Dietary Patterns in Relation to Cardiovascular Disease Incidence and Risk Markers in a Middle-Aged British Male Population: Data from the Caerphilly Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
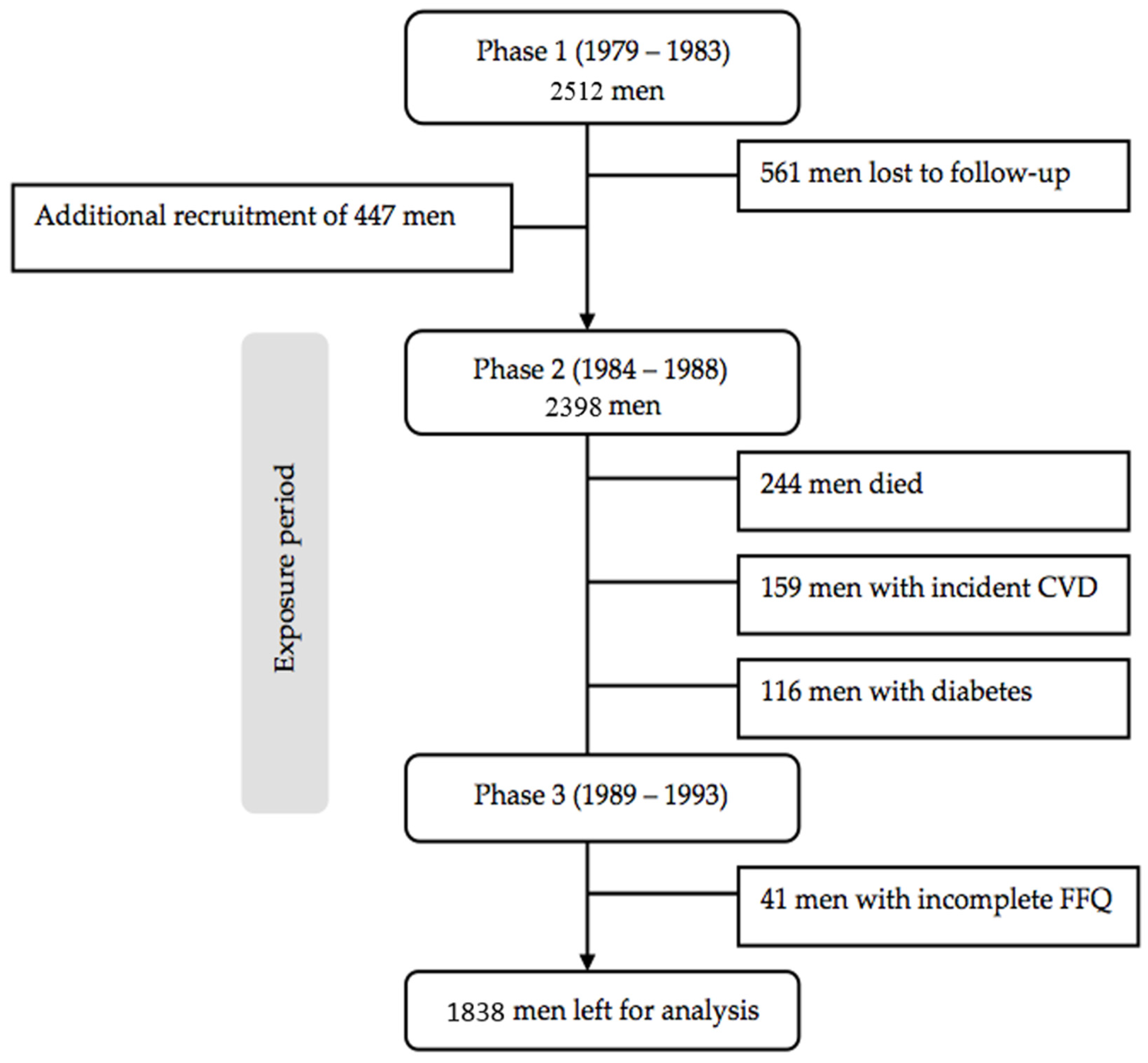
2.1. Study Design and Study Population
2.2. Exposure Assessment
2.3. Covariates
2.4. Cardiovascular Risk Markers
2.5. Verification of Outcome
2.6. Statistical Analysis
2.6.1. Identification of Dietary Patterns
2.6.2. Dietary Patterns versus Cardiovascular Events
2.6.3. Dietary Patterns versus Risk Markers
3. Results
3.1. Dietary Patterns
3.2. Descriptive Statistics
3.3. Incidence of CVD, CHD and Stroke
3.4. Cardiovascular Risk Markers
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S. Heart disease and stroke statistics-2014 update: A report from the american heart association. Circulation 2014, 129, e28. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.; Galante, J.; Pickering, J.; Palmer, S.; Bayer, A.; Ben-Shlomo, Y.; Longley, M.; Gallacher, J. Healthy lifestyles reduce the incidence of chronic diseases and dementia: Evidence from the caerphilly cohort study. PLoS ONE 2013, 8, e81877. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Ž.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Tucker, K.L. Are dietary patterns useful for understanding the role of diet in chronic disease? Am. J. Clin. Nutr. 2001, 73, 1–2. [Google Scholar] [PubMed]
- Schulze, M.B.; Hoffmann, K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br. J. Nutr. 2006, 95, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Flores-Mateo, G.; Sánchez, E. Dietary patterns and cvd: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015, 114, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
- Kimokoti, R.W.; Gona, P.; Zhu, L.; Newby, P.; Millen, B.E.; Brown, L.S.; D’Agostino, R.B.; Fung, T.T. Dietary patterns of women are associated with incident abdominal obesity but not metabolic syndrome. J. Nutr. 2012, 142, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Risk of hypertension among women in the epic-potsdam study: Comparison of relative risk estimates for exploratory and hypothesis-oriented dietary patterns. Am. J. Epidemiol. 2003, 158, 365–373. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. A Series of Systematic Reviews on the Relationship between Dietary Patterns and Health Outcomes. March 2014. Available online: http://www.nel.gov/vault/2440/web/files/DietaryPatterns/DPRptFullFinal.pdf (accessed on 8 September 2016). [Google Scholar]
- Panagiotakos, D.; Pitsavos, C.; Chrysohoou, C.; Palliou, K.; Lentzas, I.; Skoumas, I.; Stefanadis, C. Dietary patterns and 5-year incidence of cardiovascular disease: A multivariate analysis of the ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Caerphilly, T.; Group, S.C. Caerphilly and speedwell collaborative heart disease studies. J. Epidemiol. Commun. Health 1984, 38, 259–262. [Google Scholar]
- Fehily, A.; Yarnell, J.; Butland, B. Diet and ischaemic heart disease in the caerphilly study. Hum. Nutr. Appl. Nutr. 1987, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, J.; Fehily, A.; Milbank, J.; Sweetnam, P.; Walker, C. A short dietary questionnaire for use in an epidemiological survey: Comparison with weighed dietary records. Hum. Nutr. Appl. Nutr. 1983, 37, 103–112. [Google Scholar] [PubMed]
- Fehily, A.M.; Yarnell, J.W.; Sweetnam, P.M.; Elwood, P.C. Diet and incident ischaemic heart disease: The caerphilly study. Br. J. Nutr. 1993, 69, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Fehily, A.M.; Phillips, K.M.; Yarnell, J.W. Diet, smoking, social class, and body mass index in the caerphilly heart disease study. Am. J. Clin. Nutr. 1984, 40, 827–833. [Google Scholar] [PubMed]
- Taylor, H.L.; Jacobs, D.R.; Schucker, B.; Knudsen, J.; Leon, A.S.; Debacker, G. A questionnaire for the assessment of leisure time physical activities. J. Chronic Dis. 1978, 31, 741–755. [Google Scholar] [CrossRef]
- Yu, S.; Yarnell, J.W.G.; Sweetnam, P.M.; Murray, L. What level of physical activity protects against premature cardiovascular death? The caerphilly study. Heart 2003, 89, 502–506. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Mee, F.; Atkins, N.; Thomas, M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: The Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press. Monit. 1996, 1, 55–61. [Google Scholar] [PubMed]
- O’Rourke, M.F.; Pauca, A.; Jiang, X.J. Pulse wave analysis. Br. J. Clin. Pharmacol. 2001, 51, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; Fuchs, S.A.; Jansen, I.M.; Spratt, J.C.; Murray, G.D.; Cockcroft, J.R.; Webb, D.J. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J. Hypertens. 1998, 16, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical applications of arterial stiffness, task force III: Recommendations for user procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Yarnell, J.; Patterson, C.; Sweetnam, P.; Thomas, H.; Bainton, D.; Elwood, P.C.; Bolton, C.; Miller, N. Do total and high density lipoprotein cholesterol and triglycerides act independently in the prediction of ischemic heart disease? Ten-year follow-up of caerphilly and speedwell cohorts. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1340–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care the framingham heart study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.; Kupper, L.; Ke, M. Variable Reduction and Factor Analysis. Applied Regression Analysis and Other Multivariable Methods; PWS Kend Publishing Co.: Boston, MA, USA, 1988. [Google Scholar]
- Fransen, H.P.; May, A.M.; Stricker, M.D.; Boer, J.M.; Hennig, C.; Rosseel, Y.; Ocke, M.C.; Peeters, P.H.; Beulens, J.W. A posteriori dietary patterns: How many patterns to retain? J. Nutr. 2014, 144, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L. Analysis of dietary patterns in epidemiological research. Appl. Physiol. Nutr. Metab. 2010, 35, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.A.C.; Emmett, P.M.; Newby, P.K.; Northstone, K. Dietary patterns obtained through principal components analysis: The effect of input variable quantification. Br. J. Nutr. 2013, 109, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Dietary patterns and the risk of cvd and all-cause mortality in older british men. Br. J. Nutr. 2016, 116, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Lunn, J. 40th anniversary briefing paper: Food availability and our changing diet. Nutr. Bull. 2007, 32, 187–249. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Tucker, K.L.; Flores, M.; Barquera, S.; Salmerón, J. Dietary patterns are associated with predicted cardiovascular disease risk in an urban mexican adult population. J. Nutr. 2016, 146, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Copeland, W.K.; Vedanthan, R.; Grant, E.; Lee, J.E.; Gu, D.; Gupta, P.C.; Ramadas, K.; Inoue, M.; Tsugane, S.; et al. Association between body mass index and cardiovascular disease mortality in east asians and south asians: Pooled analysis of prospective data from the asia cohort consortium. Br. Med. J. 2013, 347, f5446. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Stampfer, M.J.; Hennekens, C.H.; Willett, W.C. Body weight and longevity: A reassessment. JAMA 1987, 257, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Hart, C.L.; Hole, D.J.; Davey Smith, G. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity 2006, 14, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G. Evaluation of the evidence between consumption of refined grains and health outcomes. Nutr. Rev. 2012, 70, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to hdl cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [PubMed]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, e-selectin, and blood pressure: Results from the randomized, controlled dietary intervention and vascular function (divas) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and the Prevention of Chronic Diseases. Available online: http://health.euroafrica.org/books/dietnutritionwho.pdf (accessed on 7 September 2016).
- Bates, B.; Cox, L.; Nicholson, S.; Page, P.; Prentice, A.; Steer, T.; Swan, G. National Diet and Nutrition Survey. Results from Years 5-6 (Combined) of the Rolling Programme (2012/13–2013/14). 2016. Available online: Https://www.Gov.Uk/government/statistics/ndns-results-from-years-5-and-6-combined (accessed on 21 September 2016). [Google Scholar]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, 6. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Tholstrup, T. Butter increased total and ldl cholesterol compared with olive oil but resulted in higher hdl cholesterol compared with a habitual diet. Am. J. Clin. Nutr. 2015, 102, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does dairy food intake predict arterial stiffness and blood pressure in men? Evidence from the caerphilly prospective study. Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Hu, F.B. Prospective study of major dietary patterns and stroke risk in women. Stroke 2004, 35, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus a systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Richi, E.B.; Baumer, B.; Conrad, B.; Darioli, R.; Schmid, A.; Keller, U. Health risks associated with meat consumption: A review of epidemiological studies. Int. J. Vitam. Nutr. Res. 2015, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bovalino, S.; Charleson, G.; Szoeke, C. The impact of red and processed meat consumption on cardiovascular disease risk in women. Nutrition 2016, 32, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-Q.; Xu, J.-Y.; Han, S.-F.; Zhang, Z.-L.; Zhao, Y.-Y.; Szeto, I.M. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2015, 24, 90–100. [Google Scholar] [PubMed]
- Elwood, P.C.; Pickering, J.E.; Givens, D.I.; Gallacher, J.E. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: An overview of the evidence. Lipids 2010, 45, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.C.; Strain, J.; Robson, P.J.; Fehily, A.M.; Hughes, J.; Pickering, J.; Ness, A. Milk consumption, stroke, and heart attack risk: Evidence from the caerphilly cohort of older men. J. Epidemiol. Community Health 2005, 59, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Household Consumption and Expenditure of Dairy Products from the Defra Family Food Survey. 2015. Available online: https://dairy.ahdb.org.uk/resources-library/market-information/dairy-sales-consumption/defra-family-food-survey/#.WHoO1WSLSqQ (accessed on 8 September 2016).
- Elwood, P.C.; Pickering, J.E.; Fehily, A.; Hughes, J.; Ness, A. Milk drinking, ischaemic heart disease and ischaemic stroke i. Evidence from the caerphilly cohort. Eur. J. Clin. Nutr. 2004, 58, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Steffen, L.M. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78, 508S–513S. [Google Scholar] [PubMed]
- Williams, P.G. The benefits of breakfast cereal consumption: A systematic review of the evidence base. Adv. Nutr. 2014, 5, 636S–673S. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.; Costanzo, S.; Cricelli, C. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralston, R.; Lee, J.; Truby, H.; Palermo, C.; Walker, K. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J. Hum. Hypertens. 2012, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Markey, O.; Vasilopoulou, D.; Givens, D.; Lovegrove, J. Dairy and cardiovascular health: Friend or foe? Nutr. Bull. 2014, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Verberne, L.D.; Ding, E.L.; Engberink, M.F.; Geleijnse, J.M. Dairy consumption and incidence of hypertension a dose-response meta-analysis of prospective cohort studies. Hypertension 2012, 60, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Jonnalagadda, S. Effect of whole grains on markers of subclinical inflammation. Nutr. Rev. 2012, 70, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Augustin, J.L.; Schaefer, M.M.; Rasmussen, H.; Ordovas, J.M.; Dallal, G.E.; Dwyer, J.T. Lack of efficacy of a food-frequency questionnaire in assessing dietary macronutrient intakes in subjects consuming diets of known composition. Am. J. Clin. Nutr. 2000, 71, 746–751. [Google Scholar] [PubMed]
- Ranganathan, M.; Bhopal, R. Exclusion and inclusion of nonwhite ethnic minority groups in 72 north american and european cardiovascular cohort studies. PLoS Med. 2006, 3, e44. [Google Scholar] [CrossRef] [PubMed]
- Oude Griep, L.M.; Wang, H.; Chan, Q. Empirically-derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr. Nutr. Rep. 2013, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
| Food Items/Food Groups | Empirically Derived Dietary Patterns 2 | ||
|---|---|---|---|
| Dietary Pattern 1 | Dietary Pattern 2 | Dietary Pattern 3 | |
| White bread | 0.37 | −0.04 | −0.05 |
| Wholegrain bread | −0.32 | 0.15 | 0.03 |
| White breakfast cereals | −0.04 | −0.02 | 0.09 |
| Wholegrain breakfast cereals | −0.20 | −0.01 | 0.36 |
| Red meat | 0.21 | 0.26 | 0.06 |
| Processed meat | 0.20 | 0.29 | −0.17 |
| Poultry | −0.09 | 0.35 | −0.02 |
| White fish | −0.07 | 0.31 | 0.03 |
| Oily fish | −0.05 | 0.33 | −0.05 |
| Potatoes | −0.07 | 0.08 | 0.25 |
| Chips | 0.30 | 0.10 | −0.09 |
| Vegetables | −0.17 | 0.26 | 0.19 |
| Pulses | 0.03 | 0.36 | −0.05 |
| Pasta | 0.02 | 0.26 | 0.02 |
| Rice | 0.03 | 0.29 | 0.06 |
| Sweet puddings and biscuits | 0.05 | 0.01 | 0.46 |
| Milk pudding | 0.12 | 0.13 | 0.29 |
| Fruits | −0.12 | 0.22 | 0.13 |
| Eggs | −0.12 | 0.22 | 0.13 |
| Milk | 0.22 | −0.04 | 0.28 |
| Sugar-sweetened beverages | 0.26 | 0.02 | 0.19 |
| Alcohol | 0.05 | 0.09 | −0.36 |
| Butter | 0.36 | −0.06 | 0.14 |
| Margarine | −0.18 | 0.11 | −0.10 |
| Lard | 0.33 | 0.04 | 0.01 |
| Oil | 0.04 | 0.09 | −0.04 |
| Cheese | 0.10 | 0.09 | −0.02 |
| Cream | 0.05 | −0.04 | 0.34 |
| Variance of food intake explained (%) | 10.29 | 8.40 | 6.12 |
| Descriptive Characteristics | Phase 2 | Phase 5 | p-Value 17 |
|---|---|---|---|
| Follow-up, years | 16.6 ± 7.2 | - | |
| Age, years | 56.7 ± 4.5 | 72.8 ± 4.1 13 | |
| Current smoking, n (%) | 791 (43.0%) | - | |
| Non-Manual worker, n (%) | 620 (33.7%) | - | |
| Physically active 2, n (%) | 810 (44.1%) | - | |
| Body mass index, kg/m2 | 26.3 ± 3.6 7 | - | |
| Systolic blood pressure, mmHg | 145.5 ± 22.1 8 | 141.3 ± 19.7 14 | 0.294 |
| Diastolic blood pressure, mmHg | 84.5 ± 11.7 9 | 74.4 ± 11.1 14 | <0.001 |
| Augmentation Index, % | - | 26.6 ± 9.3 14 | |
| Pulse wave velocity, m/s | - | 11.2 (9.5–12.9) 15 | |
| Total cholesterol, mmol/L | 5.6 ± 1.0 10 | - | |
| LDL cholesterol, mmol/L | 4.2 ± 0.9 10 | - | |
| HDL cholesterol, mmol/L | 1.0 ± 0.2 10 | - | |
| Total:HDL cholesterol | 5.8 ± 1.7 10 | ||
| Triacylglycerol, mmol/L | 1.6 (1.2–2.3) 10 | 1.4 (1.0–1.9) 15 | <0.001 |
| Glucose, mmol/L | 5.2 ± 0.8 10 | - | |
| C-Reactive Protein, mg/L | 1.6 (0.8–3.2) 11 | 2.9 (1.5–5.6) 16 | <0.001 |
| Framingham Risk Score 3, % | 24.5 (16.9–34.2) 12 | - | |
| Dietary intake 4 | |||
| Total energy intake, kcal/day | 1989.8 ± 529.0 | - | |
| Fat, g/day (%TE) | 79.0 ± 24.1 (35.7) | - | |
| Saturated fatty acids, g/day (%TE) | 36.4 ± 12.2 (16.5) | ||
| Carbohydrates, g/day (%TE) | 238.0 ± 70.4 (47.8) | - | |
| Total sugar, g/day (%TE) | 90.3 ± 43.1 (18.2) | ||
| Protein, g/day (%TE) | 69.7 ± 16.6 (12.0) | - | |
| Fibre, g/day | 20.6 ± 6.5 | - | |
| Sodium 5, mg/day | 2327.0 ± 615.8 | - | |
| Cholesterol, mg/day | 340.6 ± 101.5 | - | |
| Vegetable intake, g/day | 96.5 (72.4–130.1) | - | |
| Fruit intake, g/day | 44.9 (16.5–91.4) | - | |
| Dairy intake 6, g/day | 167.7 (125.4–295.8) | - | |
| Meat intake, g/day | 96.7 (70.5–123) | - | |
| Fish intake, g/day | 30.4 (19.6–42.9) | - | |
| Ethanol intake, g/day | 10.8 (2.2–22.7) | - | |
| Tertile | Cardiovascular Disease | Coronary Heart Disease | Stroke | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Crude HR (95% CI) | Adjusted HR 2 (95% CI) | Events | Crude HR (95% CI) | Adjusted HR 2 (95% CI) | Events | Crude HR (95% CI) | Adjusted HR 2 (95% CI) | |
| Dietary pattern 1 | |||||||||
| T1 (n = 612) | 233 | Reference | Reference | 136 | Reference | Reference | 60 | Reference | Reference |
| T2 (n = 613) | 233 | 1.04 (0.87, 1.25) | 1.02 (0.85, 1.24) | 128 | 0.97 (0.76, 1.24) | 0.93 (0.73, 1.20) | 66 | 1.16 (0.82, 1.65) | 1.17 (0.82, 1.68) |
| T3 (n = 612) | 249 | 1.37 (1.15, 1.64) | 1.35 (1.10, 1.67) | 138 | 1.29 (1.02, 1.63) | 1.23 (0.93, 1.63) | 79 | 1.73 (1.23, 2.42) | 1.77 (1.18, 2.63) |
| Dietary pattern 2 | |||||||||
| T1 (n = 612) | 234 | Reference | Reference | 144 | Reference | Reference | 60 | Reference | Reference |
| T2 (n = 613) | 242 | 1.03 (0.86, 1.24) | 1.01 (0.84, 1.21) | 140 | 0.97 (0.77, 1.22) | 0.96 (0.76, 1.22) | 68 | 1.15 (0.82, 1.63) | 1.08 (0.76, 1.53) |
| T3 (n = 613) | 239 | 1.06 (0.89, 1.27) | 1.12 (0.93, 1.35) | 118 | 0.85 (0.67, 1.08) | 0.93 (0.72, 1.19) | 77 | 1.36 (0.97, 1.90) | 1.32 (0.93, 1.89) |
| Dietary pattern 3 | |||||||||
| T1 (n = 612) | 248 | Reference | Reference | 139 | Reference | Reference | 78 | Reference | Reference |
| T2 (n = 613) | 245 | 0.87 (0.73, 1.04) | 0.83 (0.69, 1.00) | 148 | 0.95 (0.75, 1.20) | 0.87 (0.68, 1.11) | 58 | 0.65 (0.46, 0.91) | 0.62 (0.43, 0.88) |
| T3 (n = 613) | 222 | 0.79 (0.66, 0.95) | 0.76 (0.63, 0.93) | 115 | 0.74 (0.58, 0.95) | 0.68 (0.52, 0.90) | 69 | 0.78 (0.56, 1.08) | 0.68 (0.47, 0.99) |
| Model | Dietary Pattern 1 | Dietary Pattern 2 | Dietary Pattern 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| Systolic blood pressure, mmHg | |||||||||
| Crude | Reference | −1.28 (−4.55, 1.99) | −1.63 (−5.21, 1.94) | Reference | 0.78 (−2.63, 4.20) | 1.48 (−1.96, 4.91) | Reference | 0.21 (−3.34, 3.76) | −0.82 (−4.36, 2.73) |
| Adjusted | Reference | −1.36 (−4.81, 2.09) | −1.93 (−6.21, 2.34) | Reference | −0.19 (−3.66, 3.28) | 0.67 (−2.98, 4.33) | Reference | 1.08 (−2.62, 4.77) | 0.61 (−3.44, 4.67) |
| Diastolic blood pressure, mmHg | |||||||||
| Crude | Reference | 1.04 (−0.80, 2.89) | 1.03 (−0.99, 3.05) | Reference | −0.21 (−2.14, 1.71) | −0.36 (−2.30, 1.58) | Reference | 0.06 (−1.94, 2.06) | −0.93 (−2.93, 1.07) |
| Adjusted | Reference | 0.65 (−1.29, 2.59) | 0.53 (−1.87, 2.93) | Reference | −0.53 (−2.47, 1.42) | −1.21 (−3.26, 0.84) | Reference | 0.46 (−1.62, 2.53) | −0.64 (−2.91, 1.64) |
| Augmentation index, % | |||||||||
| Crude | Reference | −1.29 (−2.83, 0.24) | 0.63 (−1.05, 2.31) | Reference | −0.09 (−1.70, 1.51) | 1.52 (−0.10, 3.13) | Reference | 0.90 (−0.77, 2.57) | 0.09 (−1.57, 1.76) |
| Adjusted | Reference | −1.50 (−3.13, 0.12) | 0.23 (−1.78, 2.24) | Reference | −0.07 (−1.70, 1.57) | 1.74 (0.02, 3.46) | Reference | 0.31 (−1.44, 2.05) | −0.93 (−2.85, 0.99) |
| Log aortic pulse wave velocity, m/s | |||||||||
| Crude | Reference | 0.01 (−0.03, 0.06) | 0.003 (−0.04, 0.05) | Reference | 0.01 (−0.03, 0.05) | −0.02 (−0.06, 0.02) | Reference | −0.05 (−0.09, 0.00) | −0.05 (−0.09, 0.00) |
| Adjusted | Reference | 0.03 (−0.01, 0.06) | 0.03 (−0.02, 0.07) | Reference | −0.002(−0.04, 0.04) | −0.03 (−0.07, 0.01) | Reference | −0.03 (−0.07, 0.01) | −0.03 (−0.08, 0.02) |
| Log Triacylglycerol, mmol/L | |||||||||
| Crude | Reference | 0.001 (−0.08, 0.08) | 0.07 (−0.01, 0.16) | Reference | 0.05 (−0.03, 0.14) | −0.03 (−0.11, 0.06) | Reference | 0.001(−0.09, 0.09) | −0.09 (−0.18, 0.01) |
| Adjusted | Reference | −0.03 (−0.12, 0.05) | 0.03 (−0.07, 0.14) | Reference | 0.04 (−0.05, 0.12) | −0.05 (−0.14, 0.04) | Reference | 0.03 (−0.06, 0.12) | −0.06 (−0.16, 0.04) |
| Log C-Reactive Protein, mg/L | |||||||||
| Crude | Reference | 0.30 (0.09, 0.52) | 0.08 (−0.15, 0.32) | Reference | 0.16 (−0.06, 0.39) | 0.06 (−0.17, 0.28) | Reference | −0.04 (−0.27, 0.19) | −0.31 (−0.54,−0.08) |
| Adjusted | Reference | 0.26 (0.04, 0.49) | 0.03 (−0.24, 0.31) | Reference | 0.10 (−0.12, 0.33) | 0.07 (−0.17, 0.31) | Reference | 0.01 (−0.23, 0.26) | −0.18 (−0.45, 0.09) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertens, E.; Markey, O.; Geleijnse, J.M.; Givens, D.I.; Lovegrove, J.A. Dietary Patterns in Relation to Cardiovascular Disease Incidence and Risk Markers in a Middle-Aged British Male Population: Data from the Caerphilly Prospective Study. Nutrients 2017, 9, 75. https://doi.org/10.3390/nu9010075
Mertens E, Markey O, Geleijnse JM, Givens DI, Lovegrove JA. Dietary Patterns in Relation to Cardiovascular Disease Incidence and Risk Markers in a Middle-Aged British Male Population: Data from the Caerphilly Prospective Study. Nutrients. 2017; 9(1):75. https://doi.org/10.3390/nu9010075
Chicago/Turabian StyleMertens, Elly, Oonagh Markey, Johanna M. Geleijnse, David Ian Givens, and Julie A. Lovegrove. 2017. "Dietary Patterns in Relation to Cardiovascular Disease Incidence and Risk Markers in a Middle-Aged British Male Population: Data from the Caerphilly Prospective Study" Nutrients 9, no. 1: 75. https://doi.org/10.3390/nu9010075





