| Q2 (1.8–2.6) | 47 | 1.42 (0.91–2.21) | 1.27 (0.81–1.98) |
| Q3 (2.6–3.7) | 40 | 1.29 (0.81–2.07) | 1.15 (0.71–1.86) |
| Q4 (3.7–5.7) | 40 | 1.30 (0.81–2.10) | 1.26 (0.77–2.05) |
| Q5 ≥5.7 | 32 | 1.17 (0.70–1.94) | 1.04 (0.61–1.75) |
| p for trend | 0.95 | 0.73 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefèvre-Arbogast, S.; Féart, C.; Dartigues, J.-F.; Helmer, C.; Letenneur, L.; Samieri, C. Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons. Nutrients 2016, 8, 761. https://doi.org/10.3390/nu8120761
Lefèvre-Arbogast S, Féart C, Dartigues J-F, Helmer C, Letenneur L, Samieri C. Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons. Nutrients. 2016; 8(12):761. https://doi.org/10.3390/nu8120761
Chicago/Turabian StyleLefèvre-Arbogast, Sophie, Catherine Féart, Jean-François Dartigues, Catherine Helmer, Luc Letenneur, and Cécilia Samieri. 2016. "Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons" Nutrients 8, no. 12: 761. https://doi.org/10.3390/nu8120761
APA StyleLefèvre-Arbogast, S., Féart, C., Dartigues, J.-F., Helmer, C., Letenneur, L., & Samieri, C. (2016). Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons. Nutrients, 8(12), 761. https://doi.org/10.3390/nu8120761





