The Sweetener-Sensing Mechanisms of the Ghrelin Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mice
2.3. Experimental Design
2.4. Ghrelin Tissue Extraction
2.5. Ghrelin Release from Intestinal Segments
2.6. Ghrelin Release from Ghrelinoma Cells
2.7. Radioimmunoassay (RIA)
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
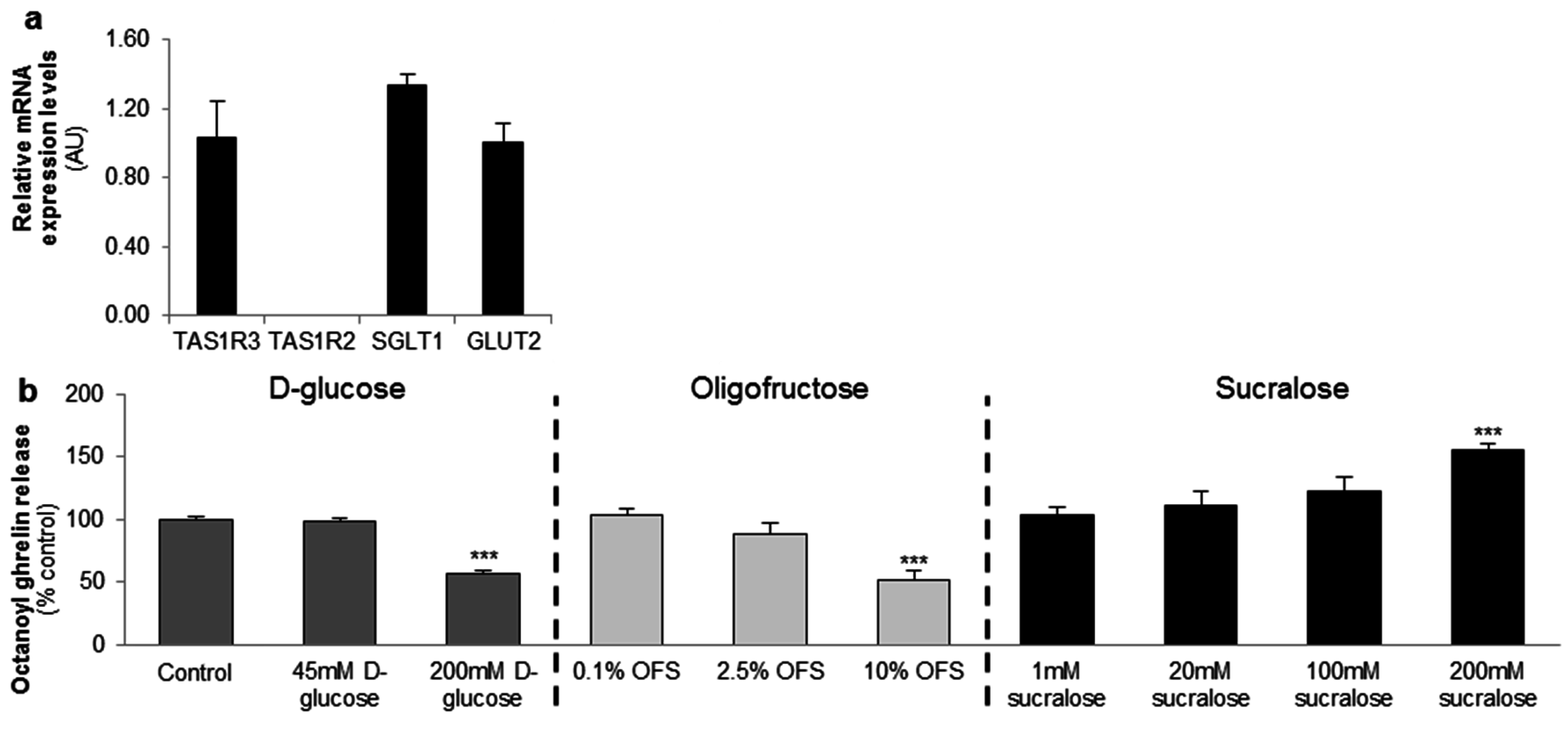
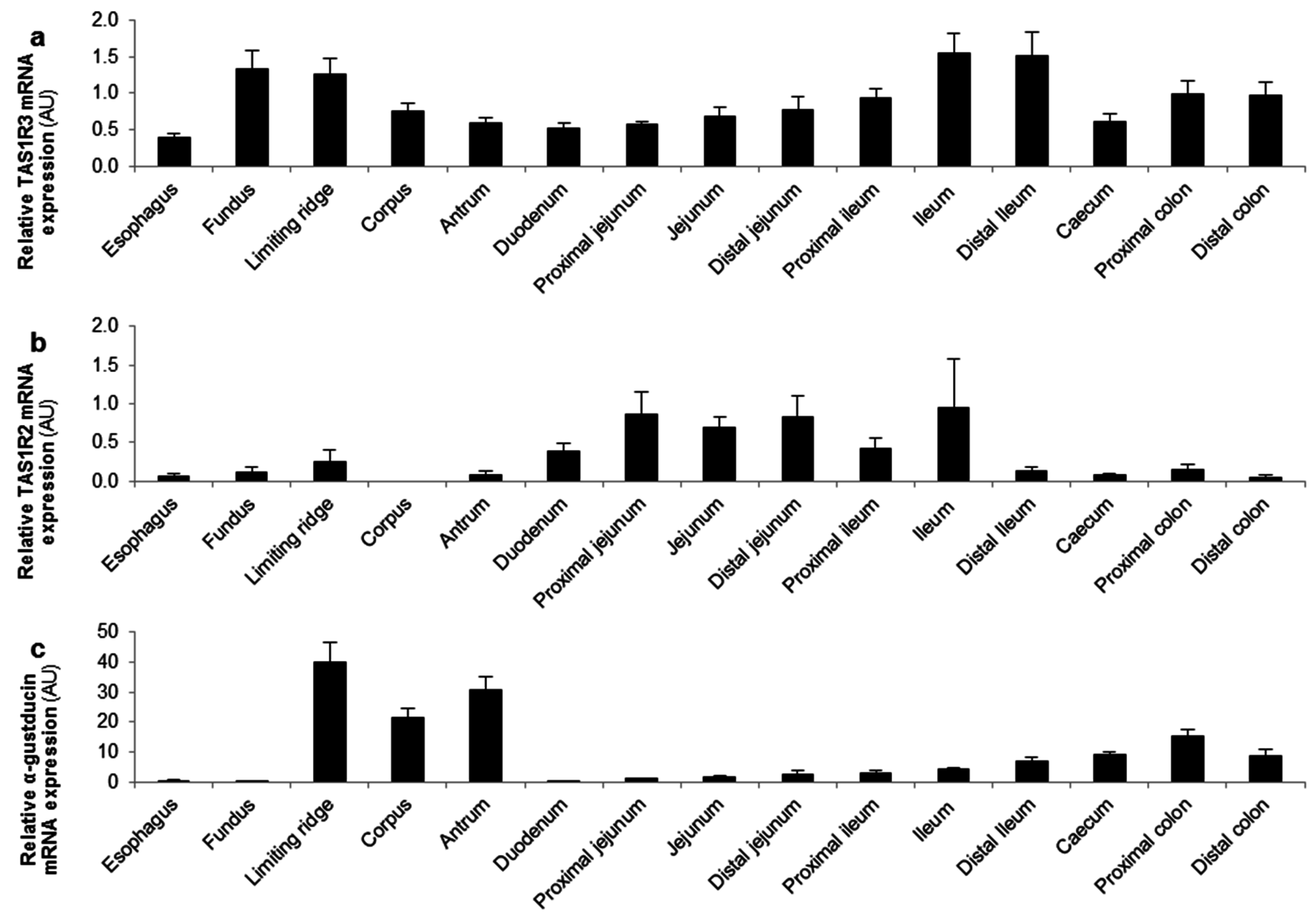
3.1. In Vitro Studies in the MGN3-1 Ghrelinoma Cell Line
3.2. Ex Vivo Studies in Intestinal Segments
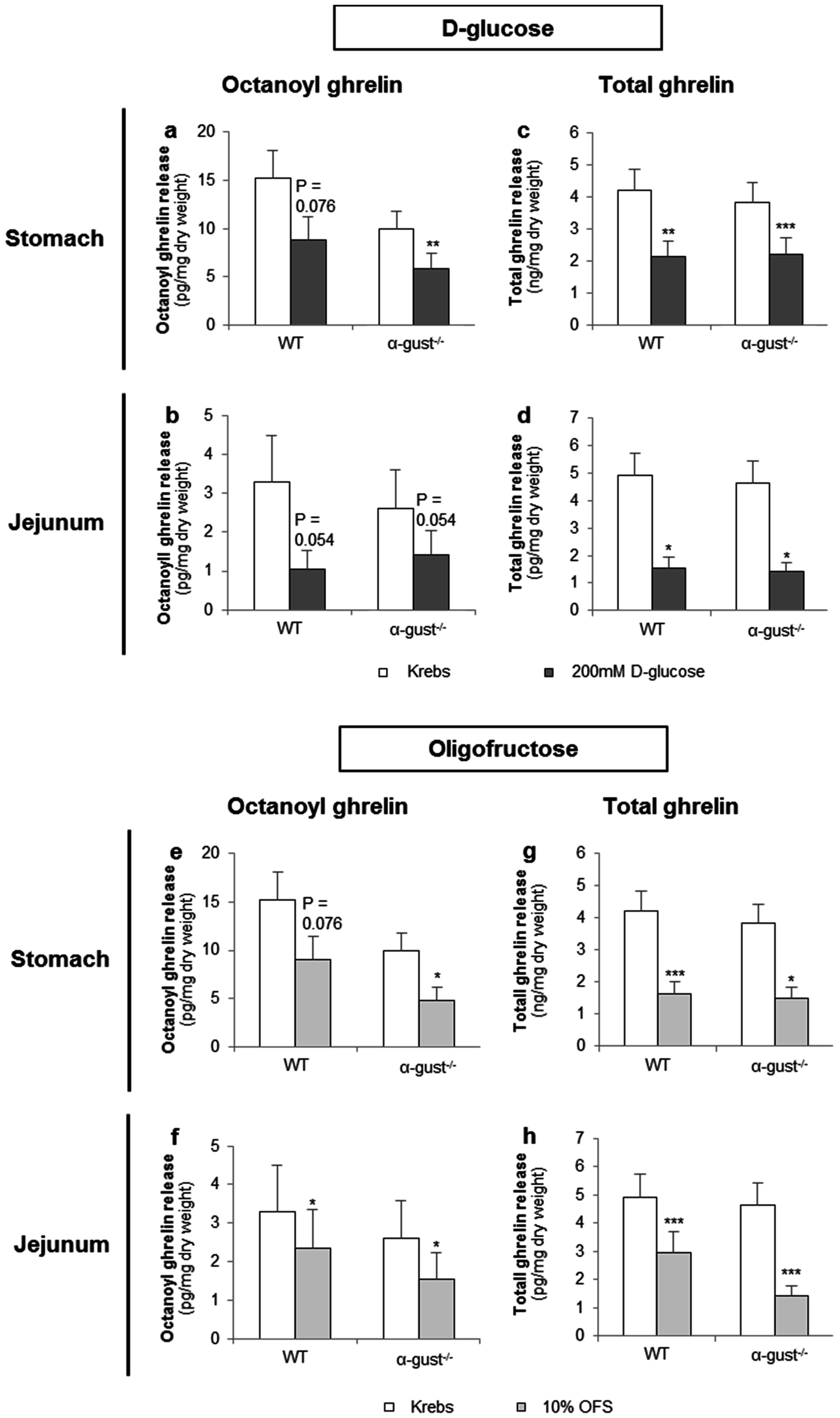
3.2.1. d-Glucose Decreased Ghrelin Release from Gastric and Jejunal Segments in an α-Gustducin Independent Manner
3.2.2. OFS Decreased Ghrelin Release from Gastric and Jejunal Segments in an α-Gustducin Independent Manner
3.2.3. Sucralose Increased Octanoyl Ghrelin, but Not Total Ghrelin Release from Gastric and Jejunal Segments in an α-Gustducin Independent Manner
3.3. In Vivo Studies in Mice
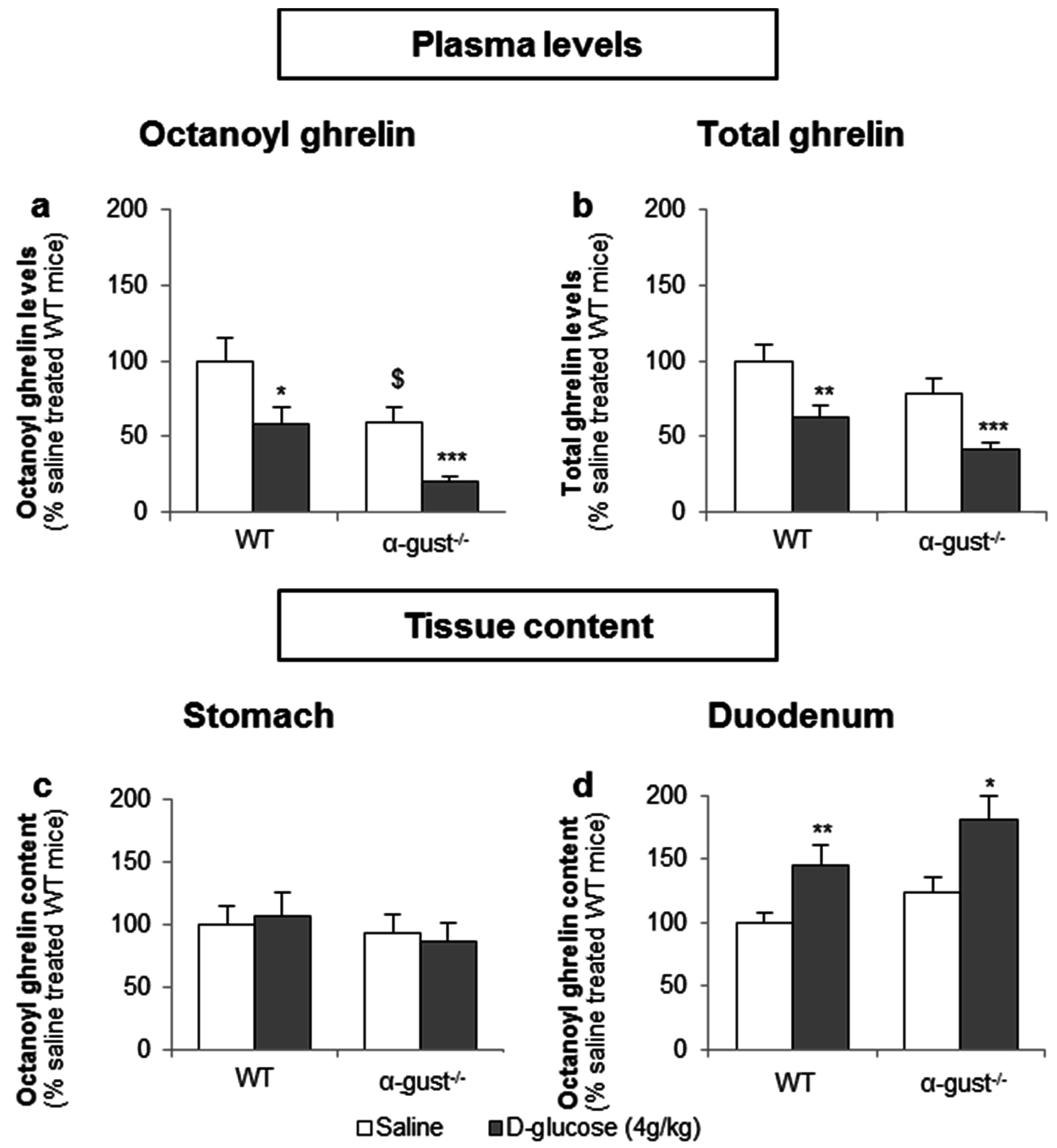
3.3.1. The Sensing of d-Glucose by the Ghrelin Cell Is Polarized and Occurs via the Lumen
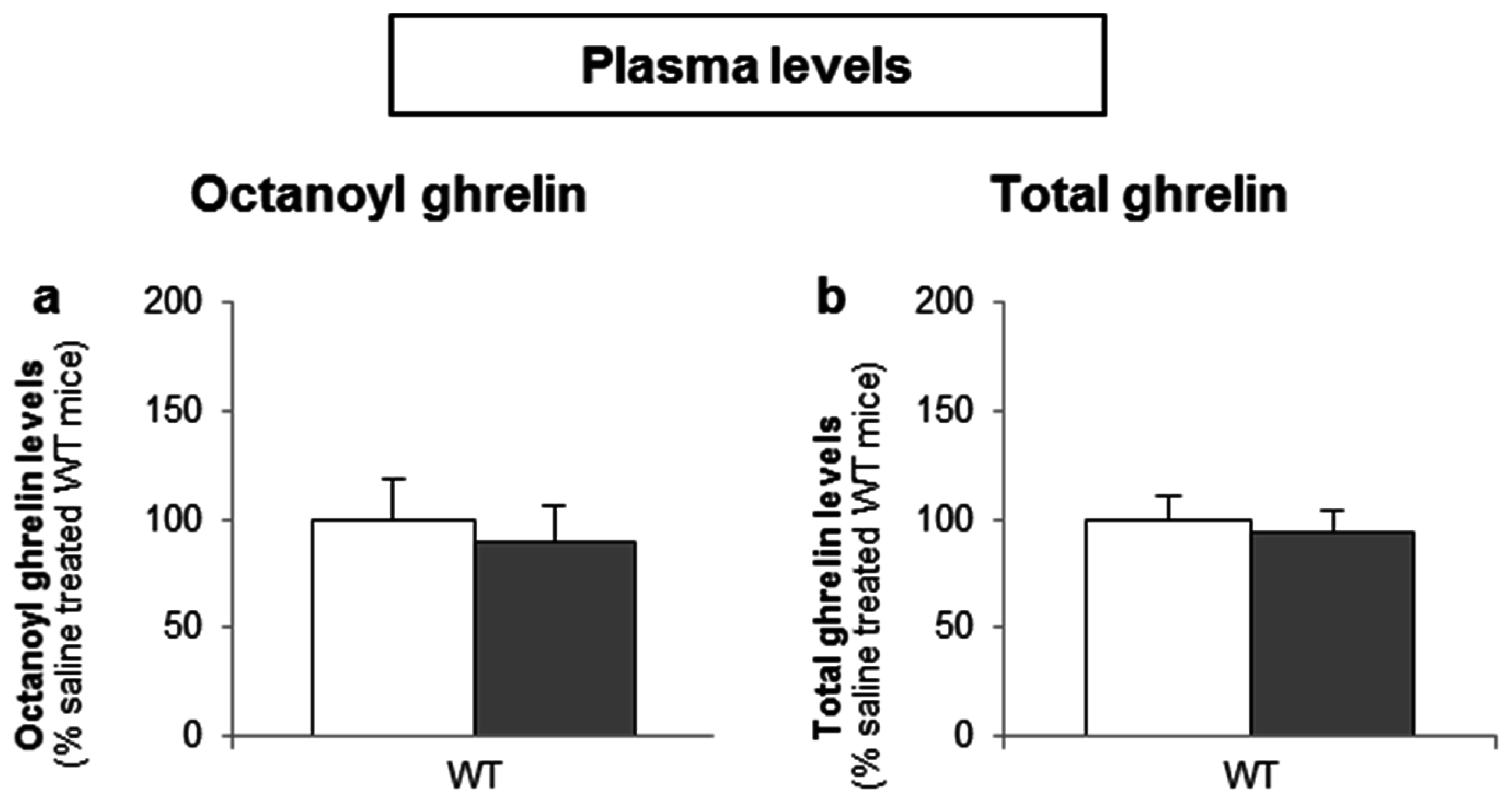
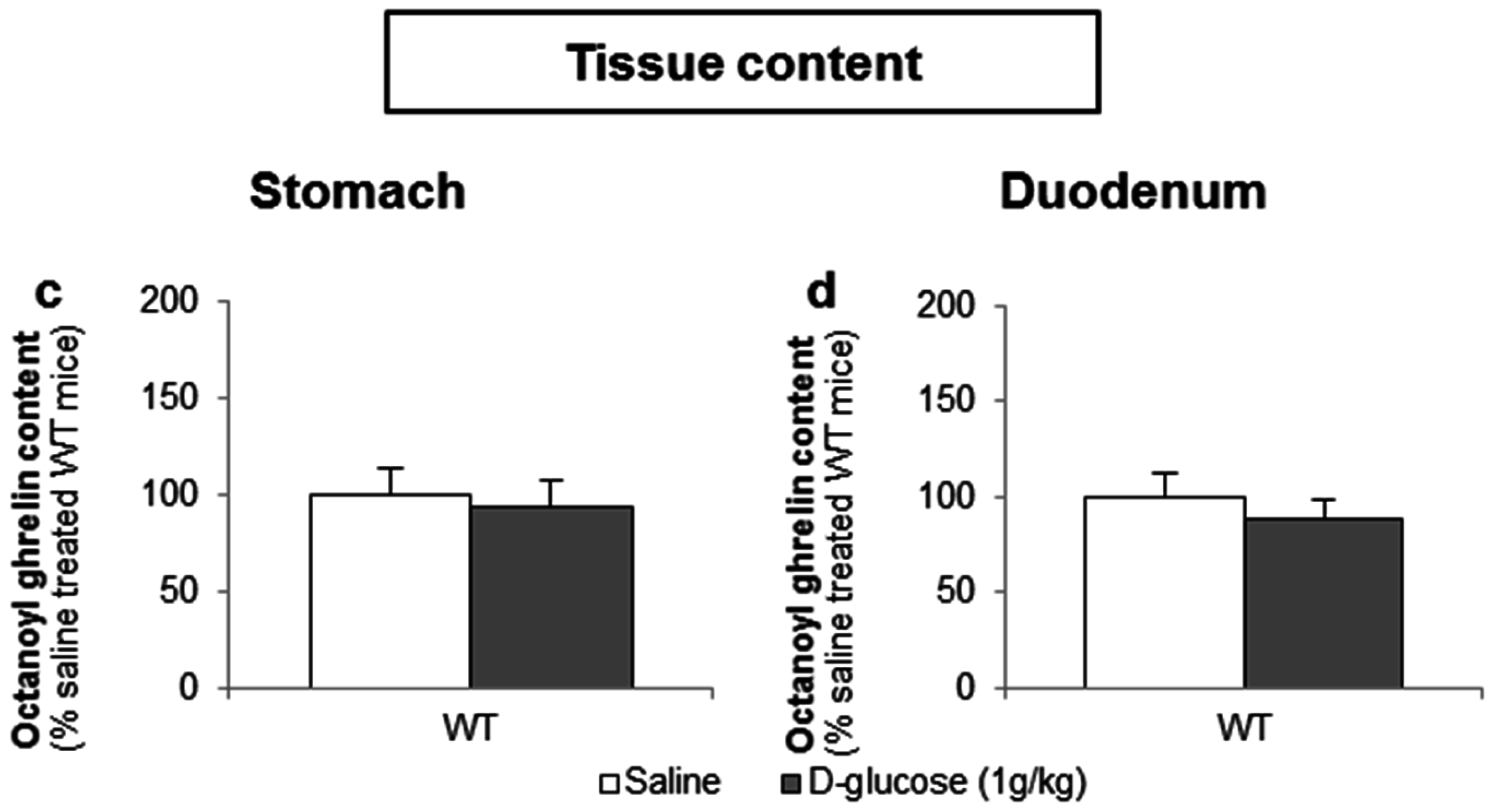
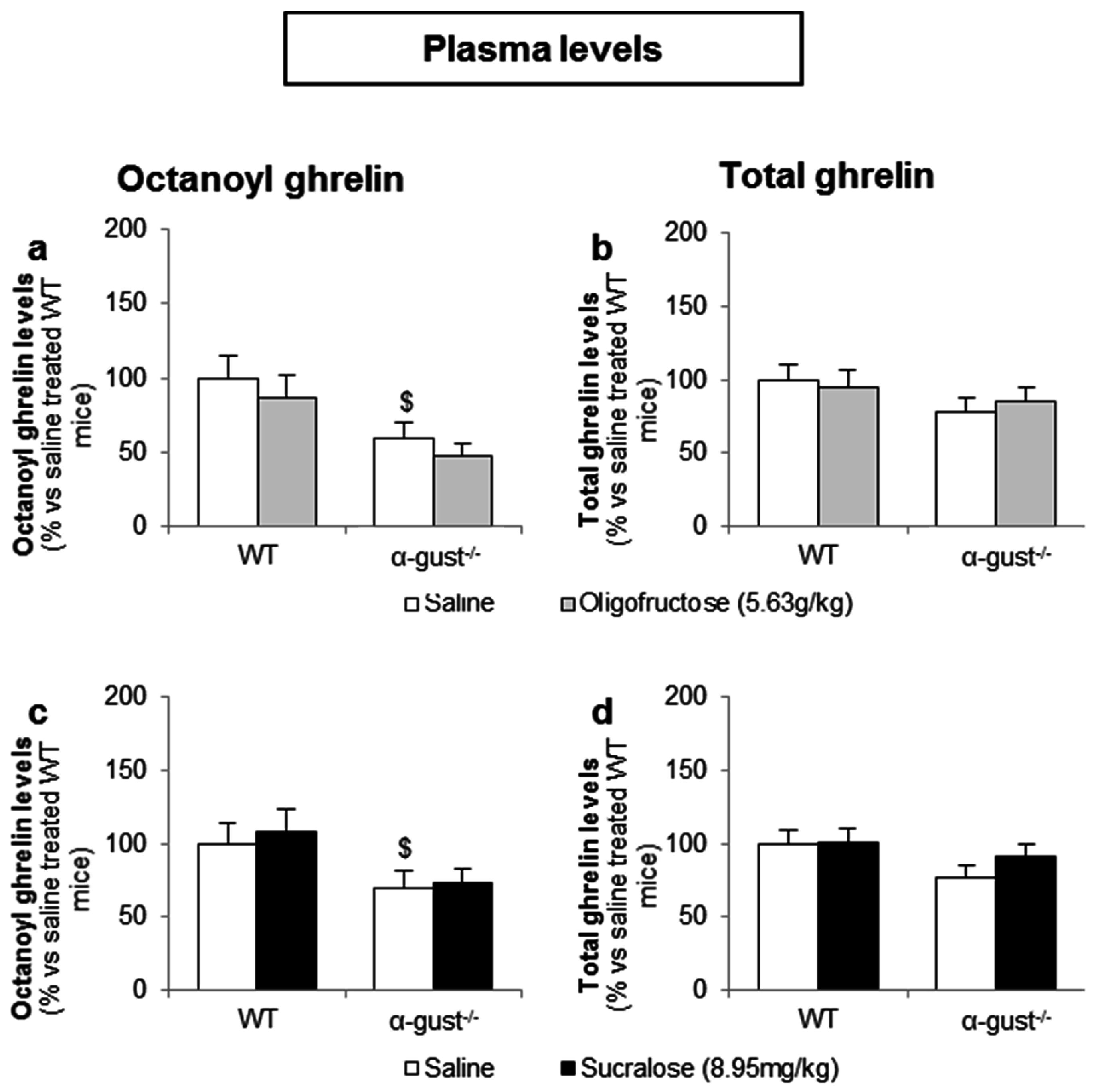
3.3.2. Intragastric Administration of Neither a Low- nor a High-Potency Sweetener Affected Plasma Ghrelin Levels
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Medina-RemOn, A.; Kirwan, R.; Lamuela-Raventos, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and mental health problems. Crit. Rev. Food Sci. Nutr. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes: Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Roberts, J.R. The paradox of artificial sweeteners in managing obesity. Curr. Gastroenterol. Rep. 2015, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Niness, K.R. Inulin and oligofructose: What are they? J. Nutr. 1999, 129, 1402S–1406S. [Google Scholar] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Fact. 2011, 10, S10. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Pfeiffer, N.; Hanske, L.; Loh, G.; Klaus, S.; Blaut, M. Alleviation of high fat diet-induced obesity by oligofructose in gnotobiotic mice is independent of presence of bifidobacterium longum. Mol. Nutr. Food Res. 2015, 59, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Eller, L.K.; Keenan, C.M.; Reimer, R.A.; Sharkey, K.A. Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity (Silver Spring) 2015, 23, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Anastasovska, J.; Arora, T.; Sanchez Canon, G.J.; Parkinson, J.R.; Touhy, K.; Gibson, G.R.; Nadkarni, N.A.; So, P.W.; Goldstone, A.P.; Thomas, E.L.; et al. Fermentable carbohydrate alters hypothalamic neuronal activity and protects against the obesogenic environment. Obesity (Silver Spring) 2012, 20, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Delmee, E.; Cani, P.D.; Gual, G.; Knauf, C.; Burcelin, R.; Maton, N.; Delzenne, N.M. Relation between colonic proglucagon expression and metabolic response to oligofructose in high fat diet-fed mice. Life Sci. 2006, 79, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C.; Iglesias, M.A.; Drucker, D.J.; Delzenne, N.M.; Burcelin, R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006, 55, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Maton, N.; Delzenne, N.M. Oligofructose promotes satiety in rats fed a high-fat diet: Involvement of glucagon-like peptide-1. Obes. Res. 2005, 13, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Liber, A.; Szajewska, H. Effects of inulin-type fructans on appetite, energy intake, and body weight in children and adults: Systematic review of randomized controlled trials. Ann. Nutr. Metab. 2013, 63, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Carbone, F.; Tack, J.; Depoortere, I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol. Motil. 2013, 25, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, P.J.; Depoortere, I. Ghrelin’s second life: From appetite stimulator to glucose regulator. World J. Gastroenterol. 2012, 18, 3183–3195. [Google Scholar] [PubMed]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.J.; Sakata, I.; Li, R.L.; Liang, G.; Richardson, J.A.; Brown, M.S.; Goldstein, J.L.; Zigman, J.M. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 15868–15873. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Overduin, J.; Prudom, C.E.; Liu, J.; Callahan, H.S.; Gaylinn, B.D.; Thorner, M.O.; Cummings, D.E. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008, 93, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Vancleef, L.; van Den Broeck, T.; Thijs, T.; Steensels, S.; Briand, L.; Tack, J.; Depoortere, I. Chemosensory signalling pathways involved in sensing of amino acids by the ghrelin cell. Sci. Rep. 2015, 5, 15725. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Iwakura, H.; Tack, J.; Depoortere, I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS ONE 2012, 7, e40168. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Hass, N.; Schwarzenbacher, K.; Breer, H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010, 339, 493–504. [Google Scholar] [CrossRef] [PubMed]
- DuBois, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2011, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A. Food Additives Permitted for Direct Addition to Food for Human Consumption; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1998; Volume 3, p. 60.
- Wildman, D.M.A.R. Advanced Human Nutrition, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2011; p. 60. [Google Scholar]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.E.; Adriaenssens, A.; Rogers, G.; Richards, P.; Koepsell, H.; Reimann, F.; Gribble, F.M. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012, 55, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-beta-glucoside in Caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Al-Rammahi, M.; Moran, A.; Marcello, M.; Ninomiya, Y.; Shirazi-Beechey, S.P. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G271–G282. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, P.J.; de Smet, B.; Saels, I.; Thijs, T.; Ver Donck, L.; Moechars, D.; Peeters, T.L.; Depoortere, I. Role of ghrelin in the relationship between hyperphagia and accelerated gastric emptying in diabetic mice. Gastroenterology 2008, 135, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Sakata, I.; Park, W.M.; Walker, A.K.; Piper, P.K.; Chuang, J.C.; Osborne-Lawrence, S.; Zigman, J.M. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1300–E1310. [Google Scholar] [CrossRef] [PubMed]
- Oya, M.; Kitaguchi, T.; Harada, K.; Numano, R.; Sato, T.; Kojima, M.; Tsuboi, T. Low glucose-induced ghrelin secretion is mediated by an ATP-sensitive potassium channel. J. Endocrinol. 2015, 226, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Yasharpour, S.; Lloyd, K.C.; Mirzayan, R.; Diamond, J.M. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. 1990, 259, G822–G837. [Google Scholar] [PubMed]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose sensing in L-cells: A primary cell study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Sanematsu, K.; Yoshida, R.; Shigemura, N.; Ninomiya, Y. Structure, function, and signaling of taste G-protein-coupled receptors. Curr. Pharm. Biotechnol. 2014, 15, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, S.J.; Naim, M.; Zehavi, U.; Lindemann, B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J. Physiol. 1996, 490, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, A.M.; Loney, G.C.; Smith, J.C.; Eckel, L.A. Examination of the perception of sweet- and bitter-like taste qualities in sucralose preferring and avoiding rats. Physiol. Behav. 2015, 140, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Booth, B.J.; Losee, M.L.; Pecore, S.D.; Warwick, Z.S. Bitterness of sweeteners as a function of concentration. Brain Res. Bull. 1995, 36, 505–513. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Nomura, M.; Shibata, A.; Ichikawa, R.; Enciso, P.L.; Wang, L.; Takayanagi, R.; Torii, K.; Uneyama, H. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem. Biophys. Res. Commun. 2010, 402, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Iwakura, H.; Dote, K.; Bando, M.; Hosoda, H.; Ariyasu, H.; Kusakabe, T.; Son, C.; Hosoda, K.; Akamizu, T.; et al. Comprehensive profiling of GPCR expression in ghrelin-producing cells. Endocrinology 2016, 157, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakagawa, Y.; Ma, J.; Sasaki, T.; Kitamura, T.; Yamamoto, Y.; Kurose, H.; Kojima, I.; Shibata, H. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS ONE 2013, 8, e54500. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ohtsu, Y.; Nagasawa, M.; Shibata, H.; Kojima, I. Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr. J. 2014, 61, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Danilova, V.; Damak, S.; Margolskee, R.F.; Hellekant, G. Taste responses to sweet stimuli in alpha-gustducin knockout and wild-type mice. Chem. Senses 2006, 31, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Cummings, D.E.; Grill, H.J.; Kaplan, J.M. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 2003, 144, 2765–2767. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Doran, S.; Wishart, J.; Horowitz, M.; Chapman, I.M. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin. Endocrinol. 2005, 62, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Doran, S.; Meyer, J.H.; Smout, A.J.; O’Donovan, D.G.; Wu, K.L.; Jones, K.L.; Wishart, J.; Rayner, C.K.; Horowitz, M.; et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E647–E655. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, R.A.; Sidani, R.M.; Garcia, A.E.; Antoun, J.; Isbell, J.M.; Albaugh, V.L.; Abumrad, N.N. Jejunal administration of glucose enhances acyl ghrelin suppression in obese humans. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E252–E259. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Frey, F.; Topfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Bohan Brown, M.M.; Onken, K.L.; Beitz, D.C. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Prodam, F.; Me, E.; Riganti, F.; Gramaglia, E.; Bellone, S.; Baldelli, R.; Rapa, A.; van der Lely, A.J.; Bona, G.; Ghigo, E.; et al. The nutritional control of ghrelin secretion in humans: The effects of enteral vs. Parenteral nutrition. Eur. J. Nutr. 2006, 45, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, Y.; Nakagawa, Y.; Nagasawa, M.; Takeda, S.; Arakawa, H.; Kojima, I. Diverse signaling systems activated by the sweet taste receptor in human GLP-1-secreting cells. Mol. Cell. Endocrinol. 2014, 394, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhao, B.R.; Bound, M.J.; Checklin, H.L.; Bellon, M.; Little, T.J.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr. 2012, 95, 78–83. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steensels, S.; Vancleef, L.; Depoortere, I. The Sweetener-Sensing Mechanisms of the Ghrelin Cell. Nutrients 2016, 8, 795. https://doi.org/10.3390/nu8120795
Steensels S, Vancleef L, Depoortere I. The Sweetener-Sensing Mechanisms of the Ghrelin Cell. Nutrients. 2016; 8(12):795. https://doi.org/10.3390/nu8120795
Chicago/Turabian StyleSteensels, Sandra, Laurien Vancleef, and Inge Depoortere. 2016. "The Sweetener-Sensing Mechanisms of the Ghrelin Cell" Nutrients 8, no. 12: 795. https://doi.org/10.3390/nu8120795
APA StyleSteensels, S., Vancleef, L., & Depoortere, I. (2016). The Sweetener-Sensing Mechanisms of the Ghrelin Cell. Nutrients, 8(12), 795. https://doi.org/10.3390/nu8120795



