Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size
2.3. Anthropometric and Clinical Measurements
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Basic Characteristics of the Study Subjects
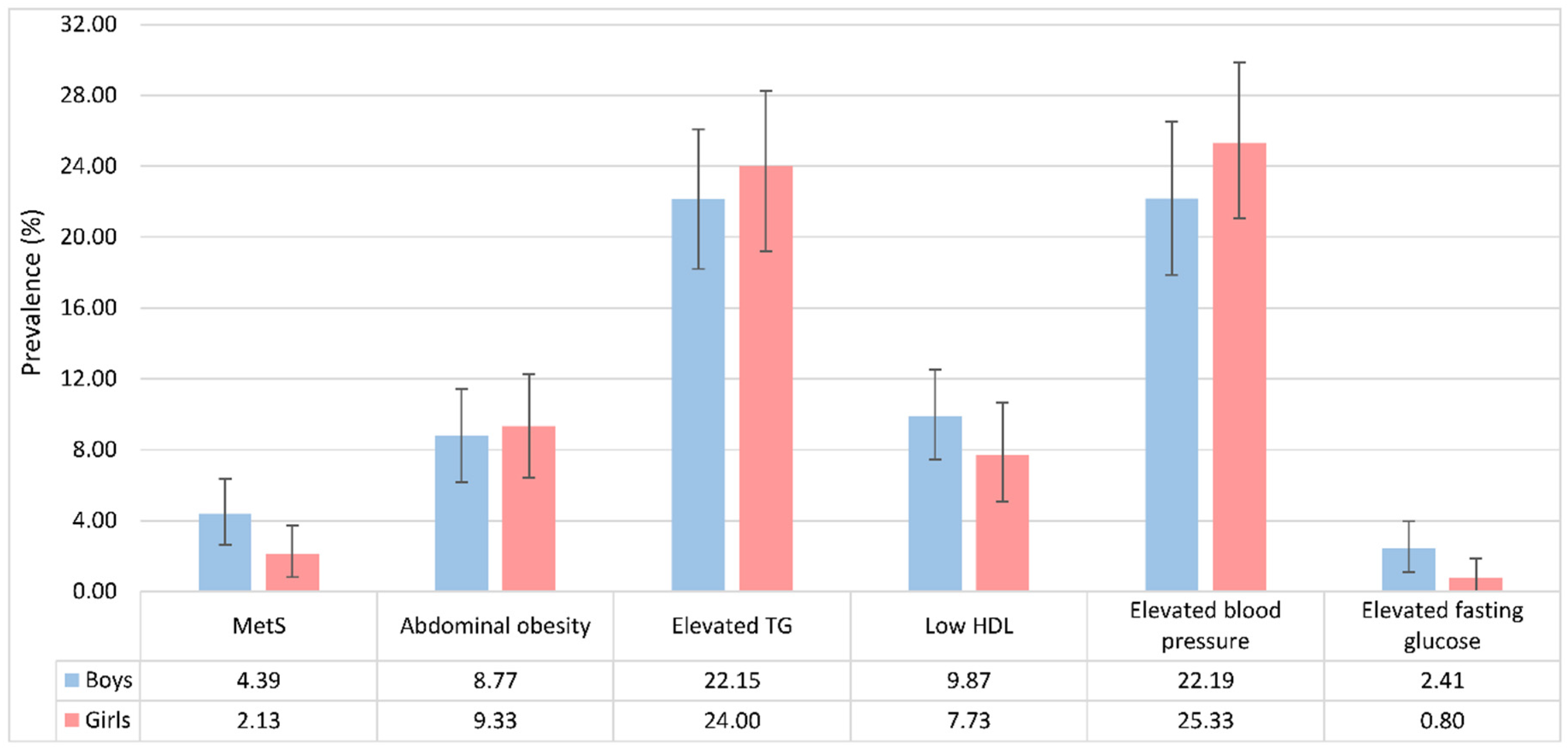
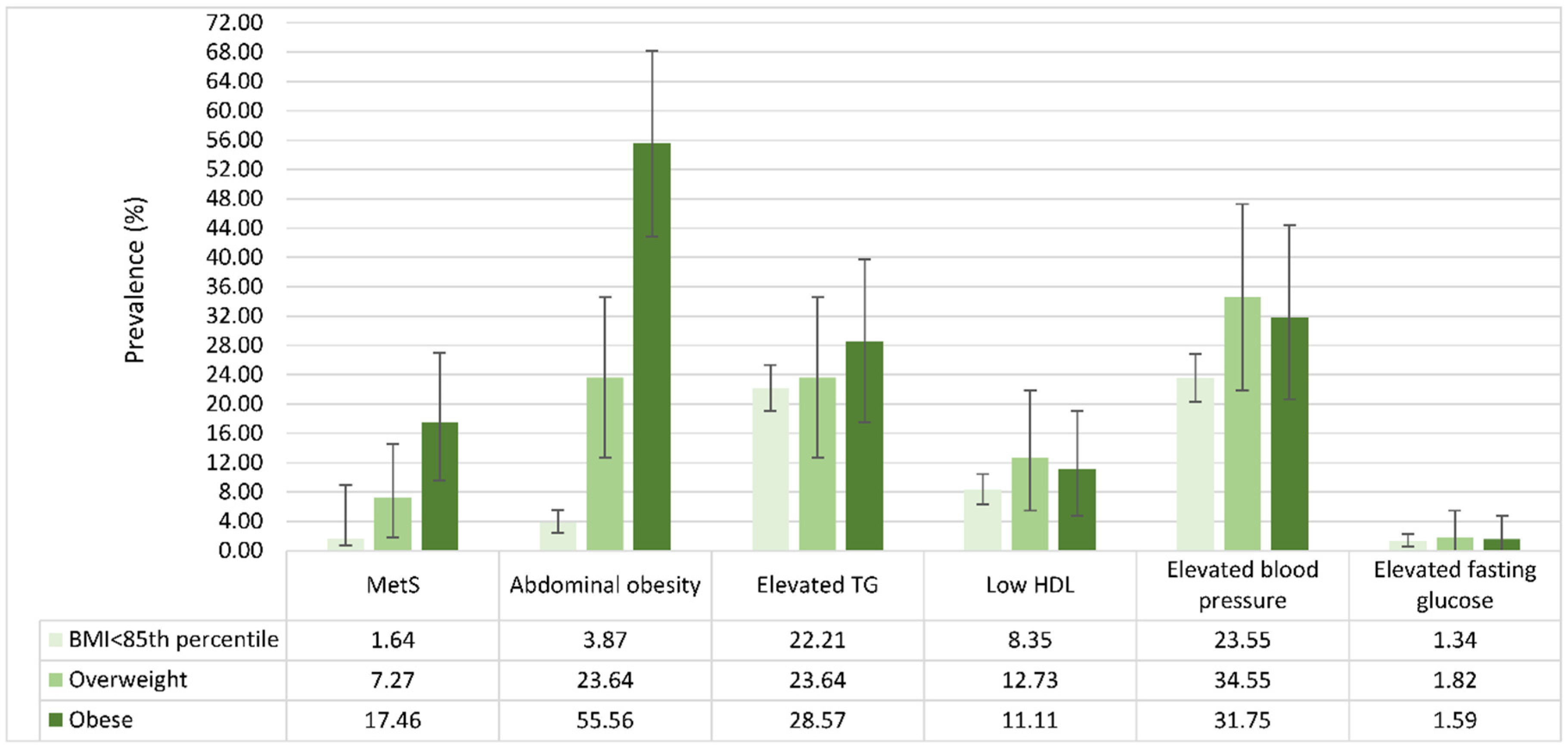
3.2. Prevalence and Distribution of MetS Components
3.3. Correlates of MetS
3.4. Agreement for Different Criteria of MetS
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Morris, R.W. Metabolic syndrome vs framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch. Intern. Med. 2005, 165, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a who consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA 2001, 285, 2486. [Google Scholar]
- Dhuper, S.; Cohen, H.W.; Daniel, J.; Gumidyala, P.; Agarwalla, V.; St Victor, R.; Dhuper, S. Utility of the modified ATP III defined metabolic syndrome and severe obesity as predictors of insulin resistance in overweight children and adolescents: A cross-sectional study. Cardiovasc. Diabetol. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents–an idf consensus report. Pediatr. Diabet. 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C. Diagnosis and management of the metabolic syndrome an american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Sánchez, H.; Harhay, M.O.; Harhay, M.M.; McElligott, S. Prevalence and trends of metabolic syndrome in the adult us population, 1999–2010. J. Am. Coll. Cardiol. 2013, 62, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mozumdar, A.; Liguori, G. Persistent increase of prevalence of metabolic syndrome among us adults: Nhanes iii to nhanes 1999–2006. Diabet. Care 2011, 34, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; He, D.; Hu, Y.; Zhou, D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: The china health and nutrition survey in 2009. Prev. Med. 2013, 57, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. N. Am. 2004, 33, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.M.; Sun, S.S.; Burns, T.L.; Morrison, J.A.; Huang, T.T.-K. Predictive ability of childhood metabolic components for adult metabolic syndrome and type 2 diabetes. J. Pediatr. 2009, 155, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Shan, X.; Cheng, H.; Hou, D.; Zhao, X.; Wang, T.; Zhao, D.; Mi, J. Association between childhood obesity and metabolic syndrome: Evidence from a large sample of chinese children and adolescents. PLoS ONE 2012, 7, e47380. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Reynolds, R.F.; Whelton, P.K.; He, J.; Group, I.C. Prevalence of the metabolic syndrome and overweight among adults in china. Lancet 2005, 365, 1398–1405. [Google Scholar] [CrossRef]
- Ji, C.Y.; Chen, T.J.; Working Group on Obesity in China (WGOC). Empirical changes in the prevalence of overweight and obesity among Chinese students from 1985 to 2010 and corresponding preventive strategies. Biomed. Environ. Sci. 2013, 26, 1–12. [Google Scholar] [PubMed]
- Ye, P.; Yan, Y.; Ding, W.; Dong, H.; Liu, Q.; Huang, G.; Mi, J. Prevalence of metabolic syndrome in Chinese children and adolescents: A meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi 2015, 36, 884–888. [Google Scholar] [PubMed]
- Zhang, B.; Zhai, F.; Du, S.; Popkin, B.M. The china health and nutrition survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort profile: The china health and nutrition survey—Monitoring and understanding socio-economic and health change in china, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, J.; Li, S.; Zhang, B.; Du, S.; Gordon-Larsen, P.; Adair, L.; Popkin, B. The expanding burden of cardiometabolic risk in china: The china health and nutrition survey. Obes. Rev. 2012, 13, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zhang, M.; Zhang, T.; Liang, Y.; Li, S.; Steffen, L.M. Hypertension screening using blood pressure to height ratio. Pediatrics 2014, 134, e106–e111. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, F.; Li, X.; Wu, L.; Zhang, Y.; Cheng, Y.; Zhou, W.; Huang, G. Blood pressure percentiles by age and height for non-overweight Chinese children and adolescents: Analysis of the china health and nutrition surveys 1991–2009. BMC Pediatr. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, C.; Zong, X.; Zhang, Y. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. Chin. J. Pediatr. 2009, 47, 493–498. [Google Scholar]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among us children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: Who Global Database on Anaemia; World Health Organization: Geneva, Switzerland, 2008; p. 4. [Google Scholar]
- Van der Aa, M.; Fazeli Farsani, S.; Knibbe, C.; De Boer, A.; van der Vorst, M. Population-based studies on the epidemiology of insulin resistance in children. J. Diabetes Res. 2015, 2015, 362375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, H.-P.; Pan, C.-W.; Xu, Y. Secular trends of age at menarche from 1985 to 2010 among Chinese urban and rural girls. Univ. J. Public Health 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Song, Y.; Ma, J.; Li, L.-B.; Dong, B.; Wang, Z.; Agardh, A. Secular trends for age at spermarche among chinese boys from 11 ethnic minorities, 1995–2010: A multiple cross-sectional study. BMJ Open 2016, 6, e010518. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, J.; Hu, P.; Zhang, B. Study of geographic distribution and 10 years’ change of spermarche in Chinese boys of han-group aged 11 to 18. Zhonghua Yu Fang Yi Xue Za Zhi 2011, 45, 522–526. [Google Scholar] [PubMed]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, X.; Gasevic, D.; Flores, A.B.; Yu, Z. Bmi, waist circumference reference values for chinese school-aged children and adolescents. Int. J. Environ. Res. Public Health 2016, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Prev. Med. 2007, 45, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Larsen, P.; Wang, H.; Popkin, B.M. Overweight dynamics in Chinese children and adults. Obes. Rev. 2014, 15, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, W.-Y. Prevalence of metabolic syndrome among Korean adolescents according to the national cholesterol education program, adult treatment panel iii and international diabetes federation. Nutrients 2016, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Ethnicity, obesity and the metabolic syndrome: Implications on assessing risk and targeting intervention. Expert Rev. Endocrinol. Metab. 2011, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
| Stage | 75th Percentile of the HOMA Index | |
|---|---|---|
| Boys | Girls | |
| Prepubertal | 2.94 | 2.62 |
| Pubertal | 4.43 | 4.56 |
| Postpubertal | 4.66 | 3.95 |
| Characteristic | Total (n = 831) | With MetS (n = 28) | With Abdominal Obesity (n = 75) | With Elevated TG (n = 191) | With Low HDL (n = 74) | With Elevated Blood Pressure (n = 199) | With Elevated Fasting Glucose (n = 14) |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Boys (%) | 456 (54.87%) | 20 (71.43%) | 40 (53.33%) | 101 (52.88%) | 45 (60.81%) | 104 (52.26%) | 11 (21.43%) |
| Girls (%) | 375 (45.13%) | 8 (28.57%) | 35 (46.67%) | 90 (47.12%) | 29 (39.19%) | 95 (47.74%) | 3 (78.57%) |
| Residence | |||||||
| Urban (%) | 347 (42.68%) | 11 (39.29%) | 31 (41.33%) | 84 (45.41%) | 28 (38.89%) | 77 (38.69%) | 8 (61.54%) |
| Rural (%) | 466 (57.32%) | 17 (60.71%) | 44 (58.67%) | 101 (54.59%) | 44 (61.11%) | 122 (61.31%) | 5 (38.46%) |
| Age (years) | 12.39 ± 3.05 | 11.83 ± 2.59 | 11.67 ± 2.52 $ | 12.96 ± 2.88 € | 13.09 ± 2.86 £ | 11.76 ± 3.00 § | 12.87 ± 3.45 |
| Weight (kg) | 39.64 ± 13.13 | 47.47 ± 14.93 * | 48.43 ± 15.11 $ | 41.97 ± 12.79 € | 42.55 ± 14.61 | 39.53 ± 14.08 | 39.48 ± 11.15 |
| Height (cm) | 147.3 ± 15.85 | 150.31 ± 11.08 | 150.34 ± 12.94 $ | 149.81 ± 13.77 € | 150.23 ± 16.02 | 145.27 ± 16.27 § | 148.85 ± 17.57 |
| BMI (kg/m2) | 17.77 ± 3.33 | 20.73 ± 5.02 * | 21.04 ± 4.48 $ | 18.32 ± 3.60 € | 18.30 ± 4.03 | 18.12 ± 3.51 | 17.57 ± 2.88 |
| WC (cm) | 63.26 ± 9.61 | 78.90 ± 10.52 * | 79.30 ± 7.77 $ | 65.51 ± 10.06 € | 66.26 ± 11.98 £ | 63.74 ± 11.30 | 64.16 ± 7.18 |
| SBP (mmHg) | 100.04 ± 13.03 | 111.04 ± 17.35 * | 104.38 ± 16.38 $ | 101.64 ± 13.83 | 99.97 ± 13.39 | 111.95 ± 10.63 § | 99.88 ± 14.52 |
| DBP (mmHg) | 66.67 ± 9.50 | 76.59 ± 11.48 * | 69.71 ± 10.59 $ | 68.00 ± 10.79 | 66.76 ± 10.06 | 76.05 ± 7.54 § | 65.67 ± 9.43 |
| Hb (g/L) | 137.65 ± 16.54 | 137.04 ± 11.29 | 136.05 ± 12.13 | 139.51 ± 17.34 | 140.36 ± 19.57 | 137.59 ± 15.99 | 145.21 ± 12.14 |
| UA (μmol/L) | 310.14 ± 84.99 | 390.18 ± 74.15 * | 339.96 ± 85.15 $ | 345.45 ± 89.49 € | 350.84 ± 92.60 £ | 308.70 ± 85.76 | 384.29 ± 92.03 ¥ |
| TC (mmol/L) | 3.88 ± 0.70 | 4.10 ± 0.82 | 3.99 ± 0.76 | 4.12 ± 0.82 € | 3.51 ± 0.62 £ | 3.92 ± 0.68 | 4.61 ± 1.31 |
| HDL (mmol/L) | 1.44 ± 0.53 | 1.13 ± 0.42 * | 1.49 ± 1.28 | 1.29 ± 0.37 € | 0.92 ± 0.09 £ | 1.42 ± 0.32 | 1.31 ± 0.33 |
| LDL (mmol/L) | 2.21 ± 0.88 | 2.18 ± 0.66 | 2.27 ± 0.59 | 2.21 ± 0.77 | 2.01 ± 0.57 | 2.18 ± 0.60 | 2.60 ± 1.41 |
| TG (mmol/L) | 1.01 ± 0.72 | 2.46 ± 1.35 * | 1.25 ± 0.84 $ | 1.94 ± 0.95€ | 1.57 ± 1.20 £ | 1.07 ± 0.66 | 2.42 ± 2.22 ¥ |
| Glucose (mmol/L) | 4.89 ± 0.80 | 5.43 ± 1.65 | 4.99 ± 0.97 | 5.15 ± 1.33 € | 4.79 ± 0.75 | 4.89 ± 0.53 | 8.59 ± 3.42 ¥ |
| Insulin (μU/mL) | 11.25 (8.08–16.68) | 23.71 (16.17–34.76) * | 18.59 (13.26–27.53) $ | 13.65 (9.83–23.45) € | 12.27 (7.50–16.91) | 12.37 (8.91–18.33) § | 27.01 (17.53–42.79) ¥ |
| HOMA-IR | 2.40 (1.71–3.66) | 4.94 (3.08–7.69) * | 3.86 (2.71–6.30) $ | 2.97 (2.15–5.33) € | 2.48 (1.51–3.66) | 2.70 (1.95–4.10) § | 9.26 (5.84–11.53) ¥ |
| Adjusted OR (95% CI) | p Value | |
|---|---|---|
| BMI category | ||
| <85th percentile r | 1.00 | |
| Overweight | 3.68 (1.09–12.50) | 0.036 |
| Obesity | 7.33 (2.84–18.90) | <0.001 |
| Hyperuricemia | ||
| No r | 1.00 | |
| Yes | 4.66 (1.93–11.25) | 0.001 |
| IR | ||
| No r | 1.00 | |
| Yes | 3.11 (1.31–7.41) | 0.010 |
| MetS Diagnosed by NCEP-ATP III | κ (95% CI) | ||||
|---|---|---|---|---|---|
| Total | + | - | |||
| MetS diagnosed by IDF | Total | 585 | 21 | 564 | 0.54 (0.30–0.74) |
| + | 8 | 8 | 0 | ||
| − | 577 | 13 | 564 | ||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Yu, J.; Chang, X.; Wang, M.; An, L. Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey. Nutrients 2017, 9, 79. https://doi.org/10.3390/nu9010079
Song P, Yu J, Chang X, Wang M, An L. Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey. Nutrients. 2017; 9(1):79. https://doi.org/10.3390/nu9010079
Chicago/Turabian StyleSong, Peige, Jinyue Yu, Xinlei Chang, Manli Wang, and Lin An. 2017. "Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey" Nutrients 9, no. 1: 79. https://doi.org/10.3390/nu9010079
APA StyleSong, P., Yu, J., Chang, X., Wang, M., & An, L. (2017). Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey. Nutrients, 9(1), 79. https://doi.org/10.3390/nu9010079




