The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
- (a)
- Children: (Biofortification OR fortification OR supplementation) AND (iron OR zinc OR micronutrient powder OR multiple micronutrients) AND (children OR infants OR toddlers) AND (trial OR study OR survey OR assessment).
- (b)
- Pregnant women: (Biofortification OR fortification OR supplementation) AND (pregnant women OR lactating women OR maternal) AND (iron OR zinc OR micronutrient powder OR multiple micronutrients) AND (trial OR study OR survey OR assessment).
- (c)
- Lactating women: (Biofortification OR fortification OR supplementation) AND lactating women AND (zinc OR iron OR micronutrient powder OR multiple micronutrients OR breast milk) AND (trial OR study OR survey OR assessment).
2.2. Inclusion and Exclusion Criteria
2.3. Study Design and Comparison Groups
2.4. Outcome Measures
2.5. Data Synthesis and Statistical Analysis
2.6. Assessment of Quality and Risk of Bias
3. Results
3.1. Literature Search
3.2. Effects of Low-Dose Iron Interventions during Pregnancy and Lactation on Child Outcomes
3.3. Iron Interventions in Children 6–23 Months of Age
3.4. Zinc Interventions during Pregnancy and Lactation
3.4.1. Effect on Birth Weight and Prevalence of Low Birth Weight
3.4.2. Effect on Infant Growth and Micronutrient Status
3.5. Zinc Interventions in Children 6–23 Months of Age
3.5.1. Effect on Serum or Plasma Zinc Concentrations and Zinc Deficiency
3.5.2. Effect on Growth
3.5.3. Effects on Diarrhea, Fever, and Respiratory Infections
3.5.4. Effects on Mental and Motor Development
3.6. The Interaction of Iron and Zinc in Interventions in Children 6–23 Months of Age
3.6.1. Effect of Zinc on Serum Ferritin
3.6.2. The Effect of Iron on Serum Zinc
3.7. Quality of the Evidence across Studies
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Guidelines for Food Fortification; World Health Organization: Geneva, Switzerland, 2006; Available online: http://www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf (accessed on 15 August 2016).
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S.; Study, M.C.U. Maternal and child undernutrition 2—Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- Black, M.M.; Quigg, A.M.; Hurley, K.M.; Pepper, M.R. Iron deficiency and iron-deficiency anemia in the first two years of life: Strategies to prevent loss of developmental potential. Nutr. Rev. 2011, 69, S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Integrated strategies needed to prevent iron deficiency and to promote early child development. J. Trace Elem. Med. Biol. 2012, 26, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Oski, F.A. Iron-Deficiency in Infancy and Childhood. N. Engl. J. Med. 1993, 329, 190–193. [Google Scholar] [PubMed]
- Christian, P.; Stewart, C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.Y.; Lonnerdal, B.; Hotz, C.; Rivera, J.A.; Brown, K.H. Recent advances in knowledge of zinc nutrition and human health. Food Nutr. Bull. 2009, 30, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Muller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Contr. Clin. Trial. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Reviews of Interventions 4.2.6. In The Cochrane Library; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 4. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. Introduction to GRADE Handbook. GRADE Working Group, 2013. Available online: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed on 28 November 2016).
- Liu, J.M.; Mei, Z.G.; Ye, R.W.; Serdula, M.K.; Ren, A.G.; Cogswell, M.E. Micronutrient Supplementation and Pregnancy Outcomes Double-Blind Randomized Controlled Trial in China. JAMA Intern. Med. 2013, 173, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ouladsahebmadarek, E.; Sayyah-Melli, M.; Taghavi, S.; Abbasalizadeh, S.; Seyedhejazie, M. The effect of supplemental iron elimination on pregnancy outcome. Pak. J. Med. Sci. 2011, 27, 641–645. [Google Scholar]
- Makrides, M.; Crowther, C.A.; Gibson, R.A.; Gibson, R.S.; Skeaff, C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 145–153. [Google Scholar] [PubMed]
- Siega-Riz, A.M.; Hartzema, A.G.; Turnbull, C.; Thorp, J.; McDonald, T.; Cogswell, M.E. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: A randomized controlled trial. Am. J. Obstet. Gynecol. 2006, 194, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [PubMed]
- Dawson, E.B.; Albers, J.; Mcganity, W.J. Serum Zinc Changes Due to Iron Supplementation in Teen-Age Pregnancy. Am. J. Clin. Nutr. 1989, 50, 848–852. [Google Scholar] [PubMed]
- Li, Q.; Yan, H.; Zeng, L.X.; Cheng, Y.; Liang, W.F.; Dang, S.N.; Wang, Q.L.; Tsuji, I. Effects of Maternal Multimicronutrient Supplementation on the Mental Development of Infants in Rural Western China: Follow-up Evaluation of a Double-Blind, Randomized, Controlled Trial. Pediatrics 2009, 123, E685–E692. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Pino, P.; Pizarro, F.; Lozoff, B. Prevention of iron-deficiency anemia: Comparison of high- and low-iron formulas in term healthy infants after six months of life. J. Pediatr. 1998, 132, 635–640. [Google Scholar] [CrossRef]
- Moffatt, M.E.; Longstaffe, S.; Besant, J.; Dureski, C. Prevention of iron deficiency and psychomotor decline in high-risk infants through use of iron-fortified infant formula: A randomized clinical trial. J. Pediatr. 1994, 125, 527–534. [Google Scholar] [CrossRef]
- Ermis, B.; Demirel, F.; Demircan, N.; Gurel, A. Effects of three different iron supplementations in term healthy infants after 5 months of life. J. Trop. Pediatr. 2002, 48, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Geltman, P.L.; Meyers, A.F.; Mehta, S.D.; Brugnara, C.; Villon, I.; Wu, Y.A.; Bauchner, H. Daily multivitamins with iron to prevent anemia in high-risk infants: A randomized clinical trial. Pediatrics 2004, 114, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Dallman, P.R.; Pizarro, F.; Velozo, L.; Pena, G.; Bartholmey, S.J.; Hertrampf, E.; Olivares, M.; Letelier, A.; Arredondo, M. Effectiveness of iron-fortified infant cereal in prevention of iron deficiency anemia. Pediatrics 1993, 91, 976–982. [Google Scholar] [PubMed]
- Gill, D.G.; Vincent, S.; Segal, D.S. Follow-on formula in the prevention of iron deficiency: A multicentre study. Acta Paediatr. 1997, 86, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Pietschnig, B.; Vanura, H.; Heil, M.; Steffan, I.; Hobiger, G.; Schuster, E.; Camaya, Z. Iron intake and iron nutritional status of infants fed iron-fortified beikost with meat. Am. J. Clin. Nutr. 1988, 47, 108–112. [Google Scholar] [PubMed]
- Ziegler, E.E.; Nelson, S.E.; Jeter, J.M. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am. J. Clin. Nutr. 2009, 90, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.; Kim, S.; Sim, B.; Gang, H.; Kim, H.; Kim, Y.; Kim, R.; Yang, M. Effect of iron fortification of nursery complementary food on iron status of infants in the DPRKorea. Asia Pac. J. Clin. Nutr. 2008, 17, 264–269. [Google Scholar] [PubMed]
- Dijkhuizen, M.A.; Wieringa, F.T.; West, C.E.; Martuti, S. Muhilal: Effects of iron and zinc supplementation in Indonesian infants on micronutrient status and growth. J. Nutr. 2001, 131, 2860–2865. [Google Scholar] [PubMed]
- Lind, T.; Lonnerdal, B.; Stenlund, H.; Ismail, D.; Seswandhana, R.; Ekstrom, E.C.; Persson, L.A. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: Interactions between iron and zinc. Am. J. Clin. Nutr. 2003, 77, 883–890. [Google Scholar] [PubMed]
- Fahmida, U.; Rumawas, J.S.; Utomo, B.; Patmonodewo, S.; Schultink, W. Zinc-iron, but not zinc-alone supplementation, increased linear growth of stunted infants with low haemoglobin. Asia Pac. J. Clin. Nutr. 2007, 16, 301–309. [Google Scholar] [PubMed]
- Untoro, J.; Karyadi, E.; Wibowo, L.; Erhardt, M.W.; Gross, R. Multiple micronutrient supplements improve micronutrient status and anemia but not growth and morbidity of Indonesian infants: A randomized, double-blind, placebo-controlled trial. J. Nutr. 2005, 135, 639S–645S. [Google Scholar] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; West, C.E.; Thurnham, D.I.; Muhilal; Van der Meer, J.W.M. Redistribution of vitamin A after iron supplementation in Indonesian infants. Am. J. Clin. Nutr. 2003, 77, 651–657. [Google Scholar] [PubMed]
- Shamah-Levy, T.; Villalpando, S.; Rivera-Dommarco, J.A.; Mundo-Rosas, V.; Cuevas-Nasu, L.; Jimenez-Aguilar, A. Ferrous gluconate and ferrous sulfate added to a complementary food distributed by the Mexican nutrition program Oportunidades have a comparable efficacy to reduce iron deficiency in toddlers. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Domellof, M.; Cohen, R.J.; Dewey, K.G.; Hernell, O.; Rivera, L.L.; Lonnerdal, B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J. Pediatr. 2001, 138, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Smuts, C.M.; Dhansay, M.A.; Faber, M.; van Stuijvenberg, M.E.; Swanevelder, S.; Gross, R.; Benade, A.J.S. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in south African infants. J. Nutr. 2005, 135, 653S–659S. [Google Scholar] [PubMed]
- Wasantwisut, E.; Winichagoon, P.; Chitchumroonchokchai, C.; Yamborisut, U.; Boonpraderm, A.; Pongcharoen, T.; Sranacharoenpong, K.; Russameesopaphorn, W. Iron and zinc supplementation improved iron and zinc status, but not physical growth, of apparently healthy, breast-fed infants in rural communities of northeast Thailand. J. Nutr. 2006, 136, 2405–2411. [Google Scholar] [PubMed]
- De Romana, G.L.; Cusirramos, S.; de Romana, D.L.; Gross, R. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, growth, and morbidity of Peruvian infants. J. Nutr. 2005, 135, 646S–652S. [Google Scholar]
- Stevens, D.; Nelson, A. The Effect of Iron in Formula Milk after 6 Months of Age. Arch. Dis. Child. 1995, 73, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.A.; Svahn, C.J.; Viinikka, L.U.; Raiha, N.C.; Siimes, M.A.; Axelsson, I.E. Iron-fortified and unfortified cow’s milk: Effects on iron intakes and iron status in young children. Acta Paediatr. 2001, 90, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Arcanjo, F.P.; Santos, P.R.; Costa Arcanjo, C.P.; Meira Magalhaes, S.M.; Madeiro Leite, A.J. Daily and Weekly Iron Supplementations are Effective in Increasing Hemoglobin and Reducing Anemia in Infants. J. Trop. Pediatr. 2013, 59, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Ninh, N.X.; Khan, N.C.; Nhien, N.V.; Lien, D.K.; Trung, N.Q.; Khoi, H.H. Efficacy of combined iron and zinc supplementation on micronutrient status and growth in Vietnamese infants. Eur. J. Clin. Nutr. 2006, 60, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Barth-Jaeggi, T.; Moretti, D.; Kvalsvig, J.D.; Holding, P.A.; Njenga, J.; Mwangi, A.; Chhagan, M.; Lacroix, C.; Zimmermann, M.B. In-home fortification with 2.5 mg iron as NaFeEDTA does not reduce anaemia but increases weight gain: A randomised controlled trial in Kenyan infants. Matern. Child Nutr. 2015, 11, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, J.; Sachdev, H.P.S.; Singh, T.; Mallika, V. A randomized placebo-controlled trial of iron supplementation in breastfed young infants initiated on complementary feeding: Effect on haematological status. J. Health Popul. Nutr. 2004, 22, 203–211. [Google Scholar] [PubMed]
- Morley, R.; Abbott, R.; Fairweather-Tait, S.; MacFadyen, U.; Stephenson, T.; Lucas, A. Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: A randomised trial. Arch. Dis. Child. 1999, 81, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hop, L.T.; Berger, J. Multiple micronutrient supplementation improves anemia, micronutrient nutrient status, and growth of Vietnamese infants: Double-blind, randomized, placebo-controlled trial. J. Nutr. 2005, 135, 660S–665S. [Google Scholar]
- Bradley, C.K.; Hillman, L.; Sherman, A.R.; Leedy, D.; Cordano, A. Evaluation of two iron-fortified, milk-based formulas during infancy. Pediatrics 1993, 91, 908–914. [Google Scholar] [PubMed]
- Yalcin, S.S.; Yurdakok, K.; Acikgoz, D.; Ozmert, E. Short-term developmental outcome of iron prophylaxis in infants. Pediatr. Int. 2000, 42, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, E.M.; Castro, I.R.; Portela, M.; Cardoso, L.O.; Monteiro, C.A. Effectiveness of daily and weekly iron supplementation in the prevention of anemia in infants. Rev. Saude Publica 2008, 42, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Massaga, J.J.; Kitua, A.Y.; Lemnge, M.M.; Akida, J.A.; Malle, L.N.; Ronn, A.M.; Theander, T.G.; Bygbjerg, I.C. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: A randomised placebo-controlled trial. Lancet 2003, 361, 1853–1860. [Google Scholar] [CrossRef]
- Lind, T.; Lonnerdal, B.; Stenlund, H.; Gamayanti, I.L.; Ismail, D.; Seswandhana, R.; Persson, L.A. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: Effects on growth and development. Am. J. Clin. Nutr. 2004, 80, 729–736. [Google Scholar] [PubMed]
- Dewey, K.G.; Domellof, M.; Cohen, R.J.; Landa Rivera, L.; Hernell, O.; Lonnerdal, B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [PubMed]
- Caulfield, L.E.; Zavaleta, N.; Figueroa, A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am. J. Clin. Nutr. 1999, 69, 1257–1263. [Google Scholar] [PubMed]
- Prawirohartono, E.P.; Nystrom, L.; Ivarsson, A.; Stenlund, H.; Lind, T. The impact of prenatal vitamin A and zinc supplementation on growth of children up to 2 years of age in rural Java, Indonesia. Public Health Nutr. 2011, 14, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Duran, C.; Marin, V.B.; Alcazar, L.S.; Iturralde, H.; Ruz, M.O. Controlled trial of zinc supplementation in Chilean pregnant adolescents. Nutr. Res. 2001, 21, 715–724. [Google Scholar] [CrossRef]
- Mahomed, K.; James, D.K.; Golding, J.; Mccabe, R. Zinc Supplementation during Pregnancy—A Double-Blind Randomized Controlled Trial. Br. Med. J. 1989, 299, 826–830. [Google Scholar] [CrossRef]
- Hafeez, A.; Mehmood, G.; Mazhar, F. Oral zinc supplementation in pregnant women and its effect on birth weight: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.E.; Faraji, B.; Swendseid, M.E.; Browdy, B.L.; Coulson, A.H.; Clark, V.A.; Settlage, R.H.; Smith, J.C. Zinc Supplementation during Pregnancy in Low-Income Teenagers of Mexican Descent—Effects on Selected Blood-Constituents and on Progress and Outcome of Pregnancy. Am. J. Clin. Nutr. 1985, 42, 815–828. [Google Scholar] [PubMed]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.E.; Faraji, B.; Swendseid, M.E.; Coulson, A.H.; Clark, V.A.; Browdy, B.L.; Cabalum, M.T.; Smith, J.C. Zinc Supplementation during Pregnancy—Effects on Selected Blood-Constituents and on Progress and Outcome of Pregnancy in Low-Income Women of Mexican Descent. Am. J. Clin. Nutr. 1984, 40, 508–521. [Google Scholar] [PubMed]
- Ross, S.M.; Nel, E.; Naeye, R.L. Differing effects of low and high bulk maternal dietary supplements during pregnancy. Early Hum. Dev. 1985, 10, 295–302. [Google Scholar] [CrossRef]
- Prawirohartono, E.P.; Nystrom, L.; Nurdiati, D.S.; Hakimi, M.; Lind, T. The Impact of Prenatal Vitamin A and Zinc Supplementation on Birth Size and Neonatal Survival—A Double-Blind, Randomized Controlled Trial in a Rural Area of Indonesia. Int. J. Vitam. Nutr. Res. 2013, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Zavaleta, N.; Leon, Z.; Shankar, A.H.; Caulfield, L.E. Maternal zinc supplementation and growth in Peruvian infants. Am. J. Clin. Nutr. 2008, 88, 154–160. [Google Scholar] [PubMed]
- Salmenpera, L.; Perheentupa, J.; Nanto, V.; Siimes, M.A. Low Zinc Intake during Exclusive Breast-Feeding Does Not Impair Growth. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.B.; Aaron, G.J.; Hess, S.Y.; Dossou, N.I.; Guiro, A.T.; Wade, S.; Brown, K.H. Plasma zinc concentration responds to short-term zinc supplementation, but not zinc fortification, in young children in Senegal. Am. J. Clin. Nutr. 2011, 93, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Bahl, R.; Taneja, S.; Strand, T.; Molbak, K.; Ulvik, R.J.; Sommerfelt, H.; Bhan, M.K. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 2002, 109, e86. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Taneja, S.; Mazumder, S.; Bahl, R.; Fontaine, O.; Bhan, M.K. Adding zinc to supplemental iron and folic acid does not affect mortality and severe morbidity in young children. J. Nutr. 2007, 137, 112–117. [Google Scholar] [PubMed]
- Chang, S.; El Arifeen, S.; Bari, S.; Wahed, M.A.; Rahman, K.M.; Rahman, M.T.; Mahmud, A.B.A.; Begum, N.; Zaman, K.; Baqui, A.H.; Black, R.E. Supplementing iron and zinc: Double blind, randomized evaluation of separate or combined delivery. Eur. J. Clin. Nutr. 2010, 64, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.Y.; Abbeddou, S.; Jimenez, E.Y.; Some, J.W.; Vosti, S.A.; Ouedraogo, Z.P.; Guissou, R.M.; Ouedraogo, J.B.; Brown, K.H. Small-Quantity Lipid-Based Nutrient Supplements, Regardless of Their Zinc Content, Increase Growth and Reduce the Prevalence of Stunting and Wasting in Young Burkinabe Children: A Cluster-Randomized Trial. PLoS ONE 2015, 10, e0122242. [Google Scholar] [CrossRef] [PubMed]
- Mazariegos, M.; Hambidge, K.M.; Westcott, J.E.; Solomons, N.W.; Raboy, V.; Das, A.; Goco, N.; Kindem, M.; Wright, L.L.; Krebs, N.F. Neither a Zinc Supplement nor Phytate-Reduced Maize nor Their Combination Enhance Growth of 6-to 12-Month-Old Guatemalan Infants. J. Nutr. 2010, 140, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, K.V.; Hemalatha, R.; Geddam, J.J.B.; Kumar, P.A.; Balakrishna, N.; Shatrugna, V. Effectiveness of Zinc Supplementation to Full Term Normal Infants: A Community Based Double Blind, Randomized, Controlled, Clinical Trial. PLoS ONE 2013, 8, e61486. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S.; Black, R.E.; Bhan, M.K.; Jalla, S.; Bhandari, N.; Sinha, A.; Majumdar, S. Zinc supplementation reduces the incidence of persistent diarrhea and dysentery among low socioeconomic children in India. J. Nutr. 1996, 126, 443–450. [Google Scholar] [PubMed]
- Schlesinger, L.; Arevalo, M.; Arredondo, S.; Diaz, M.; Lonnerdal, B.; Stekel, A. Effect of a Zinc-Fortified Formula on Immunocompetence and Growth of Malnourished Infants. Am. J. Clin. Nutr. 1992, 56, 491–498. [Google Scholar] [PubMed]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.M.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Surono, I.S.; Martono, P.D.; Kameo, S.; Suradji, E.W.; Koyama, H. Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J. Trace Elem. Med. Biol. 2014, 28, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Tielsch, J.M.; Khatry, S.K.; Stoltzfus, R.J.; Katz, J.; LeClerq, S.C.; Adhikari, R.; Mullany, L.C.; Black, R.; Shresta, S. Effect of daily zinc supplementation on child mortality in southern Nepal: A community-based, cluster randomised, placebo-controlled trial. Lancet 2007, 370, 1230–1239. [Google Scholar] [CrossRef]
- Umeta, M.; West, C.E.; Haidar, J.; Deurenberg, P.; Hautvast, J.G.A.J. Zinc supplementation and stunted infants in Ethiopia: A randomised controlled trial. Lancet 2000, 355, 2021–2026. [Google Scholar] [CrossRef]
- Walravens, P.A.; Hambidge, K.M.; Koepfer, D.M. Zinc Supplementation in Infants with a Nutritional Pattern of Failure to Thrive—A Double-Blind, Controlled-Study. Pediatrics 1989, 83, 532–538. [Google Scholar] [PubMed]
- Zlotkin, S.; Arthur, P.; Schauer, C.; Antwi, K.Y.; Yeung, G.; Piekarz, A. Home-fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. J. Nutr. 2003, 133, 1075–1080. [Google Scholar] [PubMed]
- Brown, K.H.; de Romana, D.L.; Arsenault, J.E.; Peerson, J.M.; Penny, M.E. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. Am. J. Clin. Nutr. 2007, 85, 538–547. [Google Scholar] [PubMed]
- Penny, M.E.; Marin, R.M.; Duran, A.; Peerson, J.M.; Lanata, C.F.; Lonnerdal, B.; Black, R.E.; Brown, K.H. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. Am. J. Clin. Nutr. 2004, 79, 457–465. [Google Scholar] [PubMed]
- Wessells, K.R.; Ouedraogo, Z.P.; Rouamba, N.; Hess, S.Y.; Ouedraogo, J.B.; Brown, K.H. Short-term zinc supplementation with dispersible tablets or zinc sulfate solution yields similar positive effects on plasma zinc concentration of young children in Burkina Faso: A randomized controlled trial. J. Pediatr. 2012, 160, 129.e3–135.e3. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.J.; Brown, K.H.; Lonnerdal, B.; Dewey, K.G. Zinc supplementation does not affect growth, morbidity, or motor development of US term breastfed infants at 4–10 months of age. Am. J. Clin. Nutr. 2006, 84, 594–601. [Google Scholar] [PubMed]
- Alarcon, K.; Kolsteren, P.W.; Prada, A.M.; Chian, A.M.; Velarde, R.E.; Pecho, I.L.; Hoeree, T.F. Effects of separate delivery of zinc or zinc and vitamin A on hemoglobin response, growth, and diarrhea in young Peruvian children receiving iron therapy for anemia. Am. J. Clin. Nutr. 2004, 80, 1276–1282. [Google Scholar] [PubMed]
- Gardner, J.M.M.; Powell, C.A.; Baker-Henningham, H.; Walker, S.P.; Cole, T.J.; Grantham-McGregor, S.M. Zinc supplementation and psychosocial stimulation: Effects on the development of undernourished Jamaican children. Am. J. Clin. Nutr. 2005, 82, 399–405. [Google Scholar] [PubMed]
- Ninh, N.X.; Thissen, J.P.; Collette, L.; Gerard, G.; Khoi, H.H.; Ketelslegers, J.M. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am. J. Clin. Nutr. 1996, 63, 514–519. [Google Scholar] [PubMed]
- Rivera, J.A.; Ruel, M.T.; Santizo, M.C.; Lonnerdal, B.; Brown, K.H. Zinc supplementation improves the growth of stunted rural Guatemalan infants. J. Nutr. 1998, 128, 556–562. [Google Scholar] [PubMed]
- Sur, D.; Gupta, D.N.; Mondal, S.K.; Ghosh, S.; Manna, B.; Rajendran, K.; Bhattacharya, S.K. Impact of zinc supplementation on diarrheal morbidity and growth pattern of low birth weight infants in Kolkata, India: A randomized, double-blind, placebo-controlled, community-based study. Pediatrics 2003, 112, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Walravens, P.A.; Chakar, A.; Mokni, R.; Denise, J.; Lemonnier, D. Zinc supplements in breastfed infants. Lancet 1992, 340, 683–685. [Google Scholar] [CrossRef]
- Olney, D.K.; Pollitt, E.; Kariger, P.K.; Khalfan, S.S.; Ali, N.S.; Tielsch, J.M.; Sazawal, S.; Black, R.; Allen, L.H.; Stoltzfus, R.J. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5-to 11-month old. J. Nutr. 2006, 136, 2427–2434. [Google Scholar] [PubMed]
- Umeta, M.; West, C.E.; Verhoef, H.; Haidar, J.; Hautvast, J.G.A.J. Factors associated with stunting in infants aged 5–11 months in the Dodota-Sire District, rural Ethiopia. J. Nutr. 2003, 133, 1064–1069. [Google Scholar] [PubMed]
- Wuehler, S.E.; Sempertegui, F.; Brown, K.H. Dose-response trial of prophylactic zinc supplements, with or without copper, in young Ecuadorian children at risk of zinc deficiency. Am. J. Clin. Nutr. 2008, 87, 723–733. [Google Scholar] [PubMed]
- Sazawal, S.; Black, R.E.; Bhan, M.K.; Jalla, S.; Sinha, A.; Bhandari, N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea—A community-based, double-blind, controlled trial. Am. J. Clin. Nutr. 1997, 66, 413–418. [Google Scholar] [PubMed]
- Tielsch, J.M.; Khatry, S.K.; Stoltzfus, R.J.; Katz, J.; LeClerq, S.C.; Adhikari, R.; Mullany, L.C.; Shresta, S.; Black, R.E. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: Community-based, cluster-randomised, placebo-controlled trial. Lancet 2006, 367, 144–152. [Google Scholar] [CrossRef]
- Larson, C.P.; Nasrin, D.; Saha, A.; Chowdhury, M.I.; Qadri, F. The added benefit of zinc supplementation after zinc treatment of acute childhood diarrhoea: A randomized, double-blind field trial. Trop. Med. Int. Health 2010, 15, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.N.; Mondal, S.K.; Ghosh, S.; Rajendran, K.; Sur, D.; Manna, B. Impact of zinc supplementation on diarrhoeal morbidity in rural children of West Bengal, India. Acta Paediatr. 2003, 92, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.T.; Rivera, J.A.; Santizo, M.C.; Lonnerdal, B.; Brown, K.H. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics 1997, 99, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Chhagan, M.K.; Van den Broeck, J.; Luabeya, K.K.; Mpontshane, N.; Tucker, K.L.; Bennish, M.L. Effect of micronutrient supplementation on diarrhoeal disease among stunted children in rural South Africa. Eur. J. Clin. Nutr. 2009, 63, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Sazawal, S. Daily zinc supplements reduced the incidence and severity of acute lower respiratory infections in children in India. Evid. Based Nurs. 1999, 2, 12. [Google Scholar]
- Ramakrishnan, U.; Gonzalez-Cossio, T.; Neufeld, L.M.; Rivera, J.; Martorell, R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: A randomized controlled trial in a semirural community in Mexico. Am. J. Clin. Nutr. 2003, 77, 720–725. [Google Scholar] [PubMed]
- Taneja, S.; Bhandari, N.; Bahl, R.; Bhan, M.K. Impact of zinc supplementation on mental and psychomotor scores of children aged 12 to 18 months: A randomized, double-blind trial. J. Pediatr. 2005, 146, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Duran, C.; Perales, C.G.; Hertrampf, E.D.; Marin, V.B.; Rivera, F.A.; Icaza, G. Effect of zinc supplementation on development and growth of Chilean infants. J. Pediatr. 2001, 138, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, 2, CD000230. [Google Scholar]
- Chaffee, B.W.; King, J.C. Effect of Zinc Supplementation on Pregnancy and Infant Outcomes: A Systematic Review. Paediatr. Perinat. Epidemiol. 2012, 26, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 7, CD004736. [Google Scholar]
- Eichler, K.; Wieser, S.; Ruthemann, I.; Brugger, U. Effects of micronutrient fortified milk and cereal food for infants and children: A systematic review. BMC Public Health 2012, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Hayes, E.; Kalumba, K.; Biggs, B.A. Effect of daily iron supplementation on health in children aged 4–23 months: A systematic review and meta-analysis of randomised controlled trials. Lancet Glob. Health 2013, 1, E77–E86. [Google Scholar] [CrossRef]
- Cook, J.D.; Lipschit, D.A.; Miles, L.E.M.; Finch, C.A. Serum Ferritin as a Measure of Iron Stores in Normal Subjects. Am. J. Clin. Nutr. 1974, 27, 681–687. [Google Scholar] [PubMed]
- Moretti, D.; Zimmermann, M.B.; Wegmuller, R.; Walczyk, T.; Zeder, C.; Hurrell, R.F. Iron status and food matrix strongly affect the relative bioavailability of ferric pyrophosphate in humans. Am. J. Clin. Nutr. 2006, 83, 632–638. [Google Scholar] [PubMed]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Donahue Angel, M.; Rohner, F. The Proportion of Anemia Associated with Iron Deficiency in Low, Medium, and High Human Development Index Countries: A Systematic Analysis of National Surveys. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- De Brito, N.J.; Rocha, E.D.; de Araujo Silva, A.; Costa, J.B.; Franca, M.C.; das Gracas Almeida, M.; Brandao-Neto, J. Oral zinc supplementation decreases the serum iron concentration in healthy schoolchildren: A pilot study. Nutrients 2014, 6, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Dassenko, S.A.; Lynch, S.R. Assessment of the role of nonheme-iron availability in iron balance. Am. J. Clin. Nutr. 1991, 54, 717–722. [Google Scholar] [PubMed]
- Reddy, M.B.; Hurrell, R.F.; Cook, J.D. Estimation of nonheme-iron bioavailability from meal composition. Am. J. Clin. Nutr. 2000, 71, 937–943. [Google Scholar] [PubMed]
- Singla, P.N.; Tyagi, M.; Shankar, R.; Dash, D.; Kumar, A. Fetal iron status in maternal anemia. Acta Paediatr. 1996, 85, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [PubMed]
- Nakamori, M.; Ninh, N.X.; Isomura, H.; Yoshiike, N.; Hien, V.T.; Nhug, B.T.; Nhien, N.V.; Nakano, T.; Khan, N.C.; Yamamoto, S. Nutritional status of lactating mothers and their breast milk concentration of iron, zinc and copper in rural Vietnam. J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Nikniaz, L.; Gayemmagami, S.J. Association between Zinc, Copper, and Iron Concentrations in Breast Milk and Growth of Healthy Infants in Tabriz, Iran. Biol. Trace Elem. Res. 2010, 135, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Domellof, M.; Lonnerdal, B.; Dewey, K.G.; Cohen, R.J.; Hernell, O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 2004, 79, 111–115. [Google Scholar] [PubMed]
- Shashiraj; Faridi, M.M.A.; Singh, O.; Rusia, U. Mother’s iron status, breastmilk iron and lactoferrin—Are they related? Eur. J. Clin. Nutr. 2006, 60, 903–908. [Google Scholar] [CrossRef] [PubMed]

| Variables | Mean Difference 1 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups |
|---|---|---|---|---|---|
| Birth outcomes | |||||
| Birthweight (g) | 38 (−16; 91) | 6, 13,627 | 58.2 | 0.17 | |
| Low birth weight (%) | 0.69 (0.38; 1.26) | 5, 12,845 | 63.1 | 0.23 | |
| Variables | Mean Difference 2 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups (Bold Font) and Subgroups (Regular Font) |
|---|---|---|---|---|---|
| Hb overall (g/dL) | 4.1 (2.8; 5.3) | 30, 6569 | 81.5 | <0.001 | |
| Iron dose | |||||
| <6 mg/day | −0.7 (−6.1; 4.7) | 2, 220 | 73.1 | 0.12 | |
| 6–8 mg/day | 4.4 (2.1; 6.8) | 7, 1864 | 83.9 | ||
| >8–10 mg/day | 5.5 (3.4; 7.6) | 13, 3068 | 83.8 | ||
| 11–15 mg/day | 2.7 (1.2; 4.2) | 4, 403 | 80.7 | ||
| Type of intervention | |||||
| Supplementation | 5.6 (3.4; 7.7) | 15, 3516 | 86.4 | <0.01 | |
| Fortification 3 | 2.6 (1.3; 3.9) | 16, 3053 | 67.4 | ||
| RCT, quality rating | |||||
| highest | 5.5 (3.3; 7.6) | 12, 3403 | 87.1 | <0.05 | |
| intermediate | 3.2 (1.6; 4.8) | 14, 2623 | 73.1 | ||
| lowest | 1.3 (−2.9; 5.4) | 4, 375 | 80.3 | ||
| Anemia overall | 0.59 (0.49; 0.70) | 22, (5647) | 73.8 | <0.0001 | |
| Iron dose | |||||
| 6–8 mg/day | 0.54 (0.44; 0.66) | 7, 2089 | 18.4 | 0.32 | |
| >8–10 mg/day | 0.59 (0.45; 0.77) | 9, 2575 | 85.2 | ||
| 11–15 mg/day | 0.82 (0.51; 1.30) | 3, 489 | 31.0 | ||
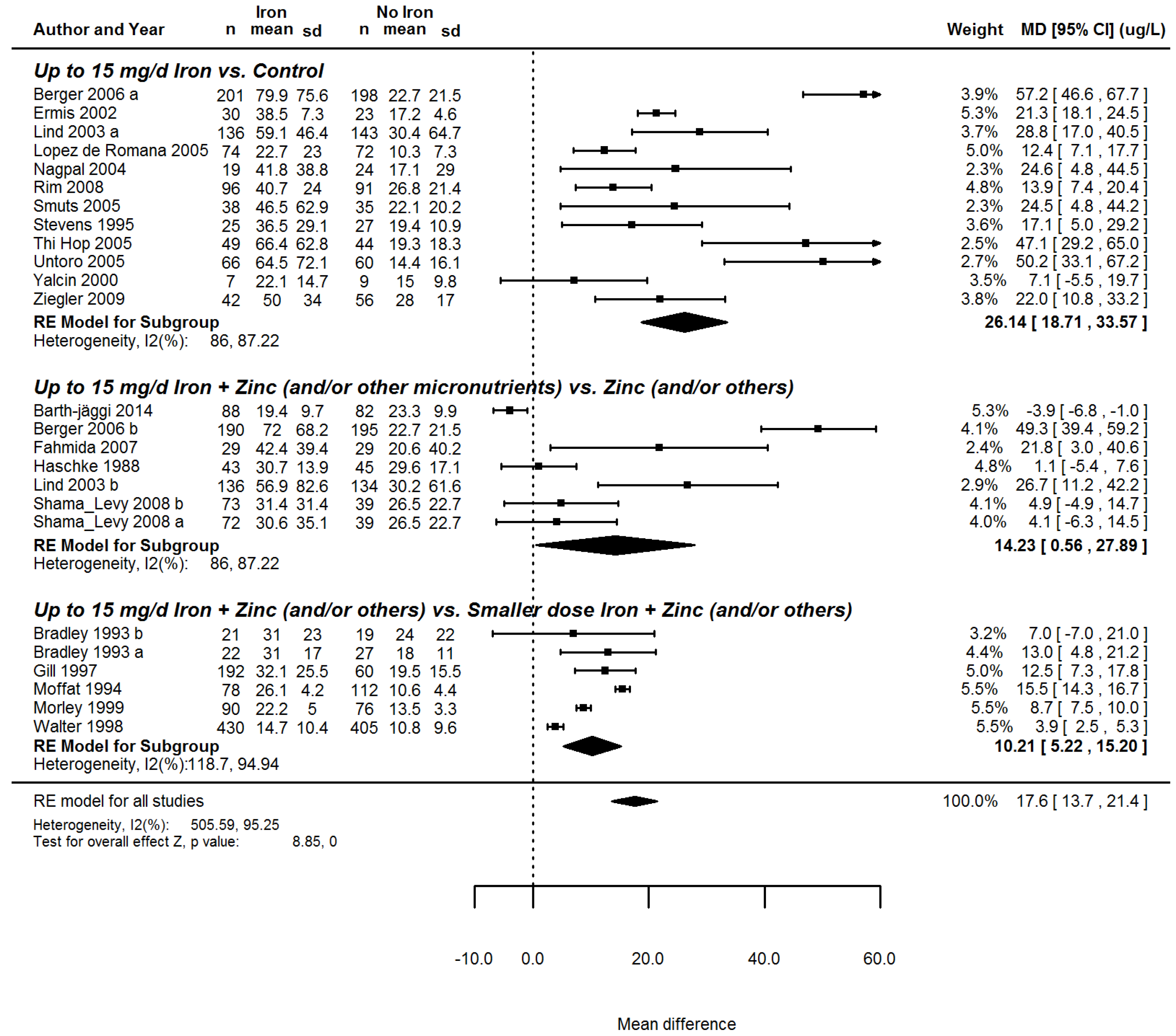
| Serum ferritin (µg/dL) | 17.3 (13.5; 21.2) | 21, (4291) | 95.1 | <0.0001 | |
| Iron dose | |||||
| <6 mg/day | 5.8 (−14.8; 26.3) | 2, 222 | 90.9 | <0.01 | |
| 6–8 mg/day | 12.1 (2.6; 21.7) | 5, 1261 | 96.4 | ||
| >8–10 mg/day | 27.5 (16.0; 39.0) | 9, 2068 | 96.4 | ||
| Type of intervention | |||||
| Supplementation | 27.2 (18.2; 36.3) | 8, 1747 | 90.1 | <0.001 | |
| Fortification | 11.3 (13.7; 21.4) | 13, 2544 | 95.2 | ||
| RCT, quality rating | |||||
| highest | 22.8 (15.2; 30.4) | 9, 2351 | 93.0 | 0.08 | |
| intermediate | 11.4 (6.6; 16.1) | 9, 1619 | 93.0 | ||
| lowest | 15.0 (7.0; 23.0) | 3, 321 | 92.0 | ||
| Baseline ID prevalence | |||||
| Low (<15%) | 27.0 (13.6; 40.4) | 4, 276 | 60.5 | 0.76 | |
| High (≥15%) | 32.4 (8.9; 55.9) | 4, 1226 | 98.3 | ||
| Baseline mean serum ferritin 4 | |||||
| Low (<29.2 µg/L) | 18.5 (11.7; 25.3) | 9, 1352 | 76.4 | 0.99 | |
| High (≥29.2 µg/L) | 21.2 (11.5; 30.9) | 8, 1698 | 94.0 | ||
| ID overall | 0.22 (0.14; 0.35) | 13, 3698 | 86.3 | <0.0001 | |
| IDA overall | 0.20 (0.11; 0.37) | 8, 3464 | 64.2 | <0.0001 | |
| Variables | Mean Difference 2 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups |
|---|---|---|---|---|---|
| Growth | |||||
| WAZ | −0.01 (−0.08; 0.05) | 10, 3511 | 12.5 | 0.69 | |
| WHZ | 0.02 (−0.06; 0.09) | 9, 3297 | 36.8 | 0.62 | |
| HAZ | −0.02 (−0.08; 0.04) | 10, 3511 | 8.2 | 0.57 | |
| Stunting | 1.09 (0.92; 1.29) | 4, 2159 | 0 | 0.33 | |
| Wasting | 1.11 (0.84; 1.47) | 4, 1975 | 0 | 0.45 | |
| Mental and motor development | |||||
| MDI | 0.4 (−0.9; 1.7) | 4, 1062 | 19.9 | 0.60 | |
| PDI | 0.6 (−1.2; 2.4) | 4, 1062 | 61.9 | 0.50 | |
| Variables | Mean Difference 1 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups |
|---|---|---|---|---|---|
| Birth outcomes | |||||
| Birthweight (g) | 1 (−32; 35) | 8, 3457 | 0 | 0.94 | |
| Low birth weight | 0.96 (0.67; 1.37) | 6, 2518 | 0 | 0.83 | |
| Variables | Mean Difference 2 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups (Bold Font) and Subgroups (Regular Font) |
|---|---|---|---|---|---|
| Serum zinc overall (µmol/L) | 2.0 (1.2; 2.9) | 23, 8848 | 96.1 | <0.0001 | |
| Zinc dose | |||||
| <4 mg/day | 0.81 (−0.07; 1.68) | 1, 256 | 55.5 | 0.05 | |
| 4–<7 mg/day | 0.9 (0.08; 1.71) | 7, 1296 | 92.4 | ||
| 7–10 mg/day | 3.0 (1.5; 4.5) | 14, 6867 | 98.5 | ||
| Type of intervention | |||||
| Supplementation | 2.4 (1.5; 3.4) | 19, 7732 | 98.5 | <0.05 | |
| Fortification 3 | 0.3 (−0.1; 0.8) | 6, 816 | 98.1 | ||
| Baseline ZD prevalence | |||||
| Low (<25%) | 2.9 (0.2; 5.7) | 4, 1231 | 97.8 | 0.15 | |
| High (≥25%) | 2.8 (1.7; 3.9) | 4, 2372 | 95.5 | ||
| Baseline mean serum zinc 4 | |||||
| Low (<10.75 µg/L) | 2.4 (0.7; 4.2) | 7, 5635 | 98.7 | 0.96 | |
| High (≥10.75 µg/L) | 2.3 (0.7; 3.9) | 9, 2200 | 96.9 | ||
| ZD overall | 0.47 (0.32; 0.69) | 12, 6666 | 92.2 | <0.001 | |
| Variables | Mean Difference 2 | Relative Risk | Studies, Participants (n) | I2 (%) | p Difference between Pooled Intervention and Control Groups |
|---|---|---|---|---|---|
| Growth | |||||
| WAZ | 0.05 (0.00; 0.10) | 21, 7440 | 39.4 | 0.04 | |
| WHZ | 0.04 (0.00; 0.08) | 16, 6875 | 22.3 | 0.04 | |
| HAZ | 0.00 (−0.04; 0.03) | 20, 7340 | 9.2 | 0.80 | |
| Stunting | 0.97 (0.90; 1.04) | 6, 5443 | 0 | 0.39 | |
| Wasting | 0.98 (0.79; 1.21) | 6, 5441 | 32.0 | 0.82 | |
| Underweight | 0.99 (0.90; 1.09) | 5, 4793 | 10.7 | 0.83 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petry, N.; Olofin, I.; Boy, E.; Donahue Angel, M.; Rohner, F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 773. https://doi.org/10.3390/nu8120773
Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients. 2016; 8(12):773. https://doi.org/10.3390/nu8120773
Chicago/Turabian StylePetry, Nicolai, Ibironke Olofin, Erick Boy, Moira Donahue Angel, and Fabian Rohner. 2016. "The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis" Nutrients 8, no. 12: 773. https://doi.org/10.3390/nu8120773
APA StylePetry, N., Olofin, I., Boy, E., Donahue Angel, M., & Rohner, F. (2016). The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients, 8(12), 773. https://doi.org/10.3390/nu8120773





