Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
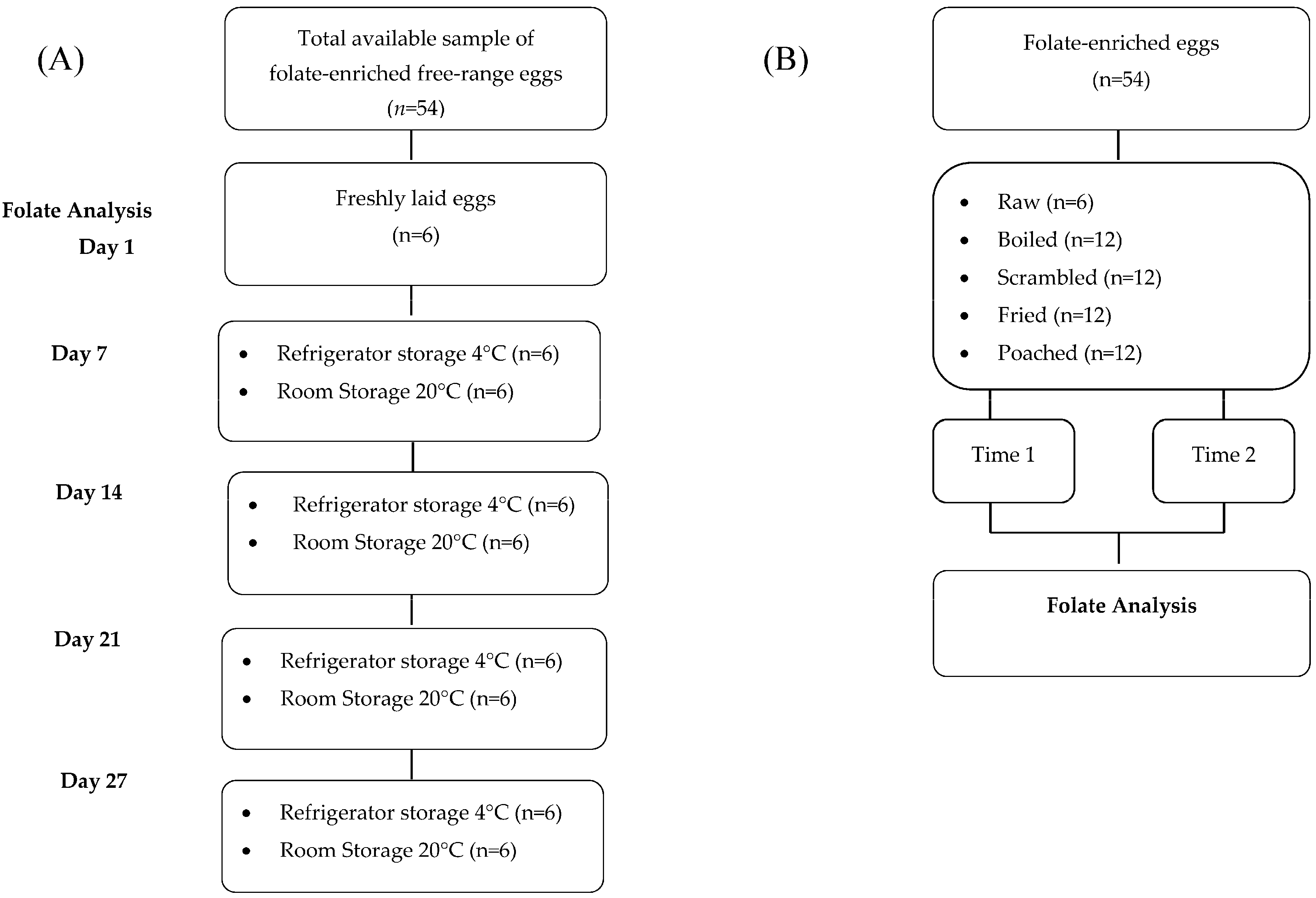
2.1.1. Stability of Folates during Storage
2.1.2. Stability of Folates during Cooking
2.2. Sampling
2.3. Determination of Total Folate Content of Samples
2.4. Thermal Extraction with Tri-Enzyme Treatment
2.5. Microbiological Assay with Lactobacillus casei
2.6. Statistical Methods
3. Results
3.1. Effect of Storage on Egg Folate Content
3.2. Effect of Cooking on Egg Folate Content
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Czeizel, A.E.; Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Eng. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003768. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.J.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Smith, D.A.; Refsum, H.; Homocysteine, B. Vitamins, and Cognitive Impairment. Ann. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Honda, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Effect of folate and mecobalamin on hip fractures in patients with stroke: A randomized controlled trial. JAMA 2005, 293, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Ward, M.; Dickey, W.; Hoey, L.; Molloy, A.M.; Waldron, L.; Varghese, A.; McCann, A.; Blayney, J.K.; McNulty, H. B-vitamin status in relation to bone mineral density in treated celiac disease patients. Scand. J. Gastroenterol. 2015, 50, 975–984. [Google Scholar] [CrossRef] [PubMed]
- McNulty, B.; Pentieva, K.; Marshall, B; Ward, M.; Molloy, A.M.; Scott, J.M.; McNulty, H. Women’s compliance with current folic acid recommendations and achievement of optimal vitamin status for preventing neural tube defects. Hum. Reprod. 2011, 26, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland. Report of the Scientific Committee of the Food Safety Authority of Ireland: Update Report on Folic Acid and the Prevention of Birth Defects in Ireland; Food Safety Authority of Ireland: Dublin, Ireland, 2016. [Google Scholar]
- Khoshnood, B.; Loane, M.; de Walle, H.; Arriola, L.; Addor, M.; Barisic, I.; Beres, J.; Bianchi, F.; Dias, C.; Draper, E.; et al. Long term trends in prevalence of neural tube defects in Europe: Population based study. Br. Med. J. 2015, 351, h5949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choumenkovitch, S.F.; Jacques, P.F.; Nadeau, M.R.; Wilson, P.W.; Rosenberg, I.H.; Selhub, J. Folic acid fortification increases red blood cell folate concentrations in the Framingham Study. J. Nutr. 2001, 131, 3277–3280. [Google Scholar] [PubMed]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Eng. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Paulozzi, L.J.; Mathews, T.J.; Erickson, J.D.; Wong, L.Y. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001, 285, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Botto, L.D.; Erickson, D.; Berry, R.J.; Sambell, C.; Johansen, H.; Friedman, J.M. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 2006, 113, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [PubMed]
- Sweeney, M.R.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.G.; Lindenbaum, J. Folate-cobalamin interactions. In Folate in Health and Disease, 1st ed.; Bailey, L.B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 237–285. [Google Scholar]
- Cole, B.F.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.I.; Burke, C.A.; et al. Folic acid for the prevention of colorectal adenomas: A randomised clinical trial. JAMA 2007, 297, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.; House, J.D.; Guenter, W. Effect of dietary folic acid supplementation on egg folate content and the performance and folate status of two strains of laying hens. Poult. Sci. 2005, 84, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hoey, L.; McNulty, H.; McCann, E.M.E.; McCracken, K.J.; Scott, J.M.; Marc, B.B.; Molloy, A.M.; Graham, C.; Pentieva, K. Laying hens can convert high doses of folic acid added to the feed into natural folates in eggs providing a novel source of food folate. Br. J. Nutr. 2009, 101, 206–212. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Braun, K.; Balance, D.M.; O’Connor, C.P.; Guenter, W. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poult. Sci. 2002, 81, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Arcot, J.; Shrestha, A. Folate retention in selected processed legumes. Food Chem. 2000, 68, 295–298. [Google Scholar] [CrossRef]
- Hawkes, J.G.; Villota, R. Folates in food: Reactivity, stability during processing, and nutritional implications. Crit. Rev. Food Sci. Nutr. 1989, 28, 439–538. [Google Scholar] [PubMed]
- McKillop, D.J.; Pentieva, K.; Daly, D.; McPartlin, J.M.; Hughes, J.; Strain, J.J.; Scott, J.M.; McNulty, H. The effect of different cooking methods on folate retention in various foods that are amongst the major contributors to folate intake in the UK diet. Br. J. Nutr. 2002, 88, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T. Determination of food folate. J. Nutr. Biochem. 1998, 9, 285–293. [Google Scholar] [CrossRef]
- Molloy, A.; Scott, J. Microbiological assay for serum, plasma and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [PubMed]
- McNulty, H.; Pentieva, K. Folate bioavailability. In Folate in Health and Disease, 2nd ed.; Bailey, L.B., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 25–48. [Google Scholar]
- DeSouza, S.C.; Eitenmiller, R.R. Effects of processing and storage on the folate content of spinach and broccoli. J. Food Sci. 1986, 51, 626–628. [Google Scholar] [CrossRef]
- Phillips, K.M.; Wunderlich, K.M.; Holden, J.M.; Exler, J.; Gebhardt, S.E.; Haytowitz, D.B.; Beecher, G.R.; Doherty, R.F. Stability of 5-methyltetrahydrofolate in frozen fresh fruits and vegetables. Food Chem. 2005, 92, 587–595. [Google Scholar] [CrossRef]
- Puupponen-Piniä, R.; Häkkinen, S.T.; Aarni, M.; Suortti, T.; Lampi, A.M.; Eurola, M.; Piironen, V.; Nuutila, A.M.; Oksman-Caldentey, K.M. Blanching and long-term freezing affect various bioactive compounds of vegetables in different ways. J. Sci. Food Agric. 2003, 83, 1389–1402. [Google Scholar] [CrossRef]
- Vahteristo, L.T.; Lehikoinen, K.E.; Ollilainen, V.; Koivistoinen, P.E.; Varo, P. Oven-baking and frozen storage affect folate vitamer retention. Food Sci. Technol. 1998, 31, 329–333. [Google Scholar] [CrossRef]
- Han, Y.H.; Yon, M.; Hyun, T.H. Folate intake estimated with an updated database and its association to blood folate and homocysteine in Korean college students. Eur. J. Clin. Nutr. 2005, 59, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, E.; Selhub, J. Properties of food folate determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J. Nutr. 1998, 128, 1956–1960. [Google Scholar] [PubMed]
- Hurdle, A.D.F.; Barton, D.; Searles, I.H. A method for measuring folate in food and its application to a hospital diet. Am. J. Clin. Nutr. 1968, 21, 1202–1207. [Google Scholar] [PubMed]
- Wilson, S.D.; Horne, D.W. Evaluation of ascorbic acid in protecting labile folic acid derivatives. Proc. Natl. Acad. Sci. USA 1983, 80, 6500–6504. [Google Scholar] [CrossRef] [PubMed]
- Vahteristo, L.T.; Ollilainen, V.; Koivistoinen, P.E.; Varo, P. Improvements in the analysis of reduced folate monoglutamates and folic acid in food by high-performance liquid chromatography. J. Agric. Food Chem. 1996, 44, 477–482. [Google Scholar] [CrossRef]
- Lund, D. Effects of heat processing on nutrients. In Nuritional Evaluation of Food Processing, 3rd ed.; Harris, R.S., Karmas, E., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1988; pp. 319–354. [Google Scholar]
- Selman, J.D. Vitamin retention during blanching of vegetables. Food Chem. 1994, 49, 137–147. [Google Scholar] [CrossRef]
- Jägerstad, M.; Jastrebova, J.; Svensson, U. Folates in fermented vegetables—A pilot study. Lebensmittel-Wissenschaft und -Technologie 2004, 37, 603–611. [Google Scholar] [CrossRef]
- Sherwood, T.A.; Alphin, R.L.; Saylor, W.W.; White, H.B. Folate metabolism and deposition in eggs by laying hens. Arch. Biochem. Biophys. 1993, 307, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ball, G.F.M. Folate. In Bioavailability and Analysis of Vitamins in Foods; Chapman and Hall: London, UK, 1998; pp. 439–496. [Google Scholar]
- McKillop, D.J.; McNulty, H.; Scott, J.M.; McPartlin, J.M.; Strain, J.J.; Bradbury, I.; Girvan, J.; Hoey, L.; McCreedy, R.; Alexander, J.; et al. The rate of intestinal absorption of natural food folates is not related to the extent of folate conjugation. Am. J. Clin. Nutr. 2006, 84, 167–173. [Google Scholar] [PubMed]
- Bates, B.; Lennox, A.; Prentice, A.; Bates, C.; Page, P.; Nicholson, S.; Balmer, R.; Swan, G. National Diet and Nutrition Survey Rolling Programme (NDNS RP) Results from Years 1–4 (Combined) for Northern Ireland. Food Standard Agency in Northern Ireland and Public Health England; 2015. Available online: https://www.food.gov.uk/northern-ireland/researchni/ndns-ni-appendices-tables (accessed on 20 September 2016). [Google Scholar]
- Harman, N.L.; Leeds, A.R.; Griffin, B.A. Increased dietary cholesterol does not increase plasma low density lipoprotein when accompanied by an energy-restricted diet and weight loss. Eur. J. Nutr. 2008, 47, 287–293. [Google Scholar] [CrossRef] [PubMed]
| Folate Concentration (μg/100 g) | |||||||
|---|---|---|---|---|---|---|---|
| Un-Enriched Barn | Un-Enriched Free-Range | Folate-Enriched 2 | p-Value 3 | ||||
| 4–7 °C | 18–20 °C | 4–7 °C | 18–20 °C | 4–7 °C | 18–20 °C | ||
| Day 1 | 41.4 (2.8) a | 65.6 (18.5) b | 123.2 (12.4) c | <0.001 | |||
| Day 7 | N/A | N/A | 107.9 (9.6) | 127.0 (26.9) | 0.17 | ||
| Day 14 | N/A | N/A | 134.0 (16.9) | 115.1 (24.3) | 0.21 | ||
| Day 21 | N/A | N/A | 119.5 (19.7) | 108.2 (14.7) | 0.28 | ||
| Day 27 | 36.6 (11.3) a | 43.9 (5.9) a | 70.0 (16.4) b | 65.9 (7.2) b | 122.0 (27.7) c | 123.7 (18.3) c | <0.001 |
| Cooking Method | Folate Concentration (μg/100 g) | p-Value 2 | ||
|---|---|---|---|---|
| Raw (n = 6) | Time 1 3 (n = 6) 4 | Time 2 3 (n = 6) 4 | ||
| Boiled | 135.7 (22.5) | 125.2 (28.6) | 145.4 (20.5) | 0.402 |
| Fried | 135.7 (22.5) | 137.2 (11.7) | 139.1 (12.1) | 0.730 |
| Scrambled | 135.7 (22.5) | 133.9 (23.0) | 132.5 (35.1) | 0.616 |
| Poached | 135.7 (22.5) | 126.9 (10.8) | 132.7 (19.1) | 0.597 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altic, L.; McNulty, H.; Hoey, L.; McAnena, L.; Pentieva, K. Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers. Nutrients 2016, 8, 777. https://doi.org/10.3390/nu8120777
Altic L, McNulty H, Hoey L, McAnena L, Pentieva K. Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers. Nutrients. 2016; 8(12):777. https://doi.org/10.3390/nu8120777
Chicago/Turabian StyleAltic, Leslie, Helene McNulty, Leane Hoey, Liadhan McAnena, and Kristina Pentieva. 2016. "Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers" Nutrients 8, no. 12: 777. https://doi.org/10.3390/nu8120777





