Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Identification of GOP
2.2. Chemicals and Reagents
2.3. Animals and Treatment
2.4. Forced Swimming Test
2.5. Biochemical Assay
2.6. Quantitative Real-Time PCR and Analyses of mtDNA Content
2.7. Determination of Blood Lactic Acid
2.8. Examination of Hepatic Glycogen
2.9. Statistical Analysis
3. Results
3.1. Effects of GOP on the Body Weight of Mice
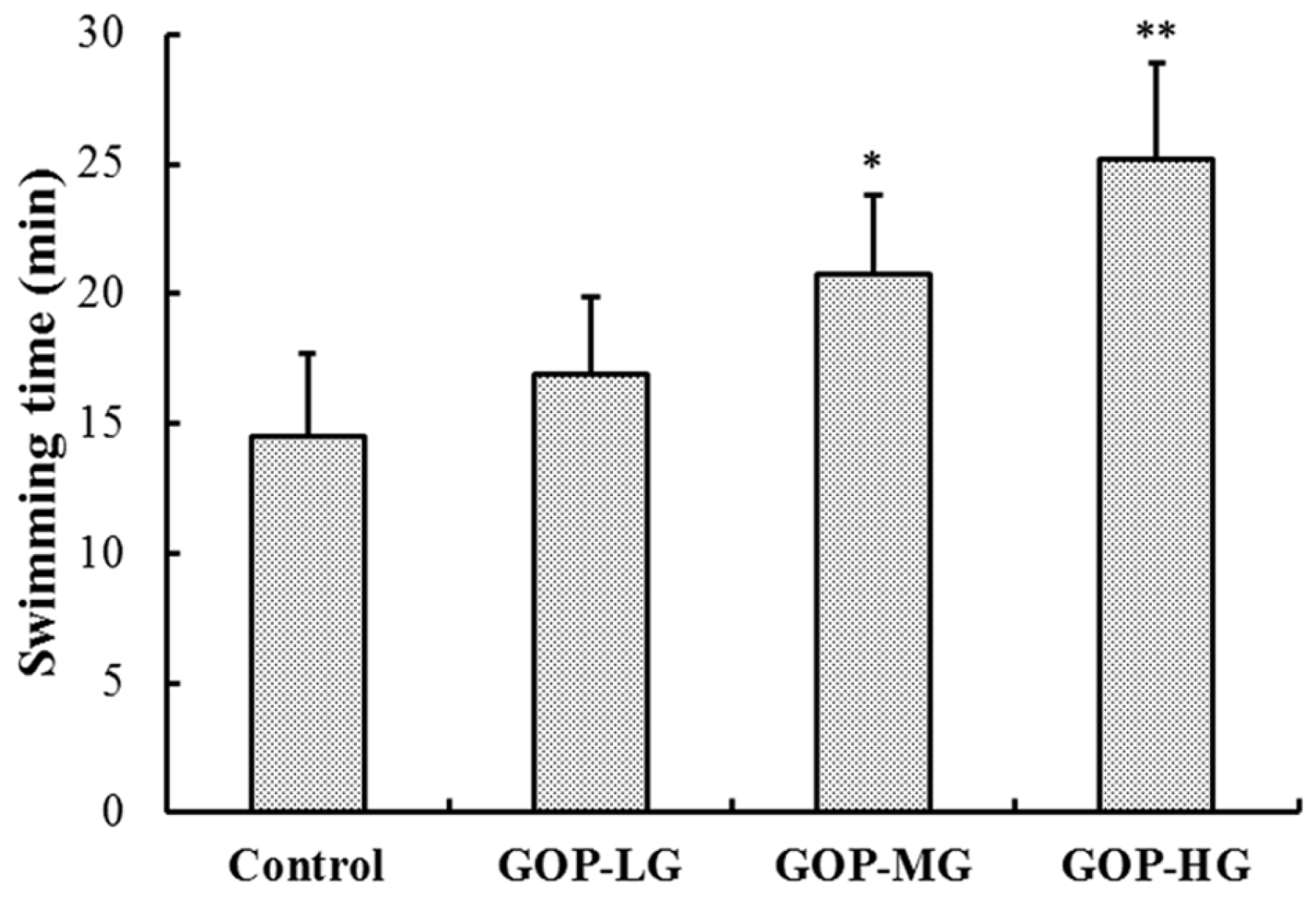
3.2. Effects of GOP in the Forced Swimming Test
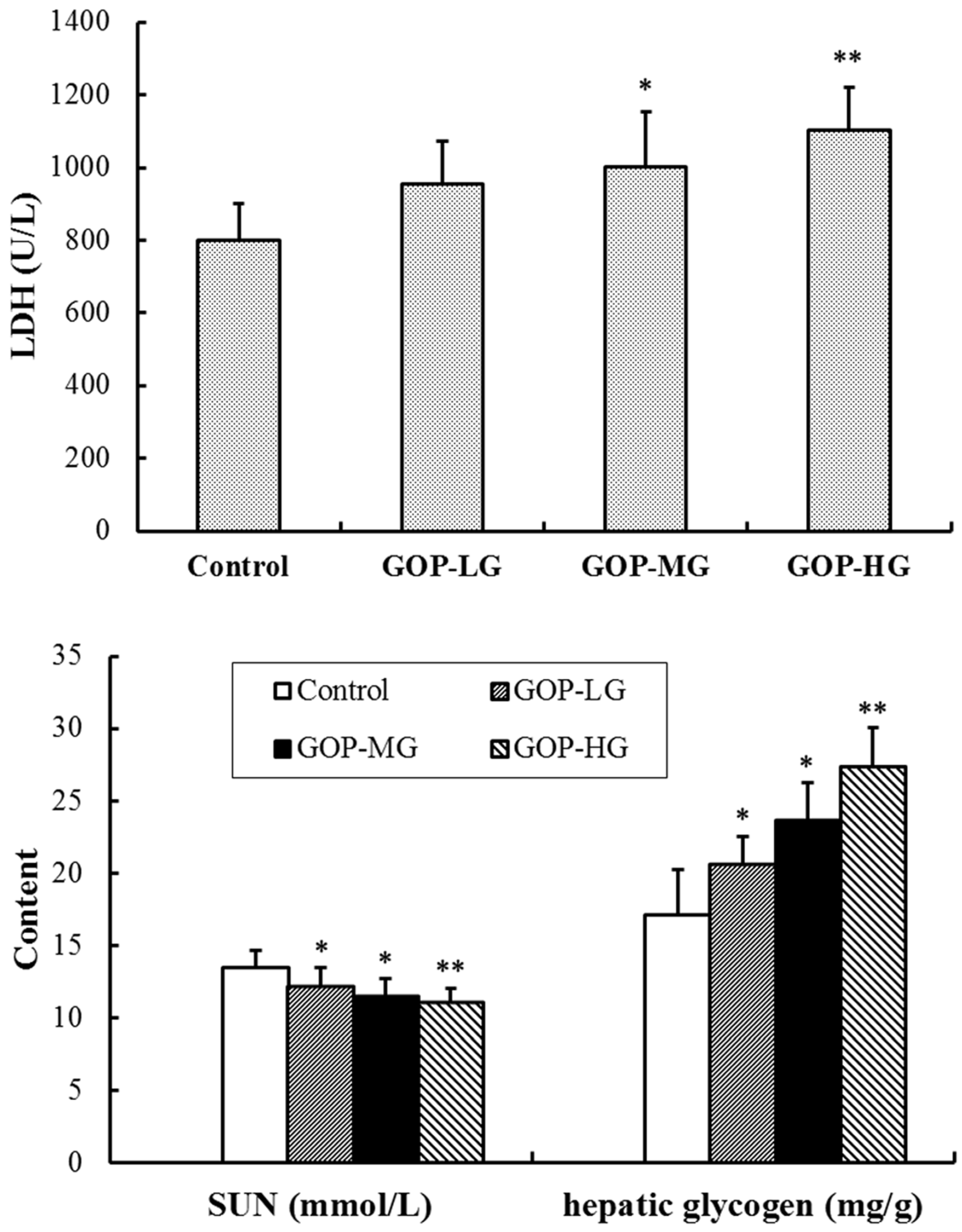
3.3. Effects of GOP on Lactate Dehydrogenase (LDH), Serum Urea Nitrogen (SUN) and Hepatic Glycogen Content in Mice
3.4. Effects of GOP on Blood Lactic Acid (BLA) Levels in Mice
3.5. Effects of GOP on Parameters of Oxidative Stress in Skeletal Muscles of Mice
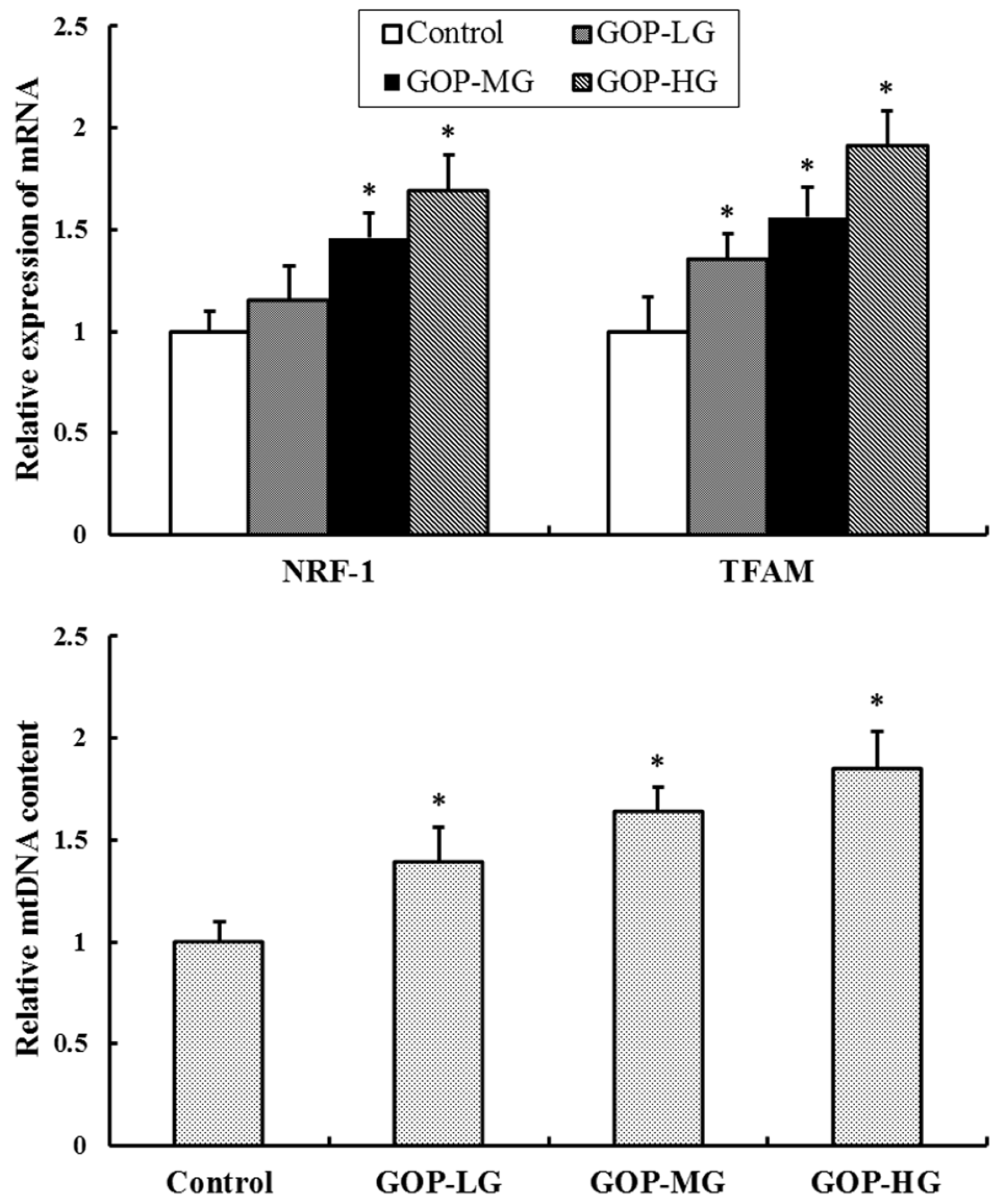
3.6. Effect of GOP on Mitochondrial Biogenesis Factors and mtDNA Content in Skeletal Muscles of Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| BLA | Blood Lactic Acid |
| CAT | Catalase |
| GOP | Ginseng Oligopeptides |
| LDH | Lactate Dehydrogenase |
| MDA | Malondialdehyde |
| mtDNA | Mitochondrial DNA |
| NRF-1 | Nuclear Respiratory Factor 1 |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| SUN | Serum Urea Nitrogen |
| TFAM | Mitochondrial Transcription Factor A |
References
- Moriura, T.; Matsuda, H.; Kubo, M. Pharmacological study on Agkistrodon blomhoffii blomhoffii BOIE. V. anti-fatigue effect of the 50% ethanol extract in acute weight-loaded forced swimming-treated rats. Biol. Pharm. Bull. 1996, 19, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Koh, J.H.; Hong, B.S.; Suh, H.J. Anti-stress and anti-fatigue effects of fermented rice bran. Biosci. Biotechnol. Biochem. 2001, 65, 2294–2296. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Yu, K.Q.; Liu, Y.Y.; Ouyang, M.Z.; Yan, M.H.; Luo, R.; Zhao, X.S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Azizbeigi, K.; Stannard, S.R.; Atashak, S.; Haghighi, M.M. Antioxidant enzymesand oxidative stress adaptation to exercise training: Comparison ofendurance, resistance, and concurrent training in untrained males. J. Exerc. Sci. Fit. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, R.; Chen, Z.; Yu, H.; Li, R.; Li, P. Effect and mechanism of mackerel (Pneumatophorus japonicus) peptides for anti-fatigue. Food Funct. 2014, 5, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Han, J.M.; Kim, Y.A.; Son, C.G. Anti-fatigue effect of Myelophil in a chronic forced exercise mouse model. Eur. J. Pharmacol. 2015, 764, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Vogler, B.K.; Pittler, M.H.; Ernst, E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur. J. Clin. Pharmacol. 1999, 55, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.O.; Kroh, M.; Kim, N.R.; Joh, Y.G.; Cho, M.Y. Effects of red ginseng upon postoperative immunity and survival in patients with stage III gastric cancer. Am. J. Chin. Med. 2002, 30, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Xi, Q.Y.; Yang, L.; Li, H.Y.; Jiang, Q.Y.; Shu, G.; Wang, S.B.; Gao, P.; Zhu, X.T.; Zhang, Y.L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C. A. Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cho, J.H.; Yoo, S.R.; Lee, J.S.; Han, J.M.; Lee, N.H.; Ahn, Y.C.; Son, C.G. Antifatigue effects of Panax ginseng C. A. Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Fan, Y.; Chen, Y.; Liu, D.; Cheng, H.; Gao, X.; Zhou, Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J. Ethnopharmacol. 2010, 130, 421–423. [Google Scholar] [CrossRef] [PubMed]
- He, L.X.; Zhang, Z.F.; Sun, B.; Chen, Q.H.; Liu, R.; Ren, J.W.; Wang, J.B.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, Y.S.; Kwak, Y.S.; Song, Y.B.; Kim, Y.S.; Park, J.D. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med. 2004, 70, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Voces, J.; Cabral de Oliveira, A.C.; Prieto, J.G.; Vila, L.; Perez, A.C.; Duarte, I.D.; Alvarez, A.I. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz. J. Med. Biol. Res. 2004, 37, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, U.; Izquierdo, J.A. Antistress and antifatigue properties of Panax ginseng: Comparison with piracetam. Acta Physiol. Lat. Am. 1982, 32, 277–285. [Google Scholar] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- You, L.; Ren, J.; Yang, B.; Regenstein, J.; Zhao, M. Antifatigue activities of loach protein hydrolysates with different antioxidant activities. J. Agric. Food Chem. 2012, 60, 12324–12331. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Xu, H. Analysis of chemical components of shiitake polysaccharides and its anti-fatigue effect under vibration. Int. J. Biol. Macromol. 2009, 45, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chiu, W.C.; Chuang, H.L.; Tang, D.W.; Lee, Z.M.; Wei, L.; Chen, F.A.; Huang, C.C. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Gibson, H.; Edwards, R.H. Muscular exercise and fatigue. Sports Med. 1985, 2, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Phani Kumar, G.; Pandareesh, M.D.; Swamy, M.S.; Khanum, F.; Bawa, A.S. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother. Res. 2012, 26, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Liu, L.; Zhang, H.; Zhou, G.X.; Wang, S.; Duan, X.Z.; Bai, X.Y.; Wang, S.M.; Zhao, D.Q. Anti-fatigue effects of proteins isolated from Panax quinquefolium. J. Ethnopharmacol. 2014, 153, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G.; Lannergren, J. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? News Physiol. Sci. 2002, 17, 17–21. [Google Scholar] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kumar, M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food Chem. Toxicol. 2009, 47, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Cemek, M.; Simsek, N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol. Environ. Saf. 2009, 72, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Lu, J.; Rodova, M.; Lezi, E.; Crafter, A.B.; Swerdlow, R.H. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim. Biophys. Acta 2010, 1802, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Signore, A.P.; Iwai, M.; Cao, G.; Gao, Y.; Chen, J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 2008, 39, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Park, K.S.; Lee, H.K. Genetic factors related to mitochondrial function and risk of diabetes mellitus. Diabetes Res. Clin. Pract. 2007, 77, S172–S177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.Y.; Zhao, J.; Dong, Z.; Feng, D.Y.; Wu, R.; Shi, M.; Zhao, G. Ginsenoside Rd Protects SH-SY5Y Cells against 1-Methyl-4-phenylpyridinium Induced Injury. Int. J. Mol. Sci. 2015, 16, 14395–14408. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Koo, Y.D.; Kim, M.; Lim, S.; Park, Y.J.; Chung, S.S.; Jang, H.C.; Park, K.S. Rg3 Improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis in C2C12 myotubes. Diabetes Metab. J. 2016, 40, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Reddy, A.; Tannir, N.M.; Chisholm, G.B.; Lee, R.T.; Lopez, G.; Escalante, C.P.; Manzullo, E.F.; Frisbee Hume, S.; Williams, J.L.; et al. High-Dose Asian ginseng (Panax Ginseng) for cancer-related fatigue: A preliminary report. Integr. Cancer Ther. 2015, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Amino Acid Composition Of GOP (g/100 g) |
|---|---|
| Asp | 0.19 |
| Glu | 0.12 |
| Ser | 0.02 |
| His | 0.06 |
| Gly | 0.02 |
| Thr | 0.05 |
| Arg | 2.26 |
| Ala | 0.13 |
| Tyr | 0.09 |
| Cys | 0.01 |
| Val | 0.06 |
| Met | 0.02 |
| Phe | 0.09 |
| Ile | 0.04 |
| Leu | 0.08 |
| Lys | 0.06 |
| Pro | 0.65 |
| Body Weight (g) | Control | GOP-LG | GOP-MG | GOP-HG |
|---|---|---|---|---|
| Set 1 | ||||
| Initial body weight | 25.22 ± 1.34 | 25.50 ± 1.12 | 25.41 ± 0.93 | 25.61 ± 1.31 |
| Terminal weight | 34.85 ± 2.65 | 34.39 ± 2.99 | 34.50 ± 2.05 | 35.83 ± 1.75 |
| Set 2 | ||||
| Initial body weight | 25.33 ± 1.43 | 25.86 ± 1.35 | 25.76 ± 1.35 | 25.90 ± 1.13 |
| Terminal weight | 36.65 ± 3.63 | 35.73 ± 2.87 | 35.28 ± 3.14 | 35.47 ± 2.43 |
| Set 3 | ||||
| Initial body weight | 25.53 ± 1.64 | 25.89 ± 1.42 | 25.34 ± 1.38 | 25.87 ± 1.32 |
| Terminal weight | 36.28 ± 3.07 | 36.81 ± 3.13 | 36.75 ± 2.79 | 36.42 ± 3.26 |
| Set 4 | ||||
| Initial body weight | 24.98 ± 1.18 | 25.55 ± 1.07 | 24.83 ± 1.27 | 24.65 ± 1.15 |
| Terminal weight | 35.47 ± 2.73 | 35.03 ± 3.05 | 35.58 ± 2.73 | 35.04 ± 2.12 |
| BLA (mg/L) | Control | GOP-LG | GOP-MG | GOP-HG |
|---|---|---|---|---|
| Baseline | 195.22 ± 38.43 | 192.50 ± 41.92 | 188.46 ± 33.66 | 195.83 ± 32.31 |
| 0 min after swimming | 437.59 ± 42.56 | 384.03 ± 42.49 | 354.05 ± 39.09 * | 355.83 ± 31.55 ** |
| 20 min after swimming | 345.23 ± 41.83 | 275.06 ± 49.05 * | 265.07 ± 41.35 ** | 247.91 ± 34.26 ** |
| Area under BLA curve | 10,997.62 ± 998.71 | 9475.45 ± 930.05 * | 8651.26 ± 901.64 * | 8457.56 ± 843.35 ** |
| Parameters | Control | GOP-LG | GOP-MG | GOP-HG |
|---|---|---|---|---|
| SOD (U/mg·pro) | 96.10 ± 9.05 | 105.19 ± 10.98 | 115.91 ± 10.30 * | 123.69 ± 11.59 ** |
| CAT (U/mg·pro) | 95.86 ± 15.23 | 111.77 ± 19.43 | 122.46 ± 11.36 * | 129.37 ± 13.92 ** |
| MDA (nmol/mg·pro) | 6.59 ± 0.26 | 6.04 ± 0.29 * | 5.97 ± 0.19 * | 5.41 ± 0.23 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients 2016, 8, 807. https://doi.org/10.3390/nu8120807
Bao L, Cai X, Wang J, Zhang Y, Sun B, Li Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients. 2016; 8(12):807. https://doi.org/10.3390/nu8120807
Chicago/Turabian StyleBao, Lei, Xiaxia Cai, Junbo Wang, Yuan Zhang, Bin Sun, and Yong Li. 2016. "Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice" Nutrients 8, no. 12: 807. https://doi.org/10.3390/nu8120807
APA StyleBao, L., Cai, X., Wang, J., Zhang, Y., Sun, B., & Li, Y. (2016). Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients, 8(12), 807. https://doi.org/10.3390/nu8120807




